Steatosis is currently the most common chronic liver disease and it can aggravate ischemia-reperfusion (IR) lesions. We hypothesized that S-nitroso-N-acetylcysteine (SNAC), an NO donor component, can ameliorate cell damage from IR injury. In this paper, we report the effect of SNAC on liver IR in rats with normal livers compared to those with steatotic livers.
METHODS:Thirty-four rats were divided into five groups: I (n=8), IR in normal liver; II (n=8), IR in normal liver with SNAC; III (n=9), IR in steatotic liver; IV (n=9), IR in steatotic liver with SNAC; and V (n=10), SHAN. Liver steatosis was achieved by administration of a protein-free diet. A SNAC solution was infused intraperitoneally for one hour, beginning 30 min. after partial (70%) liver ischemia. The volume of solution infused was 1 ml/100 g body weight. The animals were sacrificed four hours after reperfusion, and the liver and lung were removed for analysis. We assessed hepatic histology, mitochondrial respiration, oxidative stress (MDA), and pulmonary myeloperoxidase.
RESULTS:All groups showed significant alterations compared with the group that received SHAN. The results from the steatotic SNAC group revealed a significant improvement in liver mitochondrial respiration and oxidative stress compared to the steatotic group without SNAC. No difference in myeloperoxidase was observed. Histological analysis revealed no difference between the non-steatotic groups. However, the SNAC groups showed less intraparenchymal hemorrhage than groups without SNAC (p=0.02).
CONCLUSION:This study suggests that SNAC effectively protects against IR injury in the steatotic liver but not in the normal liver.
In the last thirty years, hepatic steatosis has been well-documented as the most common chronic liver disease in the general population. Its high prevalence is of epidemiological concern because it affects 31% of adults as evaluated by magnetic resonance imaging in the USA1 and 33% of living donor candidates who underwent liver biopsy.2 The prevalence is directly related to age and varies from 2.6% in children to 26% in adults who are 40 to 59 years old.3
The pathophysiology of steatosis has not been completely ascertained. The condition may be a consequence of mitochondrial β-oxidation impairment, leading to increased free fatty acids inside the hepatocyte;4 or it may be the cause of oxidative dysfunction, thus leading to structural and functional mitochondrial changes.5 Liver inflammation can also be a trigger of steatosis. Either as isolated or cooperative factors, mitochondrial dysfunction, inflammatory cytokines, free fatty acids, bacterial endotoxins, microvascular injury, and oxidative stress may all be involved in the pathogenesis of ischemia-reperfusion (IR) injury.6–8
Previous studies have demonstrated that steatosis can worsen the effect of IR lesions in liver transplantation and hepatectomy.9–12 These two factors, steatosis and IR lesions, are important because of the high prevalence of steatosis in the general population and the common risk of IR injury in liver transplantation and other hepatic surgeries. Microvascular alterations, mitochondrial dysfunction, and a lower number of sinusoids in steatosis are factors that worsen the effect of IR injury in steatotic livers.7,13,14 Moreover, fatty free acids inhibit acetyl coenzyme A, an important factor in the Krebs cycle and gluconeogenesis, thus leading to decreased production of ATP.14,15
Therefore, oxidative stress and hepatic microcirculatory damage are related to steatosis and the genesis of IR lesions.13,14,16,17 Nitric oxide (NO) has conflicting effects on IR; it can improve hepatic microcirculation at low doses.5,18–22 S-nitroso-N-acetylcysteine (SNAC) is an S-nitrosothiol that can act as an NO carrier and donor. NO has a systemic and tissue vasodilatation effect and also acts in hepatic microcirculation.23 It is suggested that NO could ameliorate hepatic microcirculation, which is a pathophysiological factor in steatotic liver IR injury.
SNAC amelioration of oxidative stress in experimental models of steatosis24 and IR injury of skeletal muscle25 can also revert and inhibit the development of non alcoholic fat liver disease (NAFLD).39,26 Although it has been studied in steatosis and IR injury of skeletal muscle, SNAC has not been studied in hepatic IR models or in steatotic liver IR models. In this study, we assessed the effect of SNAC in rats with normal and steatotic livers that had been submitted to ischemia.
METHODSAnimalsThirty-five adult male Wistar rats that weighed 250 to 300 g were used for this study. All rats were maintained in a daily 12 h light-dark cycle. The rats were randomly distributed into five groups. Rats in group I (n=8) did not have steatosis (received a balanced diet) and were treated with saline solution. Rats in group II (n=8) did not have steatosis and were treated with SNAC. Rats in group III (n=9) had steatosis (received a protein-free diet) and were treated with saline solution. Rats in group IV (n=9) had steatosis and were treated with SNAC. Rats in group V (n=9) comprised the SHAN group; the same surgery was performed but without IR injury. A protein-free diet 27 was fed to rats for 21 days to induce steatosis. The control group was fed a balanced diet (Nuvilab) for the same period. After 21 days of treatment, the animals underwent IR injury.
The animals were handled under protocols approved by the Ethics Committee of the Clinical Hospital of Sao Paulo University in Brazil. The Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences and National Research Council. National Academy Press, Washington, D.C., 1996) was used to ensure proper care and protection of the experimental animals.
SNAC preparationN-acetylcysteine (NAC) and sodium nitrite (Sigma, St. Louis, MO, USA) were used for SNAC preparation. Aqueous SNAC stock solution was prepared by combining an equimolar NAC solution with sodium nitrite. The final solution was stirred at room temperature for 20 min and was protected from light with aluminum foil. The SNAC solution was diluted in saline solution to a final concentration of 100 nM and used immediately.
Induction of liver IR injuryThe rats were anesthetized with intraperitoneal ketamine hydrochloride 5% (Park-Davis Lab, USA) and chloridrate of xylazine 2% (Rompum®, Bayer) at a dose of 30 mg/kg. Rats then underwent endotracheal intubation and were maintained at a rectal temperature of 35 to 37°C. A median laparotomy was performed, the ligaments of the liver were cut, all structures in the portal triad (hepatic artery, portal vein, and bile duct) of the median and left lateral hepatic lobes (70% of the liver) were clamped with an atraumatic microvascular clamp for 1 h (Figure 1), and the abdomen was closed. Then a second laparotomy was performed, the clamp was removed, and the animals were kept alive for 4 h after reperfusion. The SNAC solution (100 nM) was administrated intraperitonealy (i.p.) at a dose of 1 ml/100 g body weight for 1 h, starting 30 min after occlusion of the blood supply to the median and left hepatic lobes. Fifteen min before euthanasia by exsanguination, Evans blue (Sigma-Chemical Company, USA) was injected intravenously. The liver was removed, and both ischemic and non-ischemic portions were evaluated for histology, lipid peroxidation (MDA) and mitochondrial function. The lung was removed for Evans blue extraction and myeloperoxidase evaluation.
Oxidative stressThe thiobarbituric acid method was used to quantify lipid peroxidation in tissue by measuring thiobarbituric acid-reactive substances (TBARS). The tissue was homogenized in 1.15% KCl buffer (100 mg/mL) and centrifuged at 14,000 g for 20 min. The supernatant was then stored at −70°C. An aliquot of the supernatant was added to a reaction mixture of 1.5 mL 0.8% thiobarbituric acid, 200 μL 8.1% (v/v) solution (SDS), 1.5 mL 20% (v/v) acetic acid and 600 μL distilled H2O. The new solution had a pH of 3.5 and was heated to 90°C for 45 min. After cooling to room temperature, the samples underwent centrifugation at 10,000 g for 10 min, and absorbance was measured at 532 nm using 1,1,3,3-tetramethoxypropane as an external standard. The quantity of lipid peroxide was reported as nmol of malondialdehyde (MDA) equivalents/mg protein.30
Mitochondrial oxygraphic measurementLiver tissue was prepared as previously described. Mitochondrial oxygen consumption was measured polarographically using a Gilson 5/6 H Oxygraph (Gilson Medical Electronics, Inc., Middleton, WI, USA) at 28°C. Respiration states 3 and 4 were defined and calculated according to the method of Chance31 as ADP-stimulated and ADP-limited respiration, respectively. The respiratory control index (RCI) was measured and represented the ratio of state 3 to state 4 (S3/S4). Respiration states S3 and S4 were measured and reported as ng atoms of O2 min−1 mg protein−1. Mitochondrial protein content was determined using the method of Lowry et al.32 The ratio of ADP added to oxygen consumed was calculated and recorded as the ADP/O ratio. The RCI and ADP/O ratio served as indices of oxidative and phosphorylative mitochondrial function, respectively.
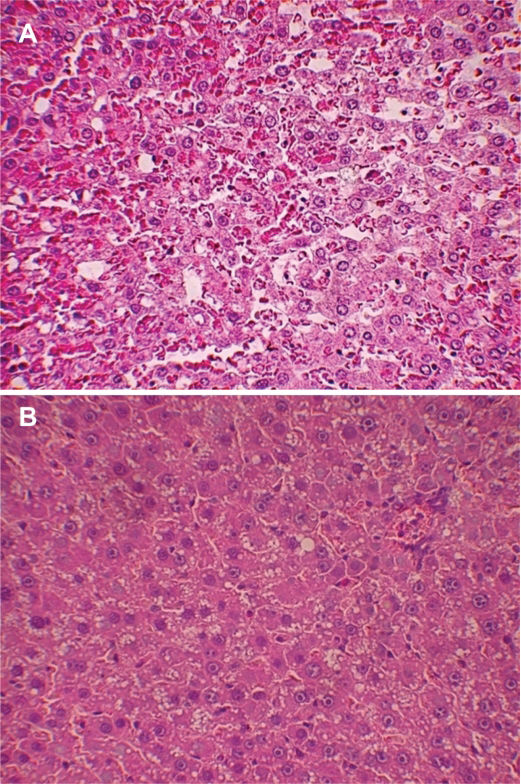
HistologyBoth ischemic and non-ischemic parts of the liver were harvested for histological examination. Liver tissue was fixed in 10% formalin solution, processed, and stained with hematoxylin-eosin and Masson Trichrome. The following histological variables were assessed and scored from 0 to 3: macro- and microvacuolar fatty change, fatty zonal distribution, foci of necrosis, portal and perivenular fibrosis, the inflammatory infiltrate and its zonal distribution.
Statistical analysisResults were expressed as the means ± standard error (SE) and were statistically analyzed using the Mann-Whitney test and Fisher's exact test. p < 0.05 was considered statistically significant.
RESULTSIn general, all experimental groups (I, II, III and IV) showed a greater response than the SHAN group for all assessments measured (p<0.05).
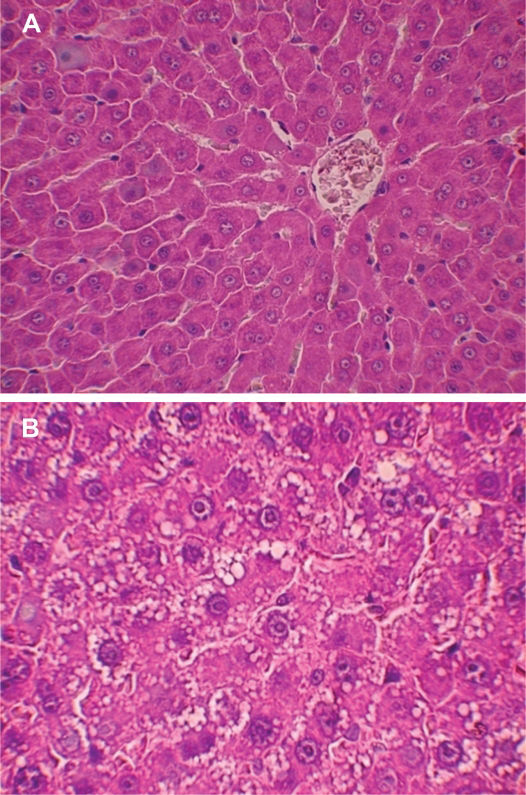
Experimental model of steatosisMicroscopically, the degree of steatosis in rats that received the protein-free diet (Figure 2a) was significantly greater than in rats that received the balanced diet (Figure 2b). The animals on the protein-free diet developed malnutrition and lost approximately 30% of their body weight after 21 days.
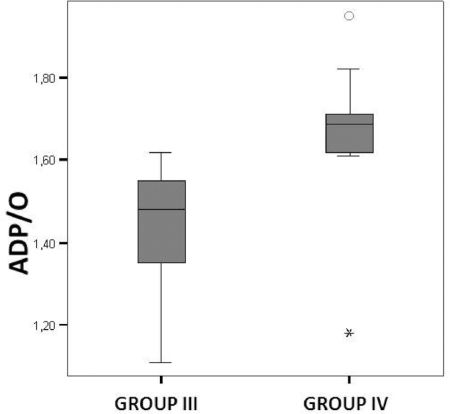
Liver mitochondrial oxygraphic measurementThe RCI indicates oxidative phosphorylation activity. In the non-steatotic condition, the RCI revealed no difference between the control and SNAC groups. The ADP/O ratio indicates the level of coupling between the phosphorylation activity and the mitochondrial respiration. In the non-steatotic groups, there was no significant difference between the control and SNAC groups. However, in steatotic groups, SNAC treatment resulted in an improved ADP/O ratio in the liver ischemic samples (p=0.01) (Figure 3).
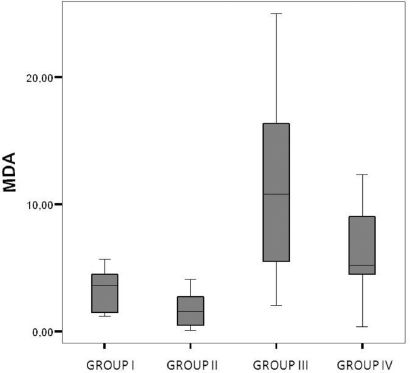
Liver oxidative stressTreatment with SNAC appeared to result in decreased liver oxidative stress. Groups treated with SNAC showed no difference in lipid peroxidation as determined by the thiobarbituric acid method (MDA) compared to controls (p=0.07). The oxidative stress was significantly higher in the steatotic groups than in the non-steatotic groups (p<0.01) (Figure 4).
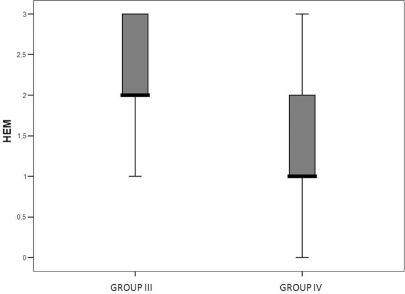
Liver histologyIn the non-steatotic condition, all histological parameters were similar between the SNAC and control groups. Conversely, the steatotic groups showed less intraparenchymal hemorrhage in the ischemic liver (p=0.02) (Figures 5a, 5b and 6). Moreover, the steatotic group had higher levels of intraparenchymal hemorrhage than did the control group (p=0.02) (Figures 2a and 2b).
Steatosis is a risk factor for liver transplantation33 and liver resection, and it is associated with higher morbidity and mortality rates.9,34 Liver damage from steatosis is caused by hepatic mitochondrial impairment and oxidative stress.35 In our study, higher levels of intraparenchymal hemorrhage and oxidative stress in the steatotic group confirmed the vulnerability and increased susceptibility of steatotic livers to IR injury (Figures 2a and 2b).
We analyzed the effects of an NO donor, SNAC, on IR injury in steatotic and non-steatotic livers. Endothelial dysfunction caused by IR injury may impair constitutive NO release37 and therefore reduce NO production in the early reperfusion period. SNAC decreases the oxidative stress in experimental models of steatosis and in skeletal muscle that undergoes IR injury.24–26 Therefore, S-nitrosothiol may be beneficial in cases of IR injury in steatotic livers. Our results also indicate that administration of SNAC after IR injury to steatotic livers can lead to improved mitochondrial function (Figures 3) and reduced oxidative stress (Figure 4).
Mitochondria are involved in fatty acid β oxidation and oxidative phosphorylation. Additionally, these organelles are an important source of reactive oxygen spieces (ROS). Impairment of their activity plays a central role in steatotic liver damage. SNAC may serve to decrease liver oxidative stress via two mechanisms. First, SNAC may improve mitochondrial function, thus leading to reduced ROS production. Second, SNAC may minimize damage to circulation in the liver, thus reducing hemorrhage in ischemic livers, as we observed in our study.
Hepatic microcirculation and oxidative stress are important factors related to IR injury. These factors are already present in steatotic livers and are aggravated in ischemic liver injury during reperfusion. An interesting finding from our study was that SNAC did not substantially alter the IR lesions in non-steatotic livers. Meanwhile, in steatotic livers that had severe lesions, administration of SNAC resulted in improvement of the IR lesions.
In this study, we confirmed that steatosis increases susceptibility to severe lesions after IR injury. Additionally, we demonstrated that SNAC can ameliorate the response of steatotic livers to IR and reduce intraparenchymal hemorrhage. Intraparenchymal hemorrhage is a histological lesion that demonstrates severe damage. Among the histological changes that we observed after IR injury, this type of lesion was significantly different between the treated and non-treated groups.
Indeed, the present study confirmed greater IR injury in steatotic livers when compared to non–steatotic livers. Furthermore, hepatic IR lesions responded differently to treatment, regardless of whether steatosis was present.
Peroxynitrite is a highly reactive free radical formed by the combination of NO with a superoxide anion. Under normal conditions, this reaction is prevented by continuous removal of NO by hemoglobin.38 Nonetheless, during the later stages of reperfusion, NO production increases as a result of the expression of intrinsic nitric oxide synthase (iNOS). Excessive NO production leads to the formation of peroxynitrite, which causes hepatic injury.37,39 SNAC is an NO donor; therefore, a large quantity of this substance can itself produce peroxynitrite and nitrative stress. Thus, the timing of the administration of this drug is crucial to ameliorate IR injury in the liver and not cause further damage. In our study, we observed that administration of the NO donor SNAC led to a benefit in the final period of ischemia and in the early period of reperfusion.
In conclusion, this study confirms a poor outcome from IR injury in steatotic livers. Additionally, we show that SNAC effectively protects against IR injury in steatotic livers but not in normal livers.
The authors thank Sandra Nassa Sampietre for her valuable technical help.