As a lifestyle-related disease, social and cultural disparities may influence the features of squamous cell carcinoma of the head and neck in different geographic regions. We describe demographic, clinical, and pathological aspects of squamous cell carcinoma of the head and neck according to the smoking and alcohol consumption habits of patients in a Brazilian cohort.
METHODSWe prospectively analyzed the smoking and alcohol consumption habits of 1,633 patients enrolled in five São Paulo hospitals that participated in the Brazilian Head and Neck Genome Project – Gencapo.
RESULTSThe patients who smoked and drank were younger, and those who smoked were leaner than the other patients, regardless of alcohol consumption. The non-smokers/non-drinkers were typically elderly white females who had more differentiated oral cavity cancers and fewer first-degree relatives who smoked. The patients who drank presented significantly more frequent nodal metastasis, and those who smoked presented less-differentiated tumors.
CONCLUSIONSThe patients with squamous cell carcinoma of the head and neck demonstrated demographic, clinical, and pathological features that were markedly different according to their smoking and drinking habits. A subset of elderly females who had oral cavity cancer and had never smoked or consumed alcohol was notable. Alcohol consumption seemed to be related to nodal metastasis, whereas smoking correlated with the degree of differentiation.
Squamous cell carcinoma of the head and neck (SCCHN) is a major health problem worldwide. More than 630,000 new cases and 350,000 deaths were expected to occur in 2008 (1). SCCHN comprises a clinically heterogeneous group of disorders because presentation, treatment, and prognosis vary widely according to the tumor site and clinical stage (2). Tobacco and alcohol are the major risk factors for its occurrence, but human papillomavirus (HPV) infection has been shown to be an increasingly important risk factor, predominantly in non-smoker/non-drinker (NSND) patients (3). As a lifestyle pattern-related disease, social and cultural disparities are expected to influence the incidence and clinical features in different geographic regions. This knowledge may be useful in establishing possible carcinogenic mechanisms and improving the development of public healthcare policies.
We describe the demographic, clinical, and pathological aspects of SCCHN according to the smoking and drinking habits of patients in a Brazilian cohort.
MATERIALS AND METHODSStudy Design: A cross-sectional observational study of the demographic, clinical, and pathological features of patients with SCCHN according to their smoking and drinking habits.
The patients studied were enrolled from January 2001 to February 2009 in the head and neck surgery departments of five hospitals of the Brazilian Head and Neck Genome Project, Gencapo, a collaborative consortium of research groups from hospitals and universities in the state of São Paulo, Brazil. The aim of the project is to develop clinical, genetic, and epidemiological analyses of head and neck squamous cell carcinomas. We selected patients with oral cavity (lips excluded), oropharynx, larynx, and hypopharynx squamous cell carcinomas that were confirmed by histology. The demographic and social information was prospectively collected by trained interviewers. The clinical and pathological data were prospectively collected by assistant clinicians using standardized forms.
The patients were considered to be smokers if they had smoked at least one cigarette, cigar, or pipe daily for at least one year during their lifetime and were classified as drinkers if they had consumed alcoholic beverages at least once a month on a regular basis. The patients were classified into four categories: non-smokers/non-drinkers (NSND); smokers/drinkers (SD); smokers-only (SND); and drinkers-only (NSD).
The data regarding gender, age, ethnicity, and body mass index (BMI) were analyzed. Familial cancer history was calculated by dividing the number of first-degree relatives with cancer by the total number of first-degree relatives. We collected data on the number of first-degree relatives who smoked as an estimated measure of home exposure to environmental tobacco smoke.
The tumor site, stage, and pathology were analyzed. The stage classification was performed according to the 5th (until 2002) and 6th editions (since 2003) of the American Joint Committee on Cancer (AJCC) TNM classification (4,5). The pathological aspects analyzed were the tumor variants, differentiation grade, vascular/lymphatic/perineural invasions, and peritumoral inflammatory infiltrate.
Statistical analysisFor the continuous variables, the means and standard deviations were calculated. The frequencies and percentages are shown for the categorical variables. Associations among the categorical variables were analyzed using Pearson's chi-square test. For the mean comparisons, we used one-way ANOVA followed by Tukey's test to detect the means that were significantly different.
EthicsThe patients provided informed consent approved by the ethics committee of each hospital and by the National Committee on Ethics in Research/CONEP.
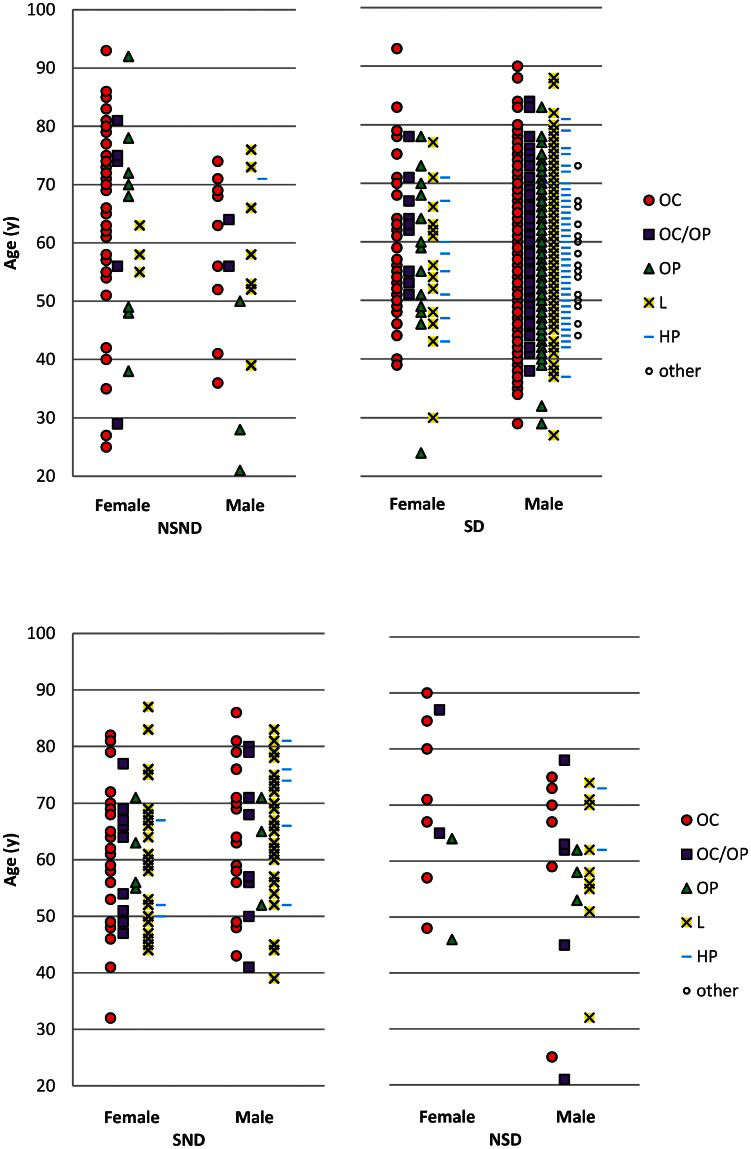
RESULTSA total of 1,659 patients with SCCHN in selected sites were identified. Twenty-six patients were excluded for incomplete data regarding smoking and alcohol consumption. From the remaining 1,633 patients, 80 were NSND (4.9%), 1,374 were SD (84.1%), 140 were SND (8.6%), and 39 were NSD (2.4%). The distributions according to age, gender, and site for each group are shown in Figure 1. The patients in the NSND, SD, SND, and NSD groups differed in gender, age, ethnicity, mean BMI, and mean number of first-degree relatives who smoked (Table 1). In an analysis of the groups, we found that the SD patients had a mean age of 57 years, whereas the mean age was 62 in the NSND, SND, and NSD groups. The patients who smoked (SD and SND) had a mean BMI of 24, whereas the NSND and NSD patients had a mean BMI of approximately 26. The NSND patients had fewer relatives who smoked than did the patients who smoked.
Age, site and gender distributions according to smoking and drinking habits in patients with SCCHN. NSND: Non-smokers/non-drinkers; SD: smokers/drinkers; SND: smokers-only; NSD: drinkers-only. OC: oral cavity; OP: oropharynx; L: larynx; HP: hypopharynx; other: other combination of sites.
Sociodemographic features according to smoking and drinking categories.
| Feature | NSND N (%) | SD N (%) | SND N (%) | NSD N (%) | p-value |
|---|---|---|---|---|---|
| Gender | <0.001∗ | ||||
| Female | 57 (71.3) | 96 (7.0) | 67 (47.9) | 11 (28.2) | |
| Male | 23 (28.8) | 1278 (93.0) | 73 (52.1) | 28 (71.8) | |
| Age (y) | <0.001† | ||||
| Mean (sd) | 62.79 (16.30)b‡ | 57.13 (9.79) a‡ | 62.21 (11.74) b‡ | 62.33 (15.12) b‡ | |
| Ethnicity | <0.001∗ | ||||
| White | 65 (82.3) | 988 (74.0) | 106 (76.8) | 32 (82.1) | |
| Mulatto | 5 (6.3) | 242 (18.1) | 22 (15.9) | 1 (2.6) | |
| Black | 4 (5.1) | 99 (7.4) | 6 (4.3) | 5 (12.8) | |
| Asian | 4 (5.1) | 4 (0.3) | 4 (2.9) | 1 (2.6) | |
| Indian | 1 (1.3) | 3 (0.2) | 0 | 0 | |
| BMI | <0.001† | ||||
| Mean (sd) | 26.81 (4.89) b‡ | 23.92 (3.88) a‡ | 24.58 (4.53) a‡ | 26.62 (4.06) b‡ | |
| Smoking 1st degree relatives | <0.001† | ||||
| Mean (sd) | 2.39 (2.38) a‡ | 4.17 (2.93) b‡ | 4.44 (3.76) b‡ | 3.16 (2.52) a, b ‡ | |
| Relatives with cancer/total relatives | 0.406† | ||||
| Mean (sd) | 0.08 (0.10) | 0.10 (0.14) | 0.09 (0.13) | 0.10 (0.14) |
NSND: Non-smokers/non-drinkers; SD: smokers/drinkers; SND: smokers/non drinkers; NSD: non smokers/drinkers. sd: standard deviation. BMI: body mass index. ∗Chi-square test. †Analysis of variance (ANOVA one-way). ‡Tukey's test – groups with the same letter are not different (p>0.05).
We identified differences among the groups according to site distribution, clinical/pathological stage, presence of clinical/pathological node metastasis, differentiation grade, and lymphatic invasion by histology (Tables 2 and 3).
Clinical features according to smoking and drinking categories.
| Feature | NSND N (%) | SD N (%) | SND N (%) | NSD N (%) | p-value‡ |
|---|---|---|---|---|---|
| Site∗ | <0.001 | ||||
| Oral cavity | 50 (62.5) | 493 (35.9) | 43 (30.7) | 14 (35.9) | |
| Oral cavity + oropharynx | 7 (8.8) | 114 (8.3) | 20 (14.3) | 7 (17.9) | |
| Oropharynx | 11 (13.8) | 241 (17.5) | 7 (5.0) | 5 (12.8) | |
| Larynx | 11 (13.8) | 384 (27.9) | 62 (44.3) | 11 (28.2) | |
| Hypopharynx | 1 (1.3) | 119 (8.7) | 8 (5.7) | 2 (5.1) | |
| Other combination of sites | 0 | 23 (1.7) | 0 | 0 | |
| cT stage | 0.369 | ||||
| 1 | 11 (14.9) | 129 (9.8) | 14 (10.9) | 6 (16.2) | |
| 2 | 24 (32.4) | 326 (24.8) | 40 (31.0) | 8 (21.6) | |
| 3 | 13 (17.6) | 310 (23.6) | 29 (22.5) | 10 (27.0) | |
| 4 | 26 (35.1) | 548 (41.7) | 46 (35.7) | 13 (35.1) | |
| Clinical nodal metastasis | <0.001 | ||||
| No | 46 (62.2) | 586 (44.6) | 84 (65.1) | 22 (59.5) | |
| Yes | 28 (37.8) | 727 (55.4) | 45 (34.9) | 15 (40.5) | |
| Clinical Stage† | 0.029 | ||||
| 0 | 0 | 1 (0.1) | 0 | 0 | |
| I | 10 (13.5) | 87 (6.6) | 13 (10.1) | 3 (8.1) | |
| II | 16 (21.6) | 199 (15.1) | 30 (23.3) | 6 (16.2) | |
| III | 13 (17.6) | 266 (20.2) | 29 (22.5) | 9 (24.3) | |
| IV | 35 (47.3) | 763 (58.0) | 57 (44.2) | 19 (51.4) |
NSND: Non-smokers/non-drinkers; SD: smokers/drinkers; SND: smokers/non drinkers; NSD: non smokers/ drinkers. ∗“other combination of sites” excluded from the analysis. †Clinical Stage = 0 excluded from the analysis. ‡ Chi-square test.
The categorical variables that showed significant differences among the groups were analyzed by group (Table 4). The gender distribution was different in all groups. Female patients composed a substantial majority of the NSND patients, approximately one half of the SND group, less than one-third of the NSD group, and 7% of the SD patients. The SD patients differed from the other groups according to ethnicity and had a smaller percentage of whites. The NSND patients had more oral cavity tumors and fewer larynx tumors than the other groups, whereas the SND group had more larynx tumors. Regarding clinical/pathological node metastasis and lymphatic invasion shown by histology, the SD patients were more affected than the patients who did not drink (NSND and SND). The NSD group seemed to be more affected than the other groups, but the result was not statistically different, possibly due to the small number of patients in the group. The SD patients presented at late pathological stages compared to the NSND and SND patients. The NSND patients had more differentiated tumors as shown by histology than the SND and SD patients.
Association of significant categorical variables among groups.
| Variable | NSND/SD p-value∗ | NSND/SND p-value∗ | NSND/NSD p-value∗ | SD/SND p-value∗ | SD/NSD p-value∗ | SND/NSD p-value∗ |
|---|---|---|---|---|---|---|
| Gender | <0.001 | 0.001 | <0.001 | <0.001 | <0.001 | 0.029 |
| Ethnicity | <0.001 | 0.174 | 0.453 | 0.001 | 0.014 | 0.050 |
| Site | <0.001 | <0.001 | 0.042 | <0.001 | 0.324 | 0.261 |
| cN+ | 0.003 | 0.673 | 0.783 | <0.001 | 0.074 | 0.528 |
| pN+ | 0.062 | 0.658 | 0.578 | 0.002 | 0.539 | 0.351 |
| Pathological Stage | 0.039 | 0.450 | 0.699 | 0.008 | 0.580 | 0.928 |
| Differentiation | 0.001 | 0.027 | 0.125 | 0.879 | 0.104 | 0.137 |
| Lymphatic invasion | 0.011 | 0.921 | 0.295 | 0.002 | 0.545 | 0.290 |
NSND: non smokers/non drinkers; SD: smokers/drinkers; SND: smokers/non drinkers; NSD: non smokers/drinkers. cN+: clinical nodal metastasis; pN+: pathological nodal metastasis; ∗Chi-square test.
In the analysis by tumor site, we identified significant differences in relation to the distribution of smoking and drinking habits. The most remarkable differences observed included a greater proportion of SND patients among those with larynx tumors and a greater proportion of NSND patients among those with oral cavity tumors (Figure 2) compared with the other groups.
DISCUSSIONSCCHN is a major cause of cancer morbidity and mortality in Brazil because the majority of cases are diagnosed at a late stage (6,7). Although smoking rates are decreasing, smoking is observed more frequently in individuals who are less educated and likely in those who are poorer than the general population (8,9). Alcohol abuse has increased among Brazilian men and, more significantly, among women (10).
The criteria that define smoking and drinking vary widely among studies. We understand that our criteria are strict, especially for drinking habits, but they are in accordance with large international series (11,12) and guarantee purity of the NSND group. The large majority of the patients in our study who drink consumed substantially more alcohol than the limits proposed for our group.
Previous series have shown that 2.4 to 14% of patients with SCCHN have never smoked or consumed alcohol (13–16). Long-term series are necessary to achieve a significant number of NSND patients. GENCAPO encompasses five Head and Neck Surgery Services from São Paulo, the most populous Brazilian state, which have prospectively enrolled patients in a standardized manner for several years.
In our study, the NSND patients were most frequently women – remarkably, elderly women – who presented with oral cavity cancers. The SD patients were younger than the other groups, possibly due to the synergistic effect of the carcinogens from alcohol and tobacco. Some studies have found the NSND patients to be younger than the SD patients (17) with oropharynx tumors. Other studies have found that the NSND patients are older than other patients and that they have oral cavity tumors (16,18). As the patients were enrolled primarily in surgery services, we might have included fewer young patients with oropharyngeal cancer because they are at times referred directly and exclusively to radiation and chemotherapy due to the traditionally good response to these treatment modalities in these cases.
The SND patients were more frequently affected by larynx cancer than the other groups. This finding is consistent with other studies (11,19), suggesting that the larynx is more susceptible to the carcinogens from tobacco than alcohol.
Although differences were observed in regards to ethnicity, and we believe they exist, this issue is a controversial subject in Brazilian populations due to intense miscegenation (20). The NSND patients were more frequently white compared to the SD and SND groups, a finding which has been reported in other studies (21).
The patients in the smokers group had a lower average BMI than those in the non-smokers group, which has been previously observed (22,23). Alcohol is related to malnutrition, but we may have not observed this relationship among patients in the NSD group due to the small number of patients in this group. Low BMI has been associated with increased mortality among SCCHN patients who smoke (24).
We attempted to estimate home exposure to tobacco by comparing the average number of first-degree relatives who smoke. Measuring environmental lifetime exposure to tobacco is difficult (25), and data about smoking by a spouse or workplace exposure were not routinely collected. Biological samples for urinary or serum cotinine measurements were not collected. We found that the NSND patients had fewer first-degree relatives who smoked and may have had less exposure to environmental smoking. We do not believe that passive smoking is a major risk factor for this group, but it would be necessary to compare environmental smoking in cancer-free patients to ensure this result. Family cancer history was not different among the groups.
The SD patients presented with nodal metastasis and pathological lymphatic invasion more frequently than the other groups. Our data suggest that this result may be more strongly related to alcohol than to tobacco, and patients in the SD and NSD groups showed nodal metastasis more frequently than did the patients who did not drink. This association has been described for other cancer types, but not for SCCHN (26,27). Further and more specific analysis of the data, including an analysis of the intensity of exposure, is being conducted. The underlying mechanism for this event is unknown, but suppression of the metastatic suppressor gene and decreased numbers of NK cells in the peripheral lymph nodes may partially explain these findings (26,27). Tobacco, but not alcohol, has been associated with neck metastasis for SCCHN (28). Likely as a consequence of these observations, the SD patients exhibited different distributions of SCCHN pathological stages, tending to present at more advanced stages than the NSND and SND patient. Heavy drinking and heavy smoking have been associated with diagnostic delays in T stage and nodal metastasis (29,30). We noted no statistical differences among the groups in terms of clinical or pathological T stage.
The tumors in the NSND patients appeared to be histologically more differentiated than the tumors in the SND and SD patients. The patients who were smokers seemed to have less-differentiated tumors than the non-smokers, suggesting a tobacco-related effect. A similar association has been reported for bladder cancer (31). Dahlstrom et al. found that a majority of the NSND patients presented with well-differentiated (oral cavity) or poorly differentiated (oropharynx) tumors, whereas the majority of the smoking/drinking patients presented with moderately differentiated tumors (17). The differentiation findings of both studies are likely similar because the NSND patients in our study typically had oral cavity tumors and few oropharynx tumors. Other pathological features such as tumor variants, vascular/perineural invasion and peri-tumor inflammatory infiltrate, were not different among the groups.
The exploratory nature of this study may lead to an increased type I error. Considering an alpha of 0.05, we would expect the 20 analyses performed (Tables 1–3) to have one significantly different finding due exclusively to chance.
Pathological features according to smoking and drinking categories.
| Feature | NSND N (%) | SD N (%) | SND N (%) | NSD N (%) | p-value† |
|---|---|---|---|---|---|
| pT stage | 0.160 | ||||
| 0, is | 1 (1.4) | 12 (1.1) | 1 (0.8) | 1 (3.0) | |
| 1 | 15 (20.5) | 140 (12.3) | 15 (12.1) | 4 (12.1) | |
| 2 | 26 (35.6) | 280 (24.5) | 34 (27.4) | 8 (24.2) | |
| 3 | 11 (15.1) | 196 (17.2) | 23 (18.5) | 9 (27.3) | |
| 4 | 20 (27.4) | 514 (45.0) | 51 (41.1) | 11 (33.3) | |
| Pathological nodal metastasis | 0.006 | ||||
| No | 37 (54.4) | 473 (42.8) | 67 (57.8) | 15 (48.4) | |
| Yes | 31 (45.6) | 631 (57.2) | 49 (42.2) | 16 (51.6) | |
| Pathological Stage∗ | 0.005 | ||||
| 0 | 0 | 4 (0.3) | 1 (0.8) | 0 | |
| I | 12 (16.2) | 99 (8.5) | 14 (11.1) | 3 (8.6) | |
| II | 15 (20.3) | 152 (13.0) | 21 (16.7) | 7 (20.0) | |
| III | 11 (14.9) | 169 (14.5) | 30 (23.8) | 7 (20.0) | |
| IV | 36 (48.6) | 742 (63.6) | 60 (47.6) | 18 (51.4) | |
| Tumor variants | 0.123 | ||||
| Squamous cell carcinoma | 70 (94.6) | 1151 (95.4) | 106 (89.1) | 33 (100.0) | |
| Verrucous carcinoma | 2 (2.7) | 5 (0.4) | 2 (1.7) | 0 | |
| Basaloid | 1 (1.4) | 24 (2.0) | 3 (2.5) | 0 | |
| Papillary | 1 (1.4) | 2 (0.2) | 1 (0.8) | 0 | |
| Spindle Cell | 0 | 4 (0.3) | 2 (1.7) | 0 | |
| Solid | 0 | 14 (1.2) | 2 (1.7) | 0 | |
| Other | 0 | 6 | 3 | 0 | |
| Differentiation | 0.005 | ||||
| Well differentiated | 42 (55.3) | 419 (34.0) | 46 (35.9) | 16 (45.7) | |
| Moderately differentiated | 29 (38.2) | 702 (57.0) | 70 (54.7) | 19 (54.3) | |
| Poorly differentiated | 5 (6.6) | 110 (8.9) | 12 (9.4) | 0 | |
| Vascular invasion | 0.571 | ||||
| Absent | 62 (91.2) | 934 (85.8) | 103 (88.0) | 26 (83.9) | |
| Present | 6 (8.8) | 154 (14.2) | 14 (12.0) | 5 (16.1) | |
| Lymphatic invasion | 0.001 | ||||
| Absent | 50 (78.1) | 653 (62.2) | 86 (77.5) | 19 (67.9) | |
| Present | 14 (21.9) | 396 (37.8) | 25 (22.5) | 9 (32.1) | |
| Perineural invasion | 0.495 | ||||
| Absent | 40 (58.8) | 626 (57.8) | 75 (62.5) | 22 (68.8) | |
| Present | 28 (41.2) | 457 (42.2) | 45 (37.5) | 10 (31.3) | |
| Peri-tumor inflammatory infiltrate | 0.397 | ||||
| Absent | 5 (8.5) | 100 (10.4) | 12 (12.2) | 0 | |
| Low | 21 (35.6) | 355 (36.9) | 35 (35.7) | 8 (38.1) | |
| Moderate | 23 (39.0) | 428 (44.5) | 40 (40.8) | 10 (47.6) | |
| Intense | 10 (16.9) | 79 (8.2) | 11 (11.2) | 3 (14.3) |
NSND: Non-smokers/non-drinkers; SD: smokers/drinkers; SND: smokers/non drinkers; NSD: non smokers/drinkers. ∗Pathological Stage = 0 excluded from the analysis. †Chi-square test.
Regarding the generalizability of these findings, it should be noted that these subjects belong to a cohort of patients from the public health services in São Paulo. The associations identified in this study should be validated in other populations.
We have shown several demographic, clinical and pathological differences according to smoking and drinking habits. An important subset of NSND elderly females with oral cavity cancer was identified, but the risk factors for this population are unknown. The additional and unexpected findings from our data were that alcohol may be related to the nodal metastasis of SCCHN and that tobacco may predispose patients to less-differentiated tumors. These findings may help elucidate the possible mechanisms of the carcinogenesis of alcohol and tobacco. Studies designed to answer these questions may be necessary to confirm these hypotheses.
AUTHOR CONTRIBUTIONSMoyses RA and Michaluart Jr P contributed to the conception, data collection and analysis, and manuscript elaboration. López RVM, Cury PM and Tajara EH contributed to the conception, data analysis, and manuscript review. Siqueira SAC, Curioni OA, Gois Filho JF, and Figueiredo DLA collected the data and participated in the review of the manuscript. Head and Neck Genome Project GENCAPO (group of researchers) collected the data and participated in the study conception.
The authors would like to thank the members of the GENCAPO (Head and Neck Genome) Project and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP). This study was supported by FAPESP (04/12054-9).