Bevacizumab has been widely used as a vascular endothelial growth factor antagonist in the treatment of retinal vasoproliferative disorders in adults and, more recently, in infants with retinopathy of prematurity. Recently, it has been proposed that vascular endothelial growth factor acts as a protective factor for neurons and glial cells, particularly in developing nervous tissue. The purpose of this study was to investigate the effects of bevacizumab on the developing retinas of juvenile rabbits.
METHODS:Juvenile rabbits received bevacizumab intravitreously in one eye; the other eye acted as an untreated control. Slit-lamp and fundoscopic examinations were performed both prior to and seven days after treatment. At the same time, retina samples were analyzed using immunohistochemistry to detect autophagy and apoptosis as well as proliferation and glial reactivity. Morphometric analyses were performed, and the data were analyzed using the Mann-Whitney U test.
RESULTS:No clinical abnormalities were observed in either treated or untreated eyes. However, immunohistochemical analyses revealed a reduction in the occurrence of programmed cell death and increases in both proliferation and reactivity in the bevacizumab-treated group compared with the untreated group.
CONCLUSIONS:Bevacizumab appears to alter programmed cell death patterns and promote gliosis in the developing retinas of rabbits; therefore, it should be used with caution in developing eyes.
Bevacizumab (Avastin; Genentech Inc., San Francisco, California, USA) is a humanized monoclonal antibody that recognizes all vascular endothelial growth factor (VEGF) (1). isoforms and is approved by the Food and Drug Administration for the treatment of metastatic colorectal cancer (2). Because patients with concomitant age-related macular degeneration have experienced improved visual acuity when treated intravenously with bevacizumab, the possibility of administering the drug intravitreally to treat neovascularization disorders of the eye without systemic adverse effects has been investigated (2–4). By early 2006, an accumulation of compelling functional and anatomical evidence led to a dramatic increase in the off-label intravitreal use of bevacizumab as a first-line therapy for exudative age-related macular degeneration (2). Over the last five years, intravitreal bevacizumab has been used to treat a rapidly expanding spectrum of adult retinal diseases (2). based on the well-established role of VEGF in angiogenesis and vascular development (5–7). More recently, intravitreous bevacizumab has been used to treat retinal disease in the developing retinas of premature infants; by controlling VEGF levels, bevacizumab inhibits the pathological neovascularization that is observed in retinopathy of prematurity in infants receiving oxygen therapy (8,9).
Although it is primarily known for its roles in endothelial cell growth and vascular permeability (6), VEGF has recently been recognized as a neuroprotective factor in the tissues of the central nervous system, including the retina (7,10). The widespread use of anti-VEGF drugs such as bevacizumab, combined with the fact that VEGF blockade can alter the physiological structure of the retina, has led researchers to evaluate the possible effects of these drugs on retinal tissue. Although some studies have described bevacizumab-associated ultrastructural abnormalities in the retinas of rabbits, mice, and primates (11–13), most researchers have observed no retinal toxicity in the developed retinas of adult animals (14–17). One recent in vivo study evaluated the effect of bevacizumab on the developing retina; though it described the effect of injection of the drug into 11- and 25-day-old rabbits (18), no quantitative analyses of cell death, proliferation or gliosis were performed. Further in vivo studies in developing retinas are necessary because, in addition to its significant effect on angiogenesis and vascular generation during tissue development, VEGF promotes the proliferation, differentiation, and survival of retinal glial cells and neurons, which express VEGF receptors at this developmental stage (1,9). Therefore, VEGF may act as a neuroprotective and neurotrophic factor in the developing retina, influencing the growth, differentiation, and survival of retinal cells (5,7).
The present study was designed to evaluate, both clinically and histologically, alterations in the developing retinas of rabbits resulting from intravitreal bevacizumab administration.
MATERIALS AND METHODSAnimalsAll procedures were designed in accordance with the Association for Research in Vision and Ophthalmology statement for the use of animals in ophthalmic and vision research. The study was previously approved by the Ethics Committee for Animal Research of the Federal University of Rio de Janeiro. Five juvenile (21-day-old) 500-g male New Zealand albino rabbits were maintained under a 12/12-h light/dark cycle with ad libitum access to water and food. Prior to each experiment, both eyes of each rabbit were subjected to slit-lamp evaluation, indirect ophthalmoscopy, and retinal fundus photography to exclude animals with ocular disorders that might interfere with the results.
Experimental proceduresPrior to experimentation, the rabbits were anesthetized by intramuscular injection of 25 mg/kg ketamine hydrochloride and 5 mg/kg xylazine hydrochloride, followed by instillation of 0.01 g tropicamide in each eye to promote pupil dilation. After a topical anesthetic (proxymetacaine) was administered, the left eye of each animal was washed with 5% povidone iodide and injected intravitreally with 0.03 mL (0.75 mg) of bevacizumab solution (Avastin; Genentech Inc., San Francisco, California, USA). The solution was injected into the mid-vitreous cavity, 1.5 mm posterior to the limbus at the 3-o'clock position, using a 28-gauge needle attached to a 1.0 mL tuberculin syringe. To enable observation of the inner structures of the eyes, the procedure was performed using a surgical microscope. The untreated right eye of each rabbit was used as a control. The animals were submitted to a second slit-lamp evaluation and indirect ophthalmoscopy immediately after bevacizumab administration and before being returned to their cages. This step was performed to exclude the possibility that the vehicle or route of administration produced any alteration in the retina.
Seven days after the injection, the rabbits (then 28 days old) were evaluated under a slit lamp and submitted to indirect ophthalmoscopy and retinography to detect inflammation, retinal injury, or cataract formation. The animals were then anesthetized as described above and euthanized with an intravenous overdose of 10% potassium chloride.
Histological proceduresThe eyes of each animal were enucleated, and sections of the posterior part of the eye (i.e., the sclera, choroid, and retina) were obtained by cutting the eyes through the equator zone. Subsequently, the half-eyes were sectioned through their vertical diameter, yielding material for histological analyses that contained the central and peripheral retinal areas. The tissue was then fixed in 4% paraformaldehyde, dehydrated in a graded ethanol series, cleared in xylene, and embedded in paraffin. Five-micrometer sections that were made using a rotary microtome were mounted on poly-L-lysine-coated slides. The treated- and control-eye sections were mounted on different slides. For routine optical microscopy, the sections were stained with hematoxylin and eosin (HE). For immunohistochemical analysis, the sections were treated with 3% hydrogen peroxide to inhibit endogenous peroxidases before being washed with PBS containing 0.2% Triton X-100.
The TUNEL method was used to assess cell death by autophagy and apoptosis using a rabbit polyclonal anti-beclin1 primary antibody and an ApopTag peroxidase detection kit (Chemicon International, Inc., Temecula, California, USA). To determine whether retinal cells were proliferating, a mouse monoclonal anti-proliferating cell nuclear antigen (PCNA) primary antibody (Santa Cruz Biotechnology, Inc., Santa Cruz, California, USA) was used. At this stage of rabbit development, neurogenesis has already ceased (19). Therefore, it was assumed that the glial cells were the only cell type capable of proliferation; this assumption was confirmed by glial fibrillary acidic protein detection (GFAP) using a rabbit polyclonal anti-GFAP primary antibody (Chemicon International, Inc., Temecula, California, USA), which labeled glial cells.
The first step in the immunohistochemical analysis was antigen retrieval. First, the slides were either immersed in 0.1 M sodium citrate buffer (pH 6.0) and incubated for 30 min in a steamer pot (for PCNA testing) or immersed in 0.1 M sodium citrate buffer (pH 6.0) and placed in a microwave at 90°C for 10 min (for beclin1, TUNEL staining, and glial fibrillary acidic protein [GFAP] testing). The slides were then washed with cold sodium citrate and PBS before being treated with 10% bovine serum albumin to block nonspecific binding of immunoglobulin to the tissues. A 1:100 dilution of a primary antibody against beclin1, PCNA, or GFAP was then added, and the slides were incubated in a humid chamber overnight at 4°C. Three washes with PBS containing 0.2% Triton X-100 were performed to terminate the reaction.
The TUNEL assays were performed according to the manufacturer's instructions, with the exception of antigen retrieval, which included a 10-min incubation step in 0.1 M sodium citrate buffer (pH 6.0) at 90°C.
All immunohistochemical reactions were visualized using a StreptAB Complex/HRP Duet Kit (Dakocytomation, Carpinteria, California, USA) using diamino benzidine (Chemicon International, Inc., Temecula, California, USA) as the chromogen. The sections were then counterstained with hematoxylin, dehydrated in a graded series of ethanols, cleared in xylene, mounted, and examined using a light microscope (Zeiss Axioskop 2 Plus; Carl Zeiss, Baltimore, Maryland, USA).
Quantitative AnalysisSections containing the central and peripheral retinal areas from treated and control eyes were evaluated using a light microscope (63x objective) connected to a digital camera (Coolpix 990; Nikon, Melville, New York, USA). This magnification allowed the entire thickness of the retina to be observed in one field of view. For histomorphometry analysis, five fields of view for each section of each retina (a total of 25 fields of view for each of the treated and control retinas) were chosen at random, and images were recorded using AxioVision 3.0 software (Carl Zeiss, Baltimore, Maryland, USA). Uneven illumination and background were corrected using Adobe Photoshop, version 3.0 (Adobe Systems, San Jose, California, USA).
The cytoplasm staining patterns for beclin1 and GFAP in both the control and treated retinas, as well as the nuclear TUNEL staining patterns and PCNA immunohistochemistry data, were quantified using Image Pro Plus software (MediaCybernetics, Bethesda, Maryland, USA). The presence of brown-stained cytoplasm (for beclin1 and GFAP analysis) or nuclei (for TUNEL and PCNA analysis) was marked for subsequent calculation by the software.
The measurements obtained from the two groups (control and treatment) were compared using the Mann-Whitney U test. A p-value of less than 0.05 was taken to represent statistical significance.
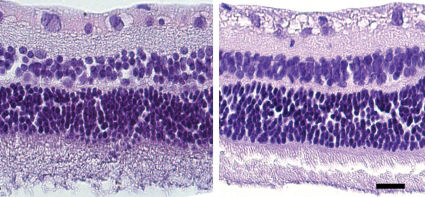
RESULTSClinical examinations that were performed either immediately following or seven days after intravitreal bevacizumab administration revealed that treatment with the drug did not cause mechanical injury to the eye tissues or produce ocular diseases such as conjunctivitis, scleritis, uveitis, or cataracts (data not shown). Figure 1 shows examples of fundus photographs of both treated and untreated animals both prior to and seven days after treatment, indicating that no abnormalities were observed in the animals. Routine light microscopy with HE staining also revealed no abnormalities in any group (Figure 2).
Beclin1 staining was observed in all of the retinal layers from both groups. However, the cytoplasms of the control retinas (Figure 3A) were more strongly stained than those of the treated retinas (Figure 3B). Quantitative analysis revealed a mean beclin1-positive cytoplasm area of 18.73 μm2 in the control group and 8.22 μm2 in the treated group, with a significant difference between the groups (p = 0.0079; Figure 1C.
First row: Immunohistochemical staining of beclin1 revealing positive staining in control retinas (A) that is visibly stronger than that observed in the bevacizumab-treated animals (B). Box plot of the staining area for beclin1 showing a significant difference (p<0.05) between groups (C). Second row: TUNEL staining showing that the number of apoptotic nuclei (brown) in the control retinas (D) is significantly higher compared with the bevacizumab-treated retinas. (E). Box plot analysis revealed a significant difference between groups (p<0.05) (F). Third row: Bevacizumab increases cell proliferation in the retinas of juvenile rabbits. PCNA immunohistochemical analysis revealed more proliferating cells (brown nuclei) in the bevacizumab-treated retinas (H) than in the control group (G). The number of proliferating cells (PCNA-positive) in the bevacizumab-treated group was significantly greater (p<0.05) than that observed in the control group (I). Fourth row: GFAP immunohistochemical analysis revealed stronger staining in the bevacizumab-treated retinas (L) than in the control retinas (J), indicating that the number of glial cells in the retinas increased after treatment (p<0.05) (M).
The TUNEL assay revealed large numbers of apoptotic nuclei in the control retinas (Figure 3D), particularly in the ganglion cell and inner nuclear layers; this is in contrast to the near-complete absence of apoptotic cells in the treated retinas (Figure 3E). The average number of apoptotic cells observed was 11.63 for the control group and 3.06 for the treated group. The difference between the groups was significant (p = 0.0286; Figure 3F.
Proliferating cell nuclei, detected by immunohistochemical analysis with the anti-PCNA antibody, were particularly numerous in the ganglion cell layers and inner nuclear layers of the treated retinas (Figure 3H). The mean number of brown-stained nuclei was higher in the treated group (21.88) (Figure 3H) than in the control group (8.88) (Figure 3G); (p = 0.0079; Figure 3I.
Immunohistochemical analysis of GFAP expression revealed that the retinal cells from both groups were labeled, though the cells of the treated retinas were more strongly stained (Figure 3L) than those of the controls (Figure 3J). Quantitative analysis revealed mean-stained cytoplasm areas of 14.13 μm2 in the treated retinas and 3.409 μm2 in the controls (p = 0.0079; Figure 3M.
DISCUSSIONThe apparent role of VEGF in a spectrum of pathological neovascular choroid and retinal diseases has led physicians to use anti-VEGF agents, including bevacizumab, off-label for the intravitreal treatment of vasoproliferative ocular disorders. However, the toxicity of bevacizumab to the retina has become an issue of great concern and has recently been the subject of intense research (2,10,11,14–17,20–23). Clinical and histological studies that tested the effect of bevacizumab on rabbit retinal tissue largely failed to detect toxicity-induced injury (4,15). However, the safety studies published thus far have, to our knowledge, only evaluated adult animals; therefore, they have been limited to assessing the safety profile of bevacizumab on fully developed retinas (11,14,16,17,20). During retinal development, VEGF is expressed by astrocytes in the retinal ganglion cell layer, Müller cells and other cells of the inner nuclear layer, and retinal pigment epithelial cells. Because the VEGF receptor is expressed in both glial cells and neurons, it has been suggested that VEGF must play a pivotal role in the proliferation, differentiation, and survival of the retina (9).
Because of the growing awareness of the neuroprotective role of VEGF, this study was designed to evaluate alterations in the developing retinal tissue of rabbits resulting from intravitreal bevacizumab administration. To this end, we clinically examined bevacizumab-treated eyes and histologically characterized them by evaluating programmed cell death patterns (24,25). and the response of glial cells to VEGF blockade. In our experiments, one eye of each rabbit received an intravitreal injection of 0.03 mL bevacizumab. The same dose is given to preterm babies to treat retinopathy of prematurity (26), although the therapeutic dosage is still unknown (27). Because intravitreal treatment with bevacizumab can only be accomplished by injection, the injected eyes represented the treated group in this study. The contralateral eyes were left untreated and used as controls. Although Nomoto et al (28). demonstrated that in rabbits, intravitreous or subconjunctival bevacizumab injection results in a high plasma concentration of the drug, the amount of bevacizumab in the contralateral coroid/retina tissue was approximately 400 times lower than that observed in the injected eye. Therefore, we believe that the contralateral eye is an adequate control for the experiment; furthermore, the same approach has been used in several studies that were published previously (11,14,16,18). The immunohistochemical changes we observed between the treatment and control groups further indicate that the contralateral eye was not affected by the absorption of bevacizumab.
In the current study, clinical ophthalmic evaluations performed using a slit-lamp, indirect ophthalmoscopy, and retinography were used to verify the absence of any abnormality or pathology either immediately or seven days after the injection of bevacizumab. This result is consistent with those obtained in studies using fully developed retinas, though it does not exclude the possibility of cellular or molecular damage to the retinal cells (4,17). Therefore, to assess the possibility of such damage, we performed histological analyses. Rabbits aged 28 days are considered juveniles, and their retinas are still undergoing programmed cell death, which lasts until the 50th postnatal day (19). During normal eye development, autophagy and apoptosis help control cell proliferation and maintain the cytoarchitecture and proper function of the retina (29–33). The intense beclin1 staining, which indicates the presence of autophagy, and the high number of apoptotic nuclei observed in our control group confirm that the morphology and physiology of the retina are still being refined to improve connectivity in juvenile rabbits (25,32,33). Interestingly, retinas treated with the anti-VEGF drug bevacizumab exhibited significantly fewer cells undergoing autophagy and apoptosis than the control retinas. Considering the importance of programmed cell death in the developing retina, the blockade of VEGF with bevacizumab may have caused the observed changes in the regulation of cell death. Therefore, our results, combined with the fact that various retinal cells express VEGF and constitutively activated VEGF receptors (9). during development, support the hypothesis that VEGF plays a role in the control of retinal cell death and differentiation.
Müller cells, the principal glial cells of the vertebrate retina, are neuron-supporting cells that span the entire thickness of the retina and interact closely with all types of neurons. Müller cells serve as an anatomical link to sites of molecular exchange, including retinal blood vessels, the vitreous body and the subretinal space (34). Müller cells synthesize and secrete important signaling molecules, such as trophic factors, during retinal development (34–36). In response to any nervous system injury that compromises tissue homeostasis, Müller cells respond with hypertrophy and hyperplasia, which characterize gliosis (10,34,37,38). This phenomenon is reflected immunohistochemically by an upregulation of the intermediate filament protein GFAP (34). In this study, the striking increase in the number of PCNA-positive nuclei in bevacizumab–treated retinas compared with the controls, together with the observed upregulation of GFAP expression, suggest high levels of gliosis. This finding is suggestive of gliosis because the retinas of juvenile rabbits, though they are still differentiating, have already undergone most of the necessary neurogenesis (19). The intense labeling with the anti-GFAP antibody that was observed in the treated retinas supports this assumption. In fact, the idea that increased Müller cell gliosis occurs upon treatment with anti-VEGF antibodies has been proposed in other studies, although these studies used a different experimental approach (i.e., rat retinal Müller glial cell culture) (10). This neuroprotective mechanism is responsible for the release of neurotrophic factors, including VEGF, in pathological conditions (10,34). The overexpression of VEGF during gliosis may explain why it is necessary to administer recurrent intravitreal injections of bevacizumab to reduce pathological neovascularization in humans (38).
The histological alterations observed in the developing rabbit retinas after the intravitreal administration of bevacizumab represent a novel finding. This preliminary study used healthy animals and short-term treatments; therefore, our results do not guarantee that the striking acute responses observed in the developing retinas will continue after long-term treatment. However, our results support the hypothesis that the intravitreal use of this anti-VEGF agent may not be completely risk-free. It is possible that the molecular abnormalities observed in the study could be the result of a combined effect of bevacizumab and the trauma of injecting the drug into the eye. We believe that the effect is the result of pharmacological action of bevacizumab because previous studies that evaluated saline-injected eyes did not reveal any histological or electrophysiological abnormalities in the retinas of rabbits (11,17,18,20). However, even if the abnormalities observed in the current study were the result of a combination of the pharmacological effect of bevacizumab and the trauma of the injection, this would not diminish the importance of the findings. Because bevacizumab is administered through intravitreous injections when used as a treatment for retinal diseases (including those of newborn infants), it is important to evaluate the effects of bevacizumab treatment under the same administration conditions. Therefore, we determined that the contralateral non-injected eye was the best possible control to assess the effect of bevacizumab injection treatment. However, future studies using different routes for the delivery of bevacizumab to the retina or in vitro models are necessary for further clarification.
One issue of particular concern is the use of bevacizumab for the treatment of preterm babies suffering from retinopathy of prematurity, a leading cause of lifelong visual impairment and blindness in premature babies (21,39). In these patients, the retina is still undergoing development, and the potential side effects of anti-VEGF drugs such as bevacizumab are still unknown (39). To our knowledge, no clinical trials that have monitored preterm infants receiving local treatment with bevacizumab have been completed, and studies of the long-term effects of this drug on the developing retina are still lacking (40). To further validate the potential consequences of such treatment in the developing retinas of premature newborns suffering from retinopathy of prematurity, our findings should be complemented by other investigative approaches.
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brasília; the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); the Rio de Janeiro State Foundation for the Advancement of Science (FAPERJ); the José Bonifácio Foundation (FUJB); and the Federal University of Rio de Janeiro Research Chamber (SR2/UFRJ), Brazil.
No potential conflict of interest was reported.
Fusco MA designed the study and was also responsible for the collection and management of data and writing of the manuscript. Portes ALF was responsible of the study design and data collection. Allodi S was responsible for the design of the study, fundraising and revision of the manuscript. Moraes Jr HV designed the study. Monteiro MLR designed the study and was also responsible for the writing and revision of the manuscript. Miguel NCO designed the study and was also responsible for collection and management of data, fundraising, writing and revision of the manuscript.