To compare the surgical outcomes of stapled and handsewn closures in loop ileostomies.
METHODS:The data of 225 patients requiring loop ileostomies from 2002 to 2007 were retrospectively evaluated. The patients underwent partial small-bowel resections and either handsewn or stapled anastomoses for the ileostomy closures. They were followed up postoperatively with routine surgical examinations.
RESULTS:The study group consisted of 124 men and 101 women with a mean age of 49.12 years. The ileostomy closure was performed with handsewn in 129 patients and with stapled in 96 patients. The mean time to the first postoperative flatus was 2.426 days in the handsewn group and 2.052 days in the stapled group (p<0.05). The mean time to the first postoperative defecation was 3.202 days in the handsewn group and 2.667 days in the stapled group (p<0.05). The mean duration of patient hospital stay was 8.581 days for the handsewn group and 6.063 days for the stapled group (p<0.05).
CONCLUSIONS:Patients who underwent ileostomy closure with stapled recovered faster in the postoperative period and required shorter hospital stays than those whose closures were performed with handsewn. In our opinion, stapled should be considered the gold standard for loop ileostomy closures.
Stoma is a Greek word for mouth or opening.1 An intestinal stoma is an opening of the intestinal tract into the abdominal wall. Ileostomies were first described by the German surgeon Baum in 1879 and later by the Bohemian surgeon Maydl in 1883.2 In 1952, Brooke published his experiences with ileostomy construction and introduced a new method for suturing the mucosa to the skin.3 Unlike the first colostomies, the first ileostomies were end stomas. Turnbull and Weakley were the first surgeons to describe the loop ileostomy (in 1971).4
The diverting loop ileostomy is a commonly used stoma that is often employed to diminish the consequences of an anastomotic leak in low-colorectal anastomoses, ileal pouch-anal anastomoses, and in situations in which reversible patient factors increase the risk of an anastomotic dehiscence.5 A defunctioning loop ileostomy is traditionally closed 6 to 12 weeks after the initial surgery.6 Once anastomotic healing is confirmed, any systemic factors are corrected, and any fistulae are controlled or corrected, these ileostomies are typically closed through the stoma site without a formal laparotomy.5 Both loop ileostomy construction and subsequent closure are generally believed to be fairly straightforward, safe procedures with relatively low associated morbidity and mortality.7
Many opinions exist about the optimal method for performing these closures. Proponents of each method claim several advantages, including a diminished risk of anastomotic complications and favorable surgical outcomes.5 Routine stoma closure can be performed either with a handsewn, end-to-end anastomosis or through various techniques using staples.8–10
Proponents of stapled (functional) anastomoses often claim that these have a larger diameter than sutured anastomoses and thus will likely have a lower risk of small bowel obstruction or anastomotic leakage. Stapling proponents also claim that these anastomoses are typically faster to construct and result in decreased operative times and potential cost reduction.5
Several studies have examined the differing surgical techniques for closing loop ileostomies to identify the method that minimizes perioperative morbidity, as measured in terms of bowel obstruction, wound infection, anastomotic leakage, operative time, and postoperative outcomes, such as the time to first flatus, time to first defecation, time to initiation of oral intake, and duration of hospital stay.5 Unfortunately, most of these studies have either not found significant differences or have reported conflicting results.7 As a result, no significant patient safety differences have been found between stapled and sutured anastomoses, and there is no consensus on the best method for loop closure.9,11 Thus, we performed a review of 225 patients to help answer this question.
METHODSThis study retrospectively evaluated the data of all patients at a single-center institution (Istanbul University, Istanbul Faculty of Medicine, Department of General Surgery) who required an ileostomy closure due to a protective loop ileostomy for coloanal or ileoanal anastomosis between 2002 and 2007. All patients who underwent ileostomy closure during this period were included in the study, without exclusion criteria. Approval for this study was obtained from the Ethics Committee of Istanbul University, Istanbul Faculty of Medicine. Signed informed consent was obtained from all patients who were included in the study. Clinical and epidemiological data on file and information on the primary and ileostomy closure operations were analyzed and correlated with complications in the first 30 postoperative days, the need for additional surgery, overall morbidity and mortality, and the surgical outcomes (i.e., the patients' early postoperative follow-up parameters, such as the time to the postoperative first flatus, the first defecation, and the initiation of oral intake, and the duration of the hospital stay).
All patients' oral intake was discontinued the night before the ileostomy closure. No special bowel preparation was performed preoperatively. Prophylactic intravenous antibiotics (second-generation cephalosporin) were administered during anesthesia induction.
The same highly experienced surgeons (a senior surgeon assisted by a resident) performed the procedures in all cases. All patients received general anesthesia. The ileostomy closure was initially attempted through a peristomal incision without midline laparotomy. To accomplish the ileostomy closures, all patients operated on between 2002 and 2005 underwent a partial small bowel resection and handsewn end-to-end anastomosis (the HA group), while every patient operated on between 2005 and 2007 underwent a partial resection and stapled functional anastomosis (the SA group).
All handsewn anastomoses were performed using a single extramucosal seromuscular layer of 3/0 polyglactin sutures (Vicryl®, Ethicon, Somerville, NJ, USA), and all the stapled anastomoses used one of two GIA 80/3.8 mm-type linear cutters (Autosuture®, Covidien, Norwalk, CT, USA). The GIA 80/3.8 mm was preferred to the GIA 60/3.8 mm because it created a larger anastomotic lumen. The mesenteric layers were not closed after the anastomoses in any of the patients.
All patients were postoperatively followed with daily routine surgical examinations that included auscultation for bowel sounds, abdominal examinations and wound care. Administration of narcotic analgesics was ceased on the first postoperative day, and analgesia was maintained with intravenous administration of nonsteroidal anti-inflammatory drugs for the next three postoperative days. The patients were offered a clear liquid diet at the time of their first flatus and were questioned to determine the time of their first postoperative defecation. On consecutive days following the initiation of the liquid diet, the patients received semi-solid and then full-solid diets. The intravenous fluid support was gradually decreased and was ceased on the day that the full-solid diet was started. When sufficient flatus and defecation were confirmed, patients with adequate oral intake and analgesia were discharged from the hospital, and the duration of their stay was recorded. All complications diagnosed within the first 30 days after surgery were considered to be postoperative, including those specifically related to the operative procedure and general complications. The complications were further classified as “surgical” or “medical” according to the specific treatment required.
A statistical analysis of the two ileostomy closure techniques compared the patients in the HA group with those in the SA group. In addition, the effects of the primary surgeries on the outcomes of the ileostomy closures were examined by dividing the patients into those who underwent a total proctocolectomy-ileal-pouch-anal anastomosis (the TPC group) and those who received a low-anterior resection (the LAR group).
Student's t-tests were used to evaluate the independent variables of postoperative surgical outcomes (the time to the first postoperative flatus, first defecation, and the initiation of oral intake, and the duration of hospital stay). Fisher's exact test and the chi-squared test were used to evaluate postoperative complications. The SPSS program was used to perform the statistical analyses. Differences were considered statistically significant when p<0.05.
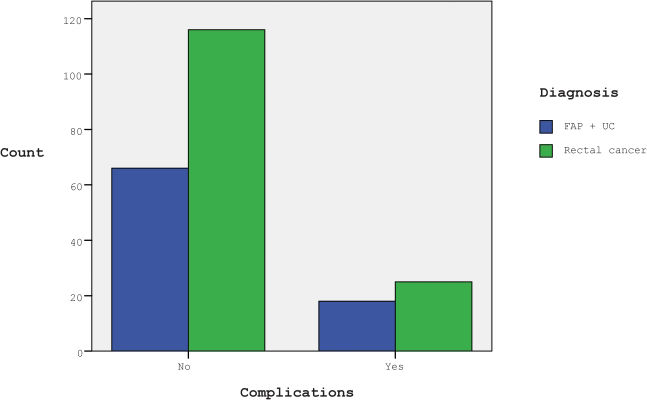
RESULTSTwo hundred twenty-five patients who underwent diverting loop ileostomy closures were retrospectively evaluated. Our study group consisted of 124 men (55.1%) and 101 women (44.9%), with a mean age of 49.12 years (range, 17 – 85). The mean time between the initial surgery and stoma closure was 10 weeks (range, 8 – 16). The distribution of the patients according to their diagnoses and surgical interventions is summarized in Table 1.
The distribution of the patients according to their diagnoses and surgical interventions.
| Primary diagnosis | Primary operation | Secondary operation (ileostomy closure) | |||||
|---|---|---|---|---|---|---|---|
| FAPa | UCb | RCc | Total proctocolectomy(TPC) | Low-anterior resection(LAR) | HA | SA | |
| Number of patients | 23 | 61 | 141 | 84 | 141 | 129 | 96 |
| % | 10.22 | 27.11 | 62.67 | 37.33 | 62.67 | 57.33 | 42.67 |
(HA, partial small bowel resection and handsewn end-to-end anastomosis; SA, partial resection and stapled functional anastomosis).
All patients with an initial diagnosis of rectal cancer underwent a low-anterior resection (LAR), and all patients with diagnoses of familial adenomatous polyposis (FAP) or ulcerative colitis (UC) underwent a total proctocolectomy-ileal-pouch-anal anastomosis (TPC).
The secondary procedure (the ileostomy closure) was performed using handsewn end-to-end anastomosis (HA) in 129 patients (57.33%) and a stapled functional anastomosis (SA) in 96 patients (42.67%).
The overall mean time to the first postoperative flatus was 2.205 days (range, 1 – 6). The mean time was 2.426 days (range, 1 – 6) in the HA group and 2.052 days (range, 1 – 6) in the SA group (p = 0.000 for the independent samples student's t-test).
The overall mean time to the first postoperative defecation was 2.878 days (range, 1 – 8). The mean time was 3.202 days (range, 1 – 8) in the HA group and 2.667 days (range, 1 – 6) in the SA group (p = 0.006 for the independent samples student's t-test).
The overall mean time to the initiation of oral intake was 3.822 days (range, 1 – 25). The mean time was 4.047 days (range, 1 – 25) in the HA group and 3.521 days (range, 2 – 13) in the SA group (p = 0.078 for the independent samples student's t-test).
The overall mean duration of the hospital stay was 7.205 days (range, 2 – 60). The mean duration was 8.581 days (range, 3 – 60) in the HA group and 6.063 days (2 – 23) in the SA group (p = 0.002 for the independent samples student's t -test).
The above results are summarized in Table 2.
A comparison of the early postoperative outcomes of stapled (SA) versus handsewn (HA) loop ileostomy closures.
| Early postoperative outcomes (days) | Overall study group | HAgroup | SAgroup | p-value |
|---|---|---|---|---|
| First flatus | 2.205 (1 – 6) | 2.426 (1 – 6) | 2.052 (1 – 6) | 0.000 |
| First defecation | 2.878 (1 – 8) | 3.202 (1 – 8) | 2.667 (1 – 6) | 0.006 |
| Initiation of oral intake | 3.822 (1 – 25) | 4.047 (1 – 25) | 3.521 (2 – 13) | 0.078 |
| Duration of hospital stay | 7.205 (2 – 60) | 8.581 (3 – 60) | 6.063 (2 – 23) | 0.002 |
The mean time to the first postoperative flatus was 2.46 days (range, 1 – 6) in the TPC group and 2.15 days (range, 1 – 6) the LAR group (p = 0.053 for the independent samples student's t-test). The mean time to the first postoperative defecation was 3.08 days (range, 1 – 8) in the TPC group and 2.95 days (range, 1 – 8) in the LAR group (p = 0.518 for the independent samples student's t-test). The mean time to the initiation of oral intake was 4.19 days (range, 1 – 10) in the TPC group and 3.60 days (range, 1 – 25) in the LAR group (p = 0.054 for the independent samples student's t-test). The mean duration of the hospital stay was 8.35 days (range, 3 – 27) in the TPC group and 7.01 days (range, 2 – 60) in the LAR group (p = 0.103 for the independent samples student's t-test). These results are summarized in Table 3.
A comparison and statistical analysis of the association between the presence of a remnant colon and the early postoperative outcomes, using independent samples student's t-tests.
| Early postoperative outcomes (days) | Overall study group | TPC Group | LAR Group | p-value |
|---|---|---|---|---|
| First flatus | 2.21 | 2.46 | 2.15 | 0.053 |
| First defecation | 2.88 | 3.08 | 2.95 | 0.518 |
| Initiation of oral intake | 3.82 | 4.19 | 3.60 | 0.054 |
| Duration of hospital stay | 7.21 | 8.35 | 7.01 | 0.103 |
The postoperative medical complications consisted of acute renal failure and early postoperative fever, and the postoperative surgical complications consisted of anastomotic leakage, small bowel obstruction and wound infection. The distribution of postoperative medical and surgical complications according to the surgical technique used for the ileostomy closure is shown in Table 4.
The postoperative medical and surgical complications according to the surgical technique used for the ileostomy closure.
| Postoperative Complications Following Ileostomy Closure | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Medical | Surgical | |||||||||
| Acute renal failure | Fever | Anastomotic leakage | Small bowel obstruction | Wound infection | ||||||
| Surgical technique | HA | SA | HA | SA | HA | SA | HA | SA | HA | SA |
| Number of patients | 1 | 0 | 4 | 1 | 5 | 2 | 8 | 6 | 7 | 9 |
| % | 0.44 | 0 | 1.78 | 0.44 | 2.22 | 0.89 | 3.56 | 2.67 | 3.11 | 4.00 |
| p-value | >0.05a | >0.05a | >0.05a | >0.05b | >0.05b | |||||
The medical complications were treated with simple, conservative therapies, which, in all cases, resulted in rapid improvement of the clinical condition and discharge without the need for any additional medical care.
The cases of small bowel obstruction and wound infection were successfully treated with conservative therapies and did not require additional surgical interventions.
Additional surgery due to anastomotic leakage was needed in seven patients (3.11%). A new ileostomy was created in four (1.78%) of these patients, and three (1.33%) patients underwent partial small bowel resection and end-to-end anastomosis. All patients with anastomotic leakage were eventually discharged from the hospital without additional medical or surgical complications in the postoperative follow-up periods for the additional surgeries.
The overall complication rate was 19.4% in the HA group and 18.8% in the SA group (p>0.05, using the chi-squared test). There were no statistically significant differences between the two groups in the rates of the individual complications (i.e., wound infection and anastomotic leakage) (p>0.05, using the chi-squared and Fisher exact tests) (Table 4).
Additionally, the overall complication rate for the TPC group was 21.4%, whereas the overall complication rate for the LAR group was 17.7%, revealing no statistically significant difference between these two groups in terms of the primary operation's effects on the secondary operation (p>0.05, using the chi-squared test).
DISCUSSIONLoop ileostomies are frequently used after ileoanal or coloanal anastomoses in colorectal surgery to prevent probable complications associated with the anastomosis itself. They are most frequently performed for colorectal cancer and inflammatory bowel disorders (IBD). Recently, the use of neoadjuvant chemoradiation therapy has resulted in an increase in sphincter-saving operations, leading to higher rates of low-colorectal and even coloanal anastomosis procedures.12–14 This decrease in the rate of abdominoperineal resection in favor of sphincter-saving operations may have led to an increase in the rate of diverting loop ileostomies. In terms of surgical complications, the postoperative morbidity and mortality rates for these low-colorectal, coloanal, and ileoanal anastomoses, when performed alone, are so remarkably high that fecal diversion has become a routine recommendation.
Since the first report of the procedure by Turnbull and Weakley15 in 1966, loop ileostomies have increased in popularity because of their technical simplicity, lack of odor, liquid discharge, and decreased rates of parastomal hernia and prolapse.12,16–21 In addition to these advantages, surgeons have also preferred protective loop ileostomies over protective colostomies because of the expected decrease in morbidity and mortality associated with the stoma closure.12,16–20
Nevertheless, ileostomy closure is not by any means a morbidity-free procedure. The reported overall complication rates for ileostomy closure range from 10% to 17%, and can reach 30% when performed to divert ileoanal pouches.17,21,22 The most frequent complication after ileostomy closure is reported to be small bowel obstruction.17,23 This complication has been particularly associated with extensive pelvic dissection during the primary surgical procedure, ileal vessel distention, and inflammatory disease affecting the remaining small bowel in patients primarily treated for IBD with proctocolectomy and ileal pouch.17,21 The relatively high rates of this complication associated with IBD have also motivated studies examining the real benefits of protective fecal diversion in this specific situation.17,19,23,24
The temporary loop ileostomy is generally thought to be simple to construct and easy to close, with low perioperative morbidity and mortality. The two principal anastomotic techniques are end-to-end handsewn (HA) and functional stapled (SA) anastomoses. Numerous studies have compared the integrity of handsewn versus stapled bowel anastomoses, and it is generally thought that their complication rates are similar.25,26
Several factors are associated with an increased risk of postoperative complications after ileostomy closure, such as the interval between primary surgery and closure, the use of bowel preparation, antibiotic prophylaxis, and technical considerations (stapled vs. handsewn suture techniques).7,8,22,27–33 In this study, our primary aim was to compare the two principal anastomotic techniques with respect to their surgical outcomes and complication rates.
Hull et al.7 compared handsewn and stapled loop ileostomy closures and found no significant differences in the time to the first defecation, solid diet, or discharge. The complications were similar for the two groups. Similar results were reported by Pittman et al.34, who found no significant difference in the anastomotic leak rate, length of surgery, or length of hospitalization in patients with sutured versus stapled anastomoses. In a systematic review of randomized controlled trials, Lustosa et al.35 found more frequent stenosis and shorter procedure times when performing colorectal anastomoses using stapling, but there was insufficient evidence to demonstrate the superiority of stapling over handsewing.
In this study, we compared the postoperative surgical outcomes of patients who underwent ileostomy closures using the two different surgical techniques. When the HA and SA groups were compared, the statistical analysis revealed a significantly shorter time to the first flatus, time to first defecation and duration of hospital stay in the SA group (p<0.05). No statistically significant difference in the time to initiation of oral intake could be demonstrated between the two groups (p>0.05); however, this outcome was not of primary importance because of its dependence on the surgeon's decisions and methods during routine postoperative follow-up. These results show a faster recovery for the patients who underwent ileostomy closure with the SA technique compared to those who underwent the HA technique (Table 2).
One of the major advantages observed with the SA technique was the shorter hospital stay. The mean durations of hospital stay were 6.063 and 8.581 days in the in the SA and HA groups, respectively (p<0.05). At first glance, these times may seem excessive following such a surgical intervention. This explanation is related to our institution's nature; being a university hospital, it is obliged to accept all patients, most of whom are from the rural areas of our country. The patients often have to travel long distances, and so the risk of any kind of postoperative complication must be ruled out before they can be sent home. Although the mean times to the initiation of oral intake were 3.521 and 4.047 days in the SA and HA groups, respectively, the patients were followed in our clinic until they received full-solid diets without any complications before they were discharged from the hospital. Despite this relatively long clinical follow-up, the results of our study still reflect a shorter hospital stay for the SA group compared with the HA group.
There have been studies comparing the operative times and costs of the two techniques in addition to their surgical outcomes. Horisberger et al.36 found that the average operative time was 17.8 minutes shorter for the SA technique than for the HA technique, and that the costs associated with operative time were significantly higher for the HA group compared to the SA group. Although the material costs for the anastomosis were significantly higher for the SA group, there were no significant differences between the HA and SA groups in the overall costs (including the surgical costs and hospital stay).36 Previous studies have shown that, in general, a reduction of the operative time by 15 minutes through the use of a stapled anastomosis reduced the overall cost per case.7 We focused on postoperative surgical outcomes and surgical complications in our study, and we did not evaluate the operative time and costs, which is a weakness of our study.
The effects of the primary surgical intervention on the secondary procedure (ileostomy closure) were also investigated in our study, with the aim of determining whether the presence of remnant colonic segments affected the surgical outcomes of the ileostomy closures. We compared the postoperative surgical outcomes of patients who had previously undergone TPC and LAR. No statistically significant differences between the TPC group and the LAR group were found in the postoperative times to first flatus, first defecation, and initiation of oral intake or in the duration of the hospital stay (p>0.05) (Table 3). These results indicate that the primary surgical technique and the presence of a remnant colon did not significantly affect the outcomes of ileostomy closures.
The complications in the post-closure interval that required surgery were small bowel obstruction and anastomotic leakage. There have been reports of obstruction and leakage frequencies after ileostomy closure of 0% to 18% and 0% to 3%, respectively.12,21,37–40 Others studies have reported obstruction rates of 0% to 9% for the enterotomy suture technique, and 0% to 0.5% for the stapled anastomosis technique.12,21,37 Advocates have suggested that the stapled anastomosis provides a larger lumen; this consideration is particularly important in the presence of a malfunctioning distal limb, which may have a smaller diameter.37,38,40
The distribution of postoperative medical and surgical complications according to the surgical techniques used for ileostomy closure is summarized in Table 4. No statistically significant differences were found between the HA and the SA groups in the overall complication rate (p>0.05, using the chi-squared test). There were also no statistically significant differences between the two groups in the rates of the individual complications (i.e., wound infection and anastomotic leakage) (p>0.05) (Figure 1).
The overall complication rates for the TPC and LAR groups were also compared, and no statistically significant differences were found between these two groups (p>0.05, using the chi-squared test) (Figure 2). These results show that the nature of the primary surgical technique and the presence of a remnant colon did not significantly increase the complications rate for the secondary procedure.
The two principal anastomotic techniques for ileostomy closures are HA and SA. Patients who underwent ileostomy closures with the SA technique recovered significantly faster in the postoperative period and required shorter hospital stays than those who underwent the HA technique.
There were no statistically significant differences between the two anastomotic techniques in postoperative complication rates.
The nature of the primary surgical intervention did not significantly alter the postoperative surgical outcomes or the complication rates of the ileostomy closure.
In our opinion, the SA technique should be considered the gold standard for loop ileostomy closures.
The authors would like to thank Sedat ZIYADE, MD for his contributions to the statistical work-up of this study. Istanbul Vakıf Gureba Training & Research Hospital, Department of Thoracic Surgery.
No potential conflict of interest was reported.
Balik E supervised and executed the study, performed most of the operations of the principal (or CPC, or RAF), and secondary operations (ileostomy closure), and operated the follow-up of patients. Eren T performed the study throughout his residence, participated in almost all operations, performed the surgery follow-up of patients in the department, collected the data, created Excel charts, performed the statistical evaluations and wrote the manuscript. Bugra D was the leader in establishing the study protocol and coordination of the surgical team during the implementation of this Protocol, held two primary operations (or CPC, or RAF), and secondary operations (ileostomy closure), and participated in the surgery follow-ups of patients in the department, read and revised the article. Buyukuncu Y performed primary operation (or CPC, or RAF), and secondary operations (ileostomy closure), and participated in the surgery follow-ups of patients in the department, revised the writing of the article. Akyuz A, chief surgeon of the surgical team, held two primary operations (or CPC, or RAF), and secondary operations (ileostomy closure), and participated in the surgery follow-ups of patients in the department, he oversaw all steps of the study and performed the last revision of the article before submission. Yamaner S had the inspiration for this work, raised the idea of such study, conducted operations both primary (or CPC, or RAF), and secondary operations (ileostomy closure), and participated in the surgery follow-ups of patients in the department, supervised every stage of the study and revised the article in order to eliminate possible errors.