Hypoxia-inducible factor 1 alpha regulates genes related to cellular survival under hypoxia. This factor is present in osteroarthritic chondrocytes, and cytokines, such as interleukin-1 beta, participate in the pathogenesis of osteoarthritis, thereby increasing the activities of proteolytic enzymes, such as matrix metalloproteinases, and accelerating cartilage destruction. We hypothesize that Hypoxia Inducible Factor-1 alpha (HIF-1α) can regulate cytokines (catabolic action) and/or growth factors (anabolic action) in osteoarthritis. The purpose of this study was to investigate the modulation of HIF-1α in human osteoarthritic chondrocytes by interleukin-1 beta (IL-1β) and insulin-like growth factors I (IGF-I) and II (IGF-II) and to determine the involvement of the phosphatidylinositol-3-kinase (PI-3K) pathway in this process.
METHODS:Human osteroarthritic chondrocytes were stimulated with IL-1β, IGF-I and IGF-II and LY294002, a specific inhibitor of PI-3K. Nuclear protein levels and gene expression were analyzed by western blot and quantitative reverse transcription-polymerase chain reaction analyses, respectively.
RESULTS:HIF-1α expression was upregulated by IL-1β at the protein level but not at the gene level. IGF-I treatment resulted in increases in both the protein and mRNA levels of HIF-1α, whereas IGF-II had no effect on its expression. However, all of these stimuli exploited the PI-3K pathway.
CONCLUSION:IL-1β upregulated the levels of HIF-1α protein post-transcriptionally, whereas IGF-I increased HIF-1α at the transcript level. In contrast, IGF-II did not affect the protein or gene expression levels of HIF-1α. Furthermore, all of the tested stimuli exploited the PI-3K pathway to some degree. Based on these findings, we are able to suggest that Hypoxia inducible Factor-1 exhibits protective activity in chondrocytes during osteoarthritis.
Articular cartilage is a highly specialized tissue present in all diarthrodial joints, and its breakdown is a crucial event in the etiopathogenesis of osteoarthritis (OA). OA is characterized by the degeneration of articular cartilage in association with subchondral bone erosions and sclerosis. Numerous inflammatory cytokines, such as interleukins 1 and 6 (IL-1 and IL-6) and tumor necrosis factor-α (TNFα), participate in the pathogenesis of this disease, increasing the expression of proteolytic enzymes and metalloproteases (MMPs) and accelerating the destruction of cartilage (1–3). Chondrocytes are cartilage cells that exist in a hypoxic microenvironment because cartilage is an avascular tissue. Hypoxia-inducible factor 1 (HIF-1) is a transcription factor that activates the expression of target genes involved in essential pathways regulating cellular survival under conditions of hypoxia, such as angiogenesis and glycolysis (4). Under normal oxygen tension, the HIF-1α subunit is marked for ubiquitination and rapid proteosome-mediated degradation by the von Hippel-Lindau tumor suppressor (pVHL). During hypoxia, ubiquitination and degradation are inhibited, increasing the steady-state level of HIF-1α protein in the cytoplasm. HIF-1α subsequently translocates to the nucleus, where it dimerizes with HIF-1β, the constitutively expressed HIF-1 subunit, forming the transcription complex HIF-1 (5,6). In addition to hypoxia, HIF-1α expression can be induced by numerous other factors, including inflammatory cytokines, reactive oxygen species (ROS), nitric oxide, and hormone-like growth factors, such as Insulin-like growth factor (IGF) and TGF-β (Transforming Growth-factor β) (7). In previous studies, we identified expression of HIF-1α associated with human OA as well as in normal chondrocytes under normal oxygen tension conditions and found that HIF-1α protein levels were increased by TNFα treatment (8). Because HIF-1α is related to cellular survival via the modulation of genes related to this function, we believe that when this transcription factor is present in osteoarthritic chondrocytes, it could be related to cytokine modulation. Specifically, HIF-1α may modulate IL-1β, a major catabolic factor involved in OA that can induce potent changes in cartilage metabolism in OA joints, thereby inhibiting the synthesis of cartilage-specific collagen II and proteoglycans and increasing the production of numerous MMPs (2,3,9).
Similarly, we chose to investigate insulin-like growth factors I and II, which are reported to be involved in HIF-1α regulation in other cell lines (10,11). In chondrocytes, the anabolic role of these growth factors, especially IGF-I, is well recognized as stimulating cellular proliferation and extracellular-matrix synthesis (12,13). However, no studies have examined whether IGF-I and II regulate the expression of HIF-1α in these cells. Thus, the aim of the present study was twofold (1): to investigate the modulation of the expression of HIF-1α in human OA chondrocytes under normal oxygen conditions in response to treatment with IL-1β, IGF-I and IGF-II; and (2). to determine whether the phosphatidylinositol-3-kinase (PI-3K) pathway participates in this modulation, as is the case in other cell types.
MATERIALS AND METHODSIsolation and culture of human OA chondrocytesHuman chondrocytes were obtained from patients with OA who underwent knee joint replacement surgery at the University Hospital of Campinas, São Paulo, Brazil. This study was analyzed and approved by the local ethics committee. The patients signed informed consent documents to allow the authors to use material from their replaced joints in the current study. Approximately 80% of the patients presented as class IV in the Kelgreen and Lawrence (14). radiographical scale. Chondrocytes were isolated from all remaining cartilage tissue as previously described (15). Briefly, the cartilage was minced and incubated in Hanks' medium containing trypsin and bacterial collagenase (2 mg/ml each) for 1 h at 37°C. The medium was subsequently discarded, and tissue fragments were incubated overnight at 37°C in Dulbecco's minimum essential medium (DMEM) containing 10% fetal bovine serum and 0.5 mg/ml bacterial collagenase. The released cells were filtered through a 70-μm nylon cell strainer, were collected by centrifugation at 250×g for 5 min and were washed with collagenase-free medium. Isolated chondrocytes were immediately frozen in freezing media (90% FBS, 10% DMSO) and stored for future experiments. For these experiments, cells were thawed and plated in suspension cultures in 6-well ultralow attachment plates (Corning, Acton, MA) at a density of 5×106/ml. The cells were allowed to recover for 48 h in DMEM containing 10% FBS, 2 mM glutamine, 1% vitamin supplements, 100 U/ml penicillin and 100 μg/ml streptomycin. Amphotericin B and ascorbic acid were avoided because they can interfere with HIF-1α expression (16). For experiments under normoxic conditions, cells were maintained at 37°C in 5% CO2 and 95% air (21% O2). The cells were stimulated for 6 h with 10 ng/ml IL-1β Pierce Endogen (N. Meridian Road, Rockford, IIL). To study PI-3K, the specific inhibitor LY 294002 Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO) was employed at a concentration of 10 μM/ml. For treatment with IGF-I or IGF-II (at 10 ng/ml each), cells were serum-depleted to 0.1% FBS 12 h before the indicated treatment, and nuclear protein extraction was performed 12 h after addition of the growth factor.
Preparation of nuclear extractsNuclear extracts were isolated from the chondrocytes according to the method of Dignam et al (17). using the CellLytic NuCLEAR extraction kit (Sigma-Aldrich, St. Louis, MO) at 4°C to avoid nuclear protein denaturation according to the manufacturer's instructions. All buffers contained a protease inhibitor cocktail with 2 mM 4-(2-aminoethyl) benzenesulfonylfluoride, 1.4 pMtrans-epoxysuccinyl-L-leucylamido (4-guanidinobutane), 130 pM bestatin, 1 μM leupeptin, 0.3 pM aprotinin (Sigma-Aldrich) and 2.6 μM calpain inhibitor (Calbiochem, San Diego CA). The obtained protein concentrations were analyzed via the Bradford method at a wavelength of 595 nm using a spectrophotometer.
Western Blot AnalysisFor western blot analysis, 30 μg of nuclear chondrocyte extract in 2x SDS buffer and distilled water (final volume of 30 μl) was denatured at 95°C for 90 sec and then separated on an 8% SDS-polyacrylamide gel. Following electrophoresis, the proteins were transferred to Hybond-P membranes (Amersham Pharmacia Biotech) in buffer containing 20 mM Tris HCl, 150 mM glycine and 20% methanol at 40 V for 18 h at 4°C. The membranes were blocked with Tris-Buffered Saline (TBS) wash solution containing 5% nonfat milk and 0.1% Tween 20 (TBSMT) for 2 h at pH 7.6. The membranes were incubated with the primary antibody, a mouse monoclonal anti-HIF-1 antibody (1/250 dilution, Transduction Laboratories, Lexington, KY), with shaking overnight at 4°C followed by incubation with the secondary antibody, a rabbit anti-mouse IgG-horseradish peroxidase conjugate (1/2,000 dilution, Amersham Pharmacia Biotech). The membranes were washed three times (1×15 min and 2×10 min) between antibody incubations with TBMST, and the blots were developed using an ECL detection kit (Amersham Pharmacia Biotech). The membranes were subjected to stripping and re-probed with a mouse monoclonal anti-β-actin antibody to normalize the results. The bands were analyzed by densitometry and normalized using ImageMaster TotalLab v.2.0 software (Amersham).
RNA Isolation and Real-Time PCR (qRT-PCR) AnalysisTotal RNA was isolated from the cells using the TRIzol reagent (Invitrogen) according to the manufacturer's specifications. The obtained RNA sample quality was evaluated by gel electrophoresis. First-strand cDNA was generated using Superscript II reverse transcriptase and oligo-dT as a primer (Invitrogen). The mRNA expression levels of HIF-1α and β-actin were obtained via relative gene expression analysis using real-time-PCR (Applied Biosystems 7500 Real Time PCR). Amplification of specific PCR products was detected using SYBR Green PCR Master Mix (Applied Biosystems). All primers employed in these experiments were designed using Vector®3.0 software and prepared by Invitrogen (São Paulo, Brazil). The primers used were as follows: HIF-1α, forward 5′ CTGACCCTGCACTCAATCAA 3′, reverse 5′ CTTTGCTTCTGTGTCTTCAGCAGCA 3′; β-actin, 5′ GCTCGTCGTCGACAA CGGCTC 3′, reverse 5′ CAAACATGATCTGGGTCATCTTCTC 3′.
The samples for qRT-PCR were prepared and analyzed in triplicate in a reaction volume of 10 μL containing 5 μL of SYBR Green PCR Master Mix, 3 μl of cDNA from each sample as a template (between 10 and 18 ng of cDNA), 2 μl of primer solution (forward and reverse) and DEPC water. The primer and cDNA concentrations were standardized. A melting curve analysis was performed to confirm the specificity of the amplification and the absence of primer dimers. Samples were heated for 10 min at 95°C and amplified for 50 cycles of 15 sec at 95°C and 60 sec at 60°C. Blank controls were run in parallel to determine the amplification efficiency within each experiment. Quantification was performed using a standard curve. Serial dilutions of cDNA in dH2O, which were used as a calibrator, were amplified to construct standard curves for target and control genes. The slopes of the standard curves ranged from -3.2 to -3.9. For each sample, the levels of the target and control genes were determined based on the appropriate standard curve. The target level was subsequently divided by the control gene level to obtain a normalized target value, which was calibrated using the standard RNA sample.
Statistical AnalysisThe results of three separate experiments were expressed as means ± standard deviations. Data were analyzed using nonparametric statistical analysis with the Kruskal-Wallis test, and in the significant cases, the Duncan test was applied to further discriminate group differences. For all analyses, a p-value of <0.05 was considered to be significant.
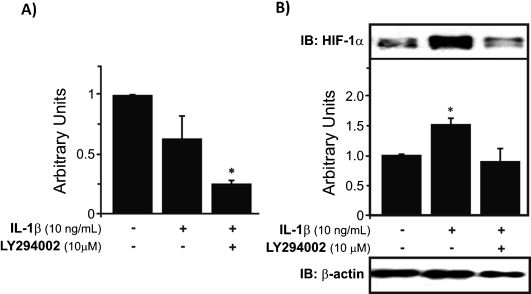
RESULTSIL-1βTreatment with IL-1β downregulated HIF-1α gene expression in OA chondrocytes, although the difference was not statistically significant. In contrast, when OA chondrocytes were incubated with both IL-1β and LY2 94002 (a PI-3K inhibitor), the observed inhibition was significantly more intense and was statistically different from the control (∗p<0.05) (Fig. 1A). In contrast to the mRNA levels of HIF-1α, the expression of HIF-1α protein assayed in nuclear extracts was upregulated in OA chondrocytes stimulated with IL-1β (∗p<0.05). When LY 294002 treatment was applied, we observed that there was a decrease in this effect of IL-1β, with HIF-1α protein levels exhibiting downregulation (∗p<0.05) (Fig. 1B).
HIF-1α mRNA expression and protein levels in human chondrocytes treated with IL-1β. A) IL-1β decreases HIF-1α mRNA expression in human chondrocytes cultured under normal oxygen conditions, and the combination of IL-1β and LY294002 downregulated HIF-1α mRNA even further (∗p<0.05). B) Upregulation of HIF-1α protein levels was observed under treatment with IL-1β (∗p<0.05), which was largely abolished by LY294002 treatment, suggesting that this upregulation occurs, at least in part, through the PI-3K pathway. This graph depicts the results normalized to endogenous β-actin protein levels.
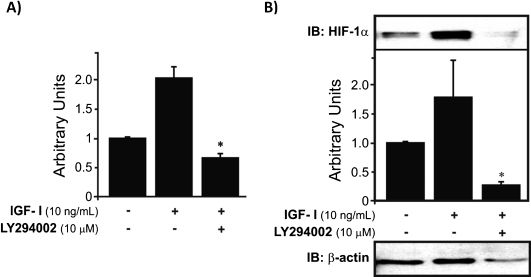
When the cells were treated with IGF-I, HIF-1α gene expression was upregulated, whereas the application of both IGF-I and LY294002 caused a significant reduction in HIF-1α levels compared to control, untreated cells (∗p<0.05). These results suggest that IGF-I stimulation of HIF-1α expression is largely mediated by the PI-3K pathway (Fig. 2A). In addition to the upregulation of HIF-1α mRNA by IGF-I, treatment with this growth factor also significantly increased HIF-1α protein levels. The combination of IGF-I and LY294002 resulted in a complete abrogation of the stimulatory effect and provoked a notable decrease in HIF-1α nuclear protein levels compared to basal levels (∗p<0.05) (Fig. 2B).
HIF-1α mRNA expression and protein levels in human chondrocytes treated with IGF-I. A) IGF-I increases the mRNA expression of HIF-1α, and the combination of LY294002 and IGF-I produces downregulation of HIF-1α mRNA to subbasal levels (∗p<0.05). B) HIF-1α protein levels were increased by IGF-I treatment, and addition of LY294002 (a PI-3K pathway inhibitor) resulted in downregulation of HIF-1α protein expression (∗p<0.05) to reduced levels compared to controls. The results were normalized to the endogenous protein β-actin.
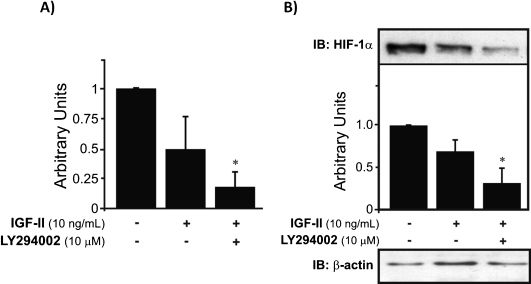
Surprisingly, we did not observe upregulation of HIF-1α mRNA expression when the cells were treated with IGF-II. However, the results further showed that the PI-3K pathway is involved in the basal regulation of HIF-1α gene expression. The involvement of the PI-3K pathway is reinforced by the finding that in cells treated with both IGF-II and LY209002, the protein levels were significantly reduced and were statistically different (∗p<0.05) from levels in the control, as well as from the levels in IGF-II-treated cells (Fig. 3A). Unlike the previously demonstrated upregulation of HIF-1α protein levels by IL-1β and IGF-I, IGF-II failed to increase the expression of this protein. In contrast, IGF-II downregulated HIF-1α protein levels. However, this result corroborates the findings from the RNA expression analysis of HIF-1α following treatment of the chondrocytes with IGF-II. Nevertheless, blocking the PI-3K pathway further decreased HIF-1α to sub-basal levels (∗p<0.05) (Fig. 3B).
HIF-1α mRNA expression and protein levels in human chondrocytes treated with IGF-II. A) Unlike IGF-I, when IGF-II treatment was applied, the mRNA expression of HIF-1α was decreased, and this downregulation was enhanced (∗p<0.05) when IGF-II and LY294002 were applied together. Inhibition of PI-3K by LY294002 caused downregulation of HIF-1α mRNA to reduced levels compared to those of the controls. B) However, HIF-1α protein expression was also decreased by IGF-II treatment. This growth factor is regulated by the PI-3K pathway, as well, because inhibition of this pathway by LY294002 decreased the expression of HIF-1α (∗p<0.05). The results were normalized to endogenous β-actin.
HIF-1α, a heterodimer transcription factor, plays a pivotal role in articular cartilage development and viability (18,19); however, its participation in the process of cartilage breakdown remains unclear. In this study, we observed that IL-1β treatment downregulated HIF-1α mRNA expression in human OA chondrocytes, though the difference was not statistically significant. However, when the cells were treated with the combination of IL-1β and LY 294002 (PI-3K pathway inhibitor), we observed stronger, statistically significant downregulation of HIF-1α mRNA expression. We found evidence of differential regulation of HIF-1α protein levels and mRNA expression. IL-1β increased HIF-1α levels through the PI-3K pathway, as IL-1β-specific upregulation was suppressed by blocking the PI-3K pathway. These findings reinforce previous observations (20–23). in other cell lines in which the regulation of HIF-1α by IL-1β occurs post-transcriptionally. In further agreement with our results, other investigators (24). who have performed real-time PCR analyses using different cartilage samples (degenerated and non-degenerated) have detected upregulation of HIF-1α, mostly in degenerated areas, suggesting that this factor could be related to a pathogenic mechanism involved in OA. As observed in the current investigation, other researchers have not found a significant effect of IL-1β treatment on mRNA levels. In contrast with our results, this effect has not previously been detected in cultured cells under normal oxygen tension. These discrepancies can be explained by the fact that other investigators have used chondrocyte monolayer cultures, whereas we used suspension cultures. We previously demonstrated that HIF-1α was not present in OA chondrocytes in monolayer culture systems (8).
Nevertheless, when other investigators have analyzed the effect of IL-1β on chondrocytes cultured under hypoxic conditions, they have also observed this increase. Additionally, the same group (25). recently reported finding elevated HIF-1α protein levels in human OA chondrocytes cultured under conditions of hypoxia and normoxia when they treated cells with IL-1β for an extended period (24 h), suggesting a delayed response in OA chondrocytes, even in cells cultured in a monolayer. Therefore, our findings suggested that in chondrocytes, as in other cell lines, IL-1β acts as a positive regulator of HIF-1α protein levels at the post-transcriptional level. Our data also confirm that the regulation of HIF-1α by IL-1β relies, at least in part, on the PI-3K pathway, given that cells treated with inhibitors of this pathway showed no effects of this cytokine. This post-transcriptional regulation may suggest that HIF-1α has a protective role because elevated protein levels may increase the possibility of the binding of DNA to HIF-1 and the subsequent transcription of genes related to cell survival.
In osteoarthritis, IGF-I expression is associated with increasing synthesis of matrix molecules in early stages of the disease and with osteophyte formation later. The lack of an IGF-I pathway could be implicated in cartilage degeneration (3,26). In this study, we demonstrated increases in HIF-1α mRNA expression and protein levels in IGF-I-treated OA chondrocytes. The PI-3K pathway was the preferred mechanistic route for this upregulation because when this pathway was blocked, HIF-1α upregulation was not observed. Moreover, without PI-3K, HIF-1α mRNA and protein levels decreased to sub-basal levels, suggesting that this pathway may be active even under baseline conditions. This upregulation has also been observed in other cell lines (27,28). Our findings suggested that IGF-I induces HIF-α expression, although additional studies will be essential to obtain a comprehensive understanding of the mechanisms involved in this induction. IGF-1 treatment increases the expression of HIF-1α at both the gene and protein levels, which reinforces its protective activity of HIF-1α because this growth factor is related to the maintenance of cartilage homeostasis.
Previous studies have shown that IGF-II promotes placental growth and function, and this process appears to be related to the regulation of HIF-1 and HIF-2α (29–31). To the best of our knowledge, this study is the first investigation that verifies the action of IGF-II on HIF-1α expression in human OA chondrocytes. Our results regarding HIF-1α protein levels are similar to previous observations made in murine throphoblast cells (29), but our findings diverge from observations in several other cell lines (27,30,31). We strongly suspect that these differences can be accounted for by length of treatment employed, given that our treatments were performed for 12 hours under low-serum conditions (0.1% FBS). In contrast, other investigators have used cells treated without serum deprivation with higher concentrations of IGF-II for a more extended period of time (30,31), and they did not use primary human OA chondrocytes. Our results may suggest that in human OA chondrocytes, IGF-II and IGF-I may play different roles; however, more studies are necessary to confirm this hypothesis. We also cannot discard the hypothesis that IGF-II may exert distinct effects in human OA chondrocytes across different stages of the disease because during our experiments, we treated cells from patients in different phases of OA. This possibility may bias our results. Interestingly, although we did not see any significant effect of IGF-II on HIF-1α mRNA or protein levels in human OA chondrocytes, blocking the PI-3K pathway produced a statistically significant downregulation of HIF-1α mRNA and protein levels. This finding suggests that expression of HIF-1α under normal O2 levels in OA chondrocytes may also occur via this pathway (31).
In conclusion, we observed that IL-1β post-transcriptionally upregulated HIF-1α protein levels in human OA chondrocytes. We also found that IGF-I upregulated HIF-1α protein and mRNA levels, indicating that this action occurs at the gene level (no effect of IGF-II on HIF-1α regulation was observed). We have demonstrated that both IL-1, the main catabolic agent, and IGF-1, an anabolic agent, are able to stabilize HIF-1α under normal oxygen conditions via the PI-3K pathway. These findings strongly suggest that Hypoxia inducible Factor-1 has a protective role in chondrocytes during osteoarthritis, and further studies are needed to clarify this relationship.
The authors are grateful to Prof Dr Antonio Condino Neto, Mss. Jussara Rehder, PhD Roseneide Conde, Prof Dr Dennys Esper Correa Cintra, Msc Lucas Rossi Sartori and PhD Michael Niehues for excellent technical assistance and to FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo) for financial support (Projects: 02/14132-1 and 05/00985-0).
No potential conflict of interest was reported.
Funding
Grants were from FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo).
Sartori-Cintra AR conceived and designed the study, performed the experiments, analyzed the data and wrote the paper. Coimbra IB conceived and designed the study and wrote the paper. Mara CS and Argolo DL performed the experiments.