The evaluation of S100B protein expression in the human heart and its correlation with drug-related death.
METHOD:Left ventricular samples were collected from 74 serial forensic autopsies (15 overdose-related deaths; 59 non-overdose-related deaths) from 2007 to 2010. Tissue sections from each sample were immunostained for S100B protein by a commercial antibody.
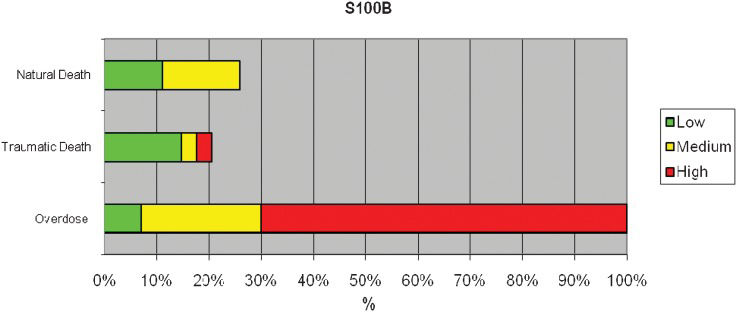
RESULTS:The S100B protein was detected in the heart samples of all 15 cases of drug-related deaths; S100B immunoreactivity was mainly observed in the cytoplasm of cardiomyocytes and as globular deposits in the interstitial spaces. No reactivity or weak reactivity was found in the cardiomyocytes of the 59 subjects who died of other causes.
CONCLUSION:Our preliminary data show that the S100B protein accumulates in injured cardiomyocytes during drug-related sudden death. Given the near absence of S100B protein in the heart of subjects who died from causes other than drug overdose, S100B immunopositivity may be used as a new ancillary screening tool for the postmortem diagnosis of overdose-related cardiac death.
Cocaine, a crystalline tropane alkaloid obtained from the leaves of the coca plant, is one of the most commonly used illicit drugs and acts as a powerful sympathomimetic agent, with local and systemic effects. Local effects are the consequence of the inhibition of membrane permeability to sodium during depolarization and the inhibition of the transmission of electrical impulses. Systemic effects are attributed to the inhibition of presynaptic reuptake of norepinephrine and dopamine and the secondary excess production of these neurotransmitters at receptor sites on the postganglionic neuron (1).
The effects of cocaine on the cardiovascular system have been extensively documented in both animal models and humans. Cocaine administered to rats produces atrial and ventricular arrhythmias and myocardial ischemia with coronary vasoconstriction (2). High-grade ventricular arrhythmias, myocardial infarction, and sudden cocaine-related death have been reported in humans (3). In the same study, cocaine use was shown to precipitate a cardiac event with myocardial infarct even after recreational use. Additionally, clinical evidence has shown that adverse cardiac effects are frequent during medicinal cocaine use, in which the drug is applied intranasally and intratracheally as a local anesthetic (4–6). Cocaine use has also been associated with other cardiac disorders, such as systemic hypertension, aortic aneurysms, torsades de pointes, arrhythmia, sudden coronary death (7), ischemia, ventricular tachycardia, and ventricular fibrillation (8–12). Cocaine may change cardiac rhythm by inhibiting sodium transport across membranes during depolarization and thus inhibiting the generation and conduction of action potentials (13,14). Cocaine-related myocardial ischemia or infarction may occur immediately after cocaine use: cocaine increases the risk of acute myocardial infarct (AMI) by up to 24-fold in the first hours after use (15). The incidence of AMI is much higher in younger patients (aged 18-45 years) (16), particularly in those with other cardiac risk factors (17).
The principal mechanism of cocaine-related heart damage appears to be myocardial ischemia. Three pharmacological mechanisms, likely acting in combination, are involved in the pathogenesis of cocaine-related ischemia: first, cocaine produces an increase in heart rate and blood pressure as a consequence of adrenergic stimulation; second, it causes coronary vasoconstriction due to the stimulation of coronary α-adrenergic receptors; and third, it leads to an increase in platelet aggregability. Furthermore, the mismatch between myocardial oxygen supply and the demand from cocaine-induced vasoconstriction and increased myocardial workload are often invoked as the major mechanisms by which cocaine might induce myocardial ischemia.
In contrast, there is limited information available on the acute effects of heroin on the heart; to the best of our knowledge, only a few cases of AMI after heroin injections have been reported (18). Experimental studies have demonstrated that heroin may cause systemic and direct effects on the heart (19,20), most likely mediated by a local coronary spasm or inflammation (21). Heroin could act directly on the vasomotor center by increasing parasympathetic activity, producing bradycardia and hypotension and stimulating the release of histamine from mast cells (22,23). Heroin-induced coronary vasospasm after heroin sniffing has also been reported (24–26). Taken together, these reports indicate that heroin may have a direct toxic effect on coronary arteries and induce acute coronary occlusion.
The S100 protein family comprises 16 low-molecular-weight (21 KDa) calcium-binding proteins that contain two calcium-binding sites. S100B, one of the two founding members of this family, is concentrated in oligodendrocytes, astrocytes, ependymocytes, Schwann cells and neurons (27). In clinical practice, it is a reliable biochemical marker of cerebral damage and is also utilized in postmortem diagnosis in medico-legal autopsy cases (28). Because S100B protein plasma levels have been shown to increase during ischemic stroke, S100 protein serum levels are used in clinical practice to monitor neurovascular status and functional outcome in subjects affected by cerebral damage (29) or subarachnoid hemorrhage (30,31). Elevated serum S100B levels have been reported in patients affected by obstructive sleep apnea syndrome (32). Moreover, S100B urine levels have been proposed as a useful tool to identify newborns that are at a higher risk of neonatal death (33) or full-term infants at risk of hypoxic-ischemic encephalopathy (34).
S100B is not restricted to the nervous system; chondrocytes, adipocytes, melanocytes, and Langerhans cells may also release S100B (35,36). In addition, recent investigations showed that elevated S100B serum levels may also be detected in patients without head injury, suggesting that it may originate from other damaged tissues (37,38), such as skeletal myofibers, muscle satellite cells and myoblasts. Isolated rat heart cells were shown to release S100B (39), and the myocardium was indicated as the putative main source for the increase in S100B serum levels in patients affected by dilated cardiomyopathy (40). Recently, S100B protein expression was shown to be induced in the heart of human subjects after myocardial infarction (41), and it has been proposed as a marker of poor prognosis in patients undergoing cardiac surgery (42).
Based on these data, the current study was aimed at verifying, with immunohistochemical data, whether the S100B protein is expressed in the hearts of subjects who underwent sudden cardiac death, including drug addicts and patients without a history of drug abuse.
MATERIALS AND METHODSSeventy-four serial autopsies conducted from 2007 to 2010 (40 males and 34 females) were included in the present study. The subjects tested were subdivided into two main groups: the overdose group (15 subjects; cocaine, heroin or polypharmacological admixtures, including ecstasy) and the non-overdose group (59 subjects; traumatic and non-traumatic deaths). The traumatic group was subdivided into three subgroups (sidearms, firearms, and drowning). The victims ranged in age from 9 to 85 years old. In each case, a full autopsy and toxicological analysis were performed within 24-72 hours after death. No macroscopic sign of congenital cardiomyopathy was found in any case. The macroscopic examination of the heart did not show any sign of severe coronary atherosclerosis. Multiple tissue samples collected from the left ventricular wall were fixed in 10% formalin, routinely processed and paraffin-embedded. The initial block was cut into 6-7 blocks approximately 2-3 mm wide. At least two histological sections were prepared from each block. The tissue sections were stained with hematoxylin-eosin or immunostained with a polyclonal rabbit anti-S100B antibody (DakoCytomation A/S, Glostrup, diluted 1/2000) followed by incubation for three hours at 37°C in with universal streptavidin/biotin immunoperoxidase detection reagents. Endogenous peroxidase was inactivated by incubation with 3% hydrogen peroxide for 5 min. To confirm the specificity of immunostaining, phosphate-buffered saline was substituted for the primary antibody in control sections. The peripheral nerves present in each section served as a positive internal control. Stained sections were read by two pathologists blinded to the clinical diagnosis.
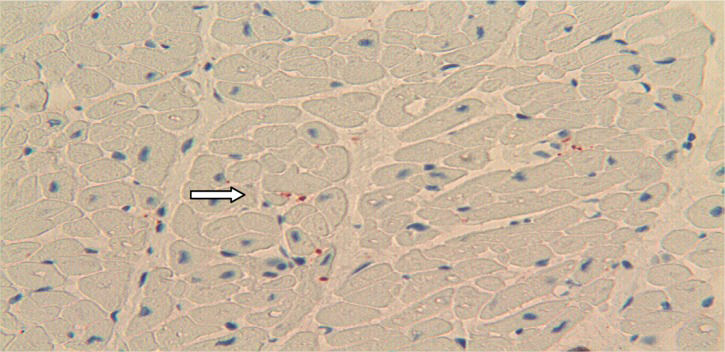
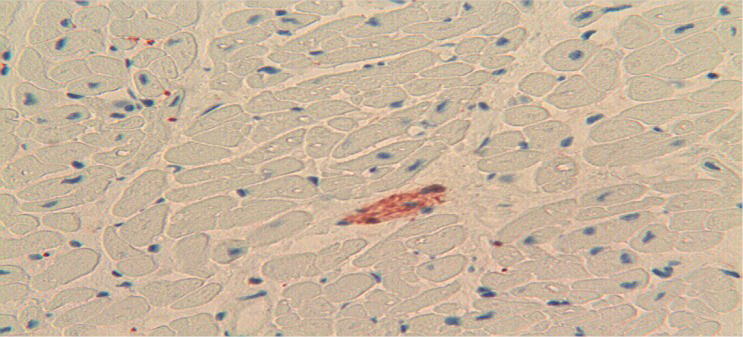
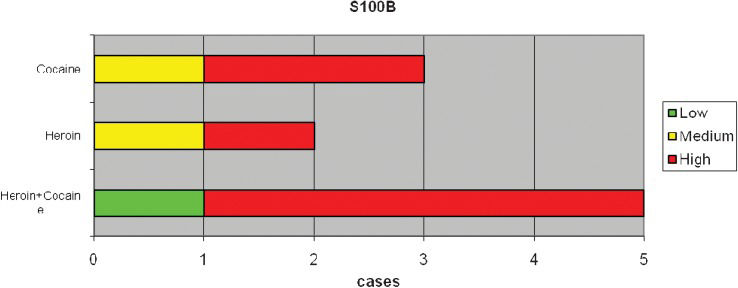
RESULTSImmunoreactivity for the S100B protein was mainly detected in the overdose group; less reactivity was detected in the 59 subjects in the non-overdose-related group (Figure 1). The pattern of S100B reactivity varied between cases, with different localization and different intensities. S100B staining was mainly observed inside the cytoplasm of scattered cardiac cells (Figure 2). The signals appeared as differently sized globular deposits and granules, often located near the nucleus. Similar globular deposits were also found in the interstitial spaces and were unevenly distributed throughout the cardiac tissue (Figure 3). Immunoreactive signals were also occasionally found in the perivascular spaces in the cytoplasm of stromal cells surrounding the wall of intra-cardiac arteries. No significant differences were found among the different subgroups in which the overdose patients were subdivided: S100B protein deposits were found in the heart in cocaine-, heroin- and other drug-related deaths. We observed the highest levels of immunoreactivity for S100B in 3 patients with a clinical history of combined cocaine and heroin abuse (Figure 4). In these cases, a large number of cardiomyocytes showed very large globular deposits of the protein.
No reactivity for the S100B protein was detected in the subpericardial fatty tissue or other soft tissues, with the exclusion of nerve fibers, which represented the internal positive control.
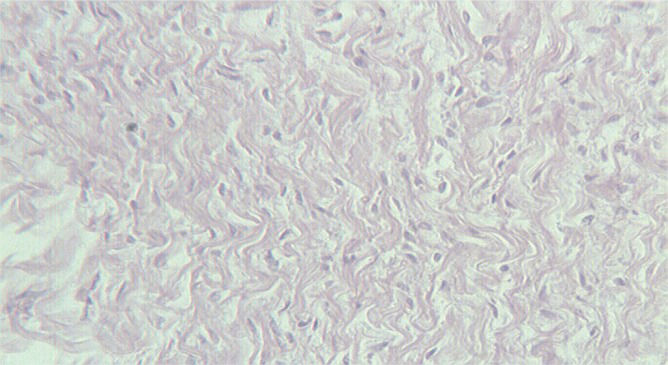
Finally, hematoxylin-eosin staining was performed to investigate the pathological status of these hearts; edema, and a wavy arrangement of cardiomyocytes were observed in all S100B-positive cases (Figure 5).
DISCUSSIONThe current study investigated whether the S100B protein is expressed and immunodetectable in the hearts of patients who died due to drug overdose and whether different drugs (heroin, cocaine, and polypharmacological admixtures, including ecstasy) could affect S100B protein retention in cardiomyocytes.
Our preliminary data clearly show that the S100B protein is significantly more expressed in the hearts of drug users, regardless of the type of drug employed, compared with patients who died from traumatic or other pathological events. From a practical point of view, this finding suggests that S100B immunostaining could be performed in all cases of cardiac death possibly related to drug abuse to confirm the role of drug use as a cause of death.
The presence of the S100B protein inside the cytoplasm of cardiomyocytes and in the interstitial spaces around cardiac cells, and the presence of myocytolysis clearly suggest cardiomyocytes as the putative main source of S100B production and release. The absence of immunoreactivity for the protein in the subepicardial fat tissue in this study is inconsistent with the previous hypothesis that cardiac adipocytes are the main source of S100B protein in the heart.
Our histological data confirm the typical histological findings previously reported in cocaine-related death; i.e., contraction band necrosis (CBN), also referred to as coagulative myocytolysis, and an anomalous hypereosinophilic crossband formed by segments of hypercontracted sarcomeres with extremely thickened Z lines (43).
Another interesting finding in our study is that a similar S100B protein expression pattern is observed in both cocaine and heroin abusers. One possible explanation for this finding could be the acute stress caused by both drugs on the central nervous system, followed by the induction of S100B protein overproduction in cardiomyocytes (44).
Particularly strong reactivity for S100B protein was observed in some cases in which toxicological analyses suggested concomitant cocaine and heroin abuse. This finding supports the assumption that both drugs may act on the same pathways, cooperating in S100B protein overproduction, storage and release. Because both of these substances are metabolized by the same enzymes, human carboxylesterases (hCEs), we might postulate that heroin and cocaine could interfere with each other at a metabolic level when taken together, giving rise to the overproduction of S100B and leading to significant cardiac storage of the protein (45). However, to the best of our knowledge, the exact mechanism by which S100B protein is overexpressed at the cardiac level in overdose-related deaths remains unclear. The over-expression of S100B protein might depend on environmental factors; it has been shown that several substances, including norepinephrine, phenylephrine, interleukin 1β (IL-1β), β-amyloid, antidepressants and pro-inflammatory cytokines, may up- or down-regulate S100B expression. Moreover, S100B expression has been attributed to catecholamines in cardiomyocytes surviving infarction (46). In fact, the S100B protein, which is undetectable in normal cardiomyocytes, is induced in the ischemic or post-infarction myocardium. These observations suggest that S100B gene expression is under complex transcriptional regulation. S100B gene expression appears to be constitutively repressed in all cell types, implying that the expression of S100B in a given cell type requires induction of an appropriate factor to counter the action of some negative regulatory elements (47).
In this study, the S100B protein was also detectable in extracellular spaces, in which it might exert multiple important effects. Previous research has shown that astrocytes may be stimulated to excrete S100B by relatively high levels of cytosolic Ca (48). A similar enhancing action of calcium ions could also be hypothesized in cardiomyocytes under hypoxic stress. A secondary hypothesis to explain the presence of S100B outside of cells is leakage from damaged cells (49). Our observation of coagulative myocytolysis in all cases of drug abuse might confirm this hypothesis.
Whatever the source of extracellular S100B and the mechanism underlying its release, extracellular S100B might attain local concentrations sufficient to affect cellular activities by paracrine, autocrine and endocrine manners.
The extracellular effects of S100B protein on cardiomyocytes and inflammatory cells could be mediated by the receptor for advanced glycation end-products (RAGE), a member of the immunoglobulin superfamily that is generally considered a central mediator of ischemia/reperfusion injury in the heart (50–53).
Dissecting the complex pattern of intracellular signaling resulting from hypoxic insults to ischemia and reperfusion (I/R) may be a considerable challenge.
In the complex environmental interactions in the injured heart, several 'danger signals’ are commonly liberated from ischemic cells 52. These signals signal the ischemic insult to other cells and mediate an inflammatory response but also promote tissue growth and remodeling that is partially mediated via RAGE activation. The S100B protein could participate in this process.
Our data confirm the hypothesized existence of a common pathway through which multiple drugs (heroin and cocaine) might cause S100B protein cardiac overload and release. The reported increase in S100B urine levels in newborns at higher risk of neonatal death (33) due to hypoxic-ischemic encephalopathy or asphyxia (34) suggest that severe hypoxia is the common mechanism of drug-induced S100B protein accumulation at the cardiac level.
Finally, our results, though limited by the small number of cases available and the numerous uncontrolled variables involved (e.g., doses, survival time, individual tolerance, post-mortem redistribution, drug stability, etc.), support the hypothesis that the immunohistochemical detection of significant S100B protein in the heart supports a suspicion of drug addiction and could distinguish overdose-related death from traumatic or ischemia-related death. Based on our preliminary data, we suggest that the immunodetection of S100B protein in the heart could be useful to not only help determine the cause of death in complicated or atypical cases but also provide evidence in individual cases to be shown in court.
Further studies are needed to clarify the metabolic link between overdose and S100B protein overload in the heart of drug abuse-related sudden deaths.
AUTHOR CONTRIBUTIONSFaa A analyzed and interpreted the data and prepared the manuscript. Senes G processed the heart tissues (hematoxylin–eosin and S100B immunostaining). Locci A processed the heart tissues (hematoxylin–eosin and S100B immunostaining). Pampaloni P provided assistance to the manuscript preparation and assembled images. Pais ME performed the experiments. Piras B performed the experiments and critically revised the manuscript. D'Aloja E critically revised the final version of the manuscript. Faa G contributed to the conception and design of the study.
The authors wish to thank Mr. Ignazio Ferru for secretarial assistance and Mrs. Sandra Serra for technical work.
No potential conflict of interest was reported.