Advances in nephron-sparing surgeries have led to an evolution in the standard of care management for small renal masses. Laparoscopic partial nephrectomy, by duplicating established principles of open surgery, has emerged as a viable alternative to open partial nephrectomy in select patients.1,2 In the continuing evolution of this minimally invasive approach to nephron-sparing surgery, laparoscopic or percutaneous renal tumor cryoablation has, in recent years, been introduced as a clinically effective option in select patients. Even though long-term follow-up and greater experience with laparoscopic cryotherapy for small renal masses is required, it has been shown to be an attractive option for treatment of patients with small renal masses in the short and intermediate term.3,4,5
Although several groups have reported complications attributable to cryotherapy, the experience levels of these groups with the use of cryothereapy has been limited, such that the scope of complications associated with renal tumor ablation is not very well known. These novel technologies give rise to potential complications previously unassociated with renal tumor treatment.6
We report here the first case, to our knowledge, of massive pulmonary thromboembolism after simultaneous renal laparoscopic cryoblation for the treatment of bilateral and multicentric papillary renal cell carcinoma.
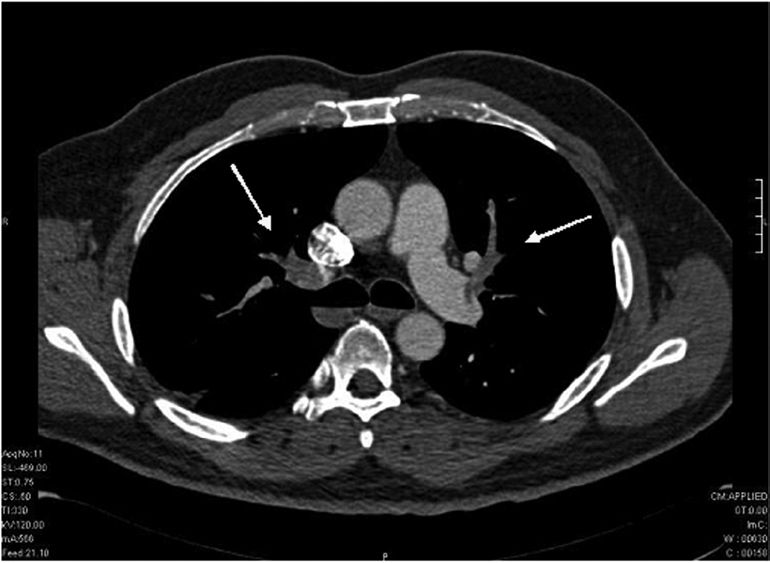
CASE REPORTA 63-year-old white male was referred to us with a familial history of papillary renal cell carcinoma. His surgical history included an open prostatectomy for treatment of benign prostatic hyperplasia 12 years before. His physical examination was normal, except for a median infraumbilical scar. Urinalysis revealed microscopic hematuria. A computed tomography and magnetic resonance imaging (MRI) showed bilateral, peripherally located enhancing renal masses. A 2 cm heterogeneous upper/mid pole mass was identified in the right kidney (Figure 1), and three contiguous masses were shown in the lower part of the left kidney (two of 3 cm and one of 4 cm) (Figure 2 - A,B,C). The patient underwent bilateral laparoscopic renal cryoablation via a transperitoneal approach under general anesthesia. Intraoperative real-time laparoscopic ultrasound was used to control the cryolesion. We used four 3 mm cryoprobes in the left kidney and one in the right kidney. A pre-cryoablation needle biopsy was performed, and the histopathological examination showed a papillary renal cell carcinoma. No intraoperative complications were seen. However, in the immediate post-operative period after extubation, the patient presented respiratory discomfort catheter. On the first post-operative day, the requiring an O2 respiratory difficulty was aggravated, and angio-tomography showed a massive pulmonary thromboembolism (Figure 3). Anticoagulation therapy with heparin was immediately started, and pulmonary function was progressively restored in subsequent weeks. The patient was discharged from the hospital on the 12th post-operative day.
Laparoscopic renal cryosurgery is considered a safe and effective minimally invasive alternative for the treatment of small renal masses.3,4 Cryotherapy is the most studied ablative alternative, and recent follow-up studies have shown encouraging oncological results.3,5 However, renal cryoablation was recently integrated into the clinical armamentarium, and some complications associated with this ablative procedure may be unique. The most common previously reported complications attributed directly to cryoablation include minor complications, like probe site pain or paresthesia, hemorrhaging and elevated serum creatinine. Some major complications, like urine leakage, liver and pancreatic lesions, secondary ureteropelvic junction obstruction and prolonged ileus, where also documented. However, some complications have been attributed by authors to the laparoscopic procedure or to surgical trauma, and are not thought to be directly related to problems seen with the cryoblation technique; these include postoperative urinary tract infections and post-operative pneumonia infections.6 To our knowledge, no previous report of post-operative pulmonary thromboembolism after renal cryoablation has been discussed in the literature. Nadler et al. reported a case of postoperative respiratory difficulty with reintubation for 24 hours, without etiologic diagnosis.4,6 In our case, the respiratory distress started during the anesthesia recovery process. The orotracheal extubation was immediately followed by a massive pulmonary thromboembolism suggesting a cause-effect relationship between this complication and the cryoblation procedure. An “ice-blood” that formed in a branch of renal vein around the tumor might have launched the coagulation phenomenon, and an embolus could have migrated to the pulmonary arteries. Such a complication may be considered to be directly associated with the location and the amount of tissue cryoablated on the left side. However, cancer alone, as well as its treatments, involves well-recognized risk factors for venous thromboembolism. Cancer alone has been associated with a 4.1-fold risk of thrombosis, whereas chemotherapy increased the risk 6.5-fold.7 Patients undergoing surgery for cancer also have a higher risk of postoperative deep vein thrombosis than those having surgery for nonmalignant diseases.8 Therefore, the massive pulmonary embolism in our patient might have resulted from an unclear deep vein thrombosis secondary to the procoagulation effect of the renal cancer.
We would advise that more comprehensive experience should be obtained with this technique before extending the indications of cryoablation therapy to large, intrarenal or bilateral simultaneous renal tumors. Aggressive prophylaxis for deep vein thrombosis should be considered, even for minimally invasive procedures in patients with cancer.