Salvia miltiorrhiza has long been used to treat systemic sclerosis. Tanshinone IIA, one of the phytochemicals derived from the roots of Salvia miltiorrhiza, exhibits multiple biological activities. The present study aimed to investigate whether tanshinone IIA has an effect on the interleukin-17A-induced functional activation of systemic sclerosis patient-derived dermal vascular smooth muscle cells.
METHODS:Systemic sclerosis patient-derived dermal vascular smooth muscle cells were incubated with various dosages of tanshinone IIA in the presence of interleukin-17A or the serum of systemic sclerosis patients. Cell proliferation was assessed using Cell Counting Kit-8. The expression of collagen 1 and 3 in cells was evaluated by immunofluorescence. Cell migration was measured using a transwell assay. The expression of phospho-extracellular signal-regulated kinase was detected by Western blotting.
RESULTS:Our data demonstrate that tanshinone IIA exerts an inhibitory effect on interleukin-17A-induced systemic sclerosis patient-derived dermal vascular smooth muscle cell proliferation, collagen synthesis and migration.
CONCLUSION:These findings suggest that tanshinone IIA might serve as a promising therapeutic agent for the treatment of systemic sclerosis.
Systemic sclerosis (SSc) is a life-threatening connective tissue disease characterized by autoimmunity, inflammation, functional and structural alterations in small blood vessels and vascular fibrosis of the skin and internal organs (1). SSc has a complex pathogenesis and protean clinical manifestations that reflect immune dysregulation, microangiopathy and systemic fibrosis, such as Raynaud phenomenon, digital ulcers, pulmonary arterial hypertension and renal crisis (2–4). Widespread intimal hypertrophy, a characteristic that SSc shares with atherosclerosis, is the result of vascular smooth muscle cell (myointimal cells) proliferation and migration as well as local collagen accumulation (5). Vascular smooth muscle cells play a crucial role in the development of SSc-related vasculopathy.
T helper cell 17 (Th 17), a subset of T lymphocytes, is implicated in the pathogenesis of SSc (6–10). Increased levels of interleukin-17A (IL-17A)-producing CD4+ T cells are observed in the lesional skin tissues and peripheral blood of SSc patients (6). Our previous studies indicated increased IL-17A in patients in the active phase of SSc. IL-17A stimulates the expression of multiplex chemokines and cellular adhesion molecules of vascular endothelial cells and promotes collagen secretion from fibroblasts, resulting in endothelial inflammation and fibrosis (9,10). Recently, our unpublished data demonstrated that serum-derived IL-17A from SSc patients promotes the proliferation, collagen synthesis and migration of SSc patient-derived dermal vascular smooth muscle cells (DVSMCs). Thus, we hypothesized that the inhibition of the IL-17A-mediated activation of DVSMCs may relieve the microangiopathy of SSc.
Tanshinone IIA (C19H18O3) is a fat-soluble pharmacologically active component of the Chinese herb Salvia miltiorrhiza, a well-known traditional Chinese medicine that is used to treat cardiovascular diseases and connective tissue diseases, such as systemic lupus erythematosus, SSc and rheumatoid arthritis (11–17). Previous studies have demonstrated that tanshinone IIA inhibits the proliferation and migration of artery smooth muscle cells (18–24), reduces pulmonary artery pressure and ameliorates hypoxia-induced pulmonary artery remodeling (25). However, whether tanshinone IIA can reverse the IL-17A-induced vessel remodeling of SSc is not fully understood. In the present study, we investigated whether tanshinone IIA inhibits IL-17A-induced DVSMC proliferation, collagen synthesis and migration.
MATERIALS AND METHODSEthics statementThis study was reviewed and approved by the Zhongshan Hospital Research Ethics Committee. The Institutional Review Board of Zhongshan Hospital of Fudan University approved the human study protocol.
ReagentsTanshinone IIA (99.0% pure; Boyun Biotech Company, Shanghai, China) was prepared as a 10-mg/ml stock solution in dimethyl sulfoxide and stored in the dark at −20°C. The following antibodies were used for immunofluorescence or Western blotting: collagen 1, collagen 3 and α-smooth muscle action (α-SMA) (Abcam, New York, USA). Myosin and calponin were purchased from Proteintech Group, Inc. (Chicago, USA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), p38, phospho-p38, Jun N-terminal kinase (JNK), phospho-JNK, extracellular signal-regulated kinase (ERK) and phospho-ERK were obtained from Cell Signaling Technology (Boston, USA). Smooth muscle cell medium-basal was obtained from Sciencell (San Diego, California, USA). Human recombinant IL-17A was purchased from R&D Systems (Minneapolis, Minnesota, USA). Other chemicals and materials were obtained from the Beyotime Institute of Biotechnology or KeyGEN Biotechnology Company (Nanjing, China).
SSc patients and healthy individualsTen patients with SSc (n ϝ 5 men and 5 women; mean age 42.9 ± 6.5 years) were enrolled in the study after providing informed and written consent. All patients met the 1980 preliminary classification criteria for SSc (26). Disease activity was assessed using the criteria proposed by Valentini et al. (27), in which the evaluation of clinical and laboratory factors are used to determine a score ranging from 0 to 10 (0 represents no disease activity, and 10 represents maximal activity). Patients were in the active phase with scores ≥ 3, and the disease durations were < 5 years. The patients had different degrees of microangiopathy-related manifestations (e.g., Raynaud phenomenon, digital ulcers, telangiectasia, pulmonary hypertension and renal crisis). Ten age- and sex-matched healthy individuals (n ϝ 4 men and 6 women; mean age 39.6 ± 5.3 years) were enrolled after providing informed consent. Blood samples were obtained from SSc patients and healthy individuals. Lesional skin tissues of SSc patients were obtained to isolate DVSMCs.
Isolation and identification of SSc patient-derived DVSMCsDVSMCs were isolated from lesional skin tissues using the trypsin digestion method. The cells were cultured with smooth muscle cell medium-basal. DVSMCs from the third to seventh passages were identified using immunofluorescence staining for myosin and calponin. α-SMA was analyzed by Western blotting. In addition, human primary dermal vascular smooth muscle cells (HDVSMCs), which were purchased from CHI Scientific, Inc. (Boston, USA), served as a positive control. Both types of cells were cultured at 37°C with 5% CO2 and 100% humidity.
DVSMC culture and stimulationThe cells were seeded in 6-well plates at a density of 3 × 105 cells/well and cultured overnight. DVSMCs were incubated with various dosages of tanshinone IIA (1, 10, and 100 µg/ml) in the presence of IL-17A (100 ng/ml) or 5% serum from SSc patients or healthy subjects.
CCK-8Cell proliferation was assessed using CCK-8. DVSMCs were plated in 96-well plates at a density of 2 × 104 per well. When cells were grown to 90 to 100% confluence, they were stimulated for 24, 48 and 72 h. Then, 10 µl of the CCK-8 solution was added to each well 2 h prior to each time point, and the optical density was read at 450 nm.
Cell fluorescence stainingCells were plated in 24-well plates with cover slips. When cells were grown to 90 to 100% confluence, they were stimulated for 24 h. After stimulation, the cells were fixed with 4% paraformaldehyde for 30 min and treated with 0.5% triton for 15 min. The cells were blocked with 1% BSA for 30 min and incubated with rabbit anti-human collagen 1 (1:200) and rabbit anti-human collagen 3 (1:300) overnight at 4°C. After incubation with FITC-goat anti-rabbit IgG at room temperature for 1 h, DAPI (4′,6-diamidino-2-phenylindole) was used to stain the cell nuclei. The images were scanned using a fluorescence microscope (Olympus, Japan).
Western blottingProteins from DVSMCs were extracted using RIPA lysis buffer with the protease inhibitor phenylmethanesulfonyl fluoride. Proteins were separated on 8% or 10% Tris-glycine gels by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). After blocking with 5% milk for 2 h at room temperature, the membranes were incubated with primary antibodies, including ERK, phospho-ERK, p38, phospho-p38, JNK, phospho-JNK and GAPDH, overnight at 4°C. The membranes were washed and incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies for 1.5 h at room temperature. The proteins were detected with ECL detection reagents.
TranswellDVSMC migration was evaluated using polycarbonate membrane transwell chambers (8 μm pore size). The cells that exhibited growth arrest for 48 h were harvested using 0.05% trypsin-EDTA solution and resuspended (10 × 105 cells/ml) in the medium. The cells were added to the upper chamber. Tanshinone IIA (1, 10, and 100 µg/ml), IL-17A (100 ng/ml), 5% healthy serum or 5% SSc serum were added to the lower well of the chamber containing Dulbecco's modified Eagle's medium with 2% serum. Cells were incubated for 6 h at 37°C and 5% CO2. After scraping the cells affixed to the inside of the membrane, the cells that had migrated through the insert were fixed with 4% paraformaldehyde and stained with Giemsa according to the manufacturer's instructions. For each well, the number of migrated cells was counted from 5 high-power fields (HPFs) at 200× magnification.
Statistical analysisResults were expressed as the mean ± SD values. The statistical analysis was performed using SPSS 17.0 statistical software (SPSS, Inc., Chicago, IL, USA). Statistical significance was determined by analysis of variance for comparisons of multiple means followed by Student's t-test or the Mann-Whitney U test. Results demonstrating P < 0.05 were considered statistically significant.
RESULTSTanshinone IIA inhibits IL-17A-induced proliferation of SSc patient-derived DVSMCsOur recent study demonstrated that a significant increase in DVSMC proliferation occurred after incubation with 100 ng/ml IL-17A and SSc patient serum for 72 h compared with the vehicle. In this study, we investigated the effect of tanshinone IIA on IL-17A-induced DVSMC proliferation. First, DVSMCs were isolated using the trypsin digestion method and identified using immunofluorescence staining for myosin and calponin (see Figure 1A). Furthermore, the presence of α-SMA, a protein produced by DVSMCs, was verified by Western blotting (see Figure 1B). In addition, tanshinone IIA inhibited IL-17A-mediated DVSMC proliferation in a dose- and time-dependent manner (see Figure 1C). A similar inhibition was observed in cells incubated with tanshinone IIA in the presence of 5% SSc serum (see Figure 1D).
The identification of SSc patient-derived DVSMCs; tanshinone IIA inhibits IL-17A-induced DVSMC proliferation. (A) The morphology and positive markers (Myosin, red fluorescence; Calponin, green fluorescence) of SSc patient-derived DVSMCs and HDVSMCs are shown. Scale bars represent 100 μm. (B) α–SMA expression of SSc patient-derived DVSMCs and HDVSMCs was examined by Western blotting. GAPDH was used as a loading control. (C) SSc patient-derived DVSMCs were treated with various dosages of tanshinone IIA (1, 10, and 100 µg/ml) in the presence of human recombinant IL-17A (100 ng/ml) for 24, 48 and 72 h. Cell proliferation (optical density) was assayed using CCK8. (D) DVSMCs were treated with various doses of tanshinone IIA in the presence of 5% serum obtained from SSc patients or healthy subjects for 24, 48, and 72 h. Cell proliferation was assayed using CCK8. The data are presented as the mean ± SD (standard deviation). The experiment was repeated three times.
Our recent study demonstrated that IL-17A derived from the serum of SSc patients might play a role in inducing collagen synthesis and secretion in DVSMCs. Here, we demonstrate that tanshinone IIA inhibits the expression of collagen 1 and 3 in the presence of IL-17A and 5% SSc serum (see Figure 2A and B). These results indicate that tanshinone IIA might play a role in attenuating IL-17A-induced collagen synthesis in DVSMCs.
Tanshinone IIA attenuates IL-17A-induced collagen synthesis in SSc patient-derived DVSMCs. (A) DVSMCs were treated with tanshinone IIA (100 µg/ml) in the presence of IL-17A (100 ng/ml) and 5% serum from SSc patients or healthy subjects for 24 h. Collagen 1 was detected by immunofluorescence. (B) DVSMCs were treated with tanshinone IIA (100 µg/ml) in the presence of IL-17A (100 ng/ml) and 5% serum from SSc patients or healthy subjects for 24 h. Collagen 3 was stained by immunofluorescence. The experiment was repeated three times.
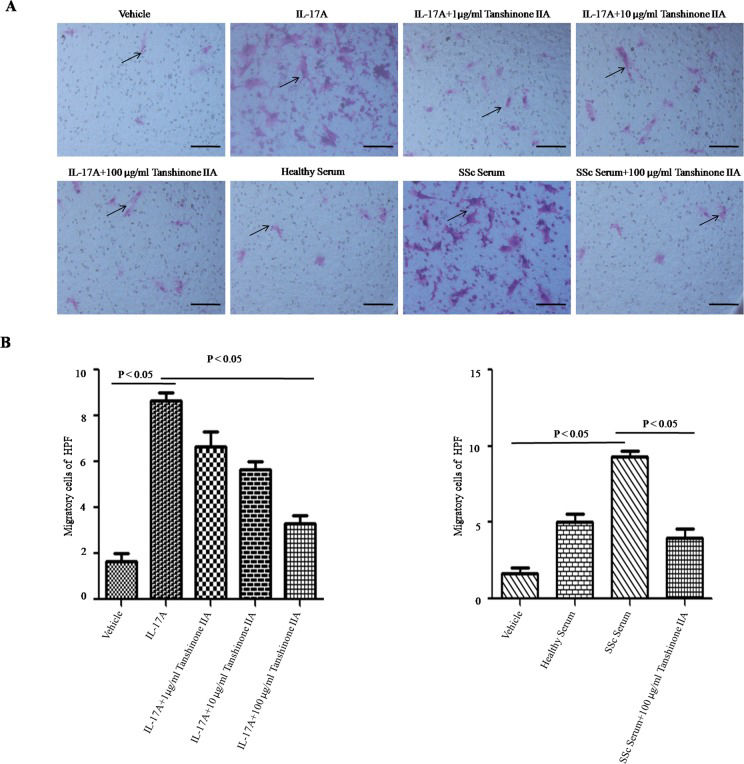
In scleroderma, an increased number of vascular smooth muscle cells migrate from media to intima, resulting in intimal thickening and vascular stenosis. In the present study, IL-17A and SSc serum promoted DVSMC migration. Tanshinone IIA significantly reduced the number of migrating cells in the presence of IL-17A and 5% SSc serum (see Figure 3A and B).
Tanshinone IIA suppresses IL-17A-induced migration of SSc patient-derived DVSMCs. (A) DVSMCs were incubated with tanshinone IIA (100 µg/ml) in the presence of IL-17A (100 ng/ml) or 5% serum from SSc patients or healthy subjects for 24 h. Cell migration was measured via the transwell assay. Black arrows represent migrated cells per high-power field (HPF) at 200× magnification. Scale bar ϝ 100 μm. (B) The number of migrated cells per HPF. The experiment was repeated three times, and data are presented as the mean ± SD.
The above results indicate that tanshinone IIA attenuated IL-17A-induced DVSMC proliferation, collagen synthesis and migration. However, the precise mechanism underlying these effects has not been established. Recently, we discovered that ERK MAPK might be an important signaling pathway in the IL-17A-induced up-regulation of DVSMC proliferation, collagen synthesis and migration. We detected phospho-ERK, phospho-JNK and phospho-p38 MAPK in these cells. We found that tanshinone IIA inhibited ERK phosphorylation induced by IL-17A or 5% SSc serum. However, JNK and p38 phosphorylation was not significantly altered (see Figure 4A-D).
Tanshinone IIA reduces IL-17A-induced ERK phosphorylation in SSc patient-derived DVSMCs. (A) DVSMCs were treated with tanshinone IIA (100 µg/ml) in the presence of IL-17A (100 ng/ml) for 15, 30, 45 and 60 min, and the phosphorylation of ERK, JNK and p38 MAPK were detected by western blotting. (B) DVSMCs were exposed to tanshinone IIA (100 µg/ml) in the presence of SSc serum for 15, 30, 45 and 60 min. The phosphorylation of ERK was detected by western blotting. (C) DVSMCs were incubated with different dosages of tanshinone IIA (1 µg/ml, 10 µg/ml, 100 µg/ml) in the presence of IL-17A (100 ng/ml) for 30 min. The phosphorylation of ERK was detected by western blotting. (D) DVSMCs were incubated with different dosage of tanshinone IIA (1, 10, and 100 µg/ml) for 30 min in the presence of SSc serum. The phosphorylation of ERK was detected by western blotting. GAPDH was used as a loading control. The experiment was repeated three times.
In the present study, we demonstrated that tanshinone IIA inhibits IL-17A-induced proliferation and migration as well as collagen synthesis in SSc patient-derived DVSMCs. Furthermore, we indicate that tanshinone IIA reduced ERK phosphorylation stimulated by IL-17A and SSc serum in a time- and dose-dependent manner. This novel information partially clarified the mechanism by which tanshinone IIA ameliorates IL-17A-induced vascular remodeling in SSc patients.
Although advances have been made in the treatment of SSc, targeted therapies with fewer side effects are still lacking. Our previous studies have demonstrated the therapeutic efficacy of Salvia miltiorrhiza on SSc (28–30). Tanshinone IIA is one of the pharmacological components of Salvia miltiorrhiza. Recently, tanshinone IIA has been shown to modulate the growth, proliferation and migration of numerous cells, including keratinocytes, cardiac fibroblasts, cardiomyocytes, tumor cells and human aortic smooth muscle cells (20,31–39). IL-17A-induced aberrant vascular smooth muscle cell proliferation may serve as a major cause of vascular remodeling. Therefore, we hypothesized that the inhibition of IL-17A-induced vascular smooth muscle cell proliferation might contribute to its anti-remodeling effects. The present study demonstrated that tanshinone IIA significantly inhibited IL-17A-induced DVSMC proliferation.
In our recent study, we demonstrated that human recombinant IL-17A and SSc serum-derived IL-17A promoted collagen 1 and 3 synthesis and secretion in SSc patient-derived DVSMCs. Here, the immunofluorescence results indicated that tanshinone IIA reduces collagen 1 and collagen 3 synthesis in DVSMCs when co-stimulated with human recombinant IL-17A or SSc serum-derived IL-17A.
Similarly, our previous transwell experiments revealed that IL-17A and SSc serum-derived IL-17A accelerated DVSMC migration. Our present results revealed that tanshinone IIA decreased the number of migrating cells in the presence of IL-17A and SSc serum.
The above results indicate that tanshinone IIA inhibits IL-17A-induced abnormal DVSMC activation. However, the exact mechanism remains unknown. ERK, a member of the MAPK family, is the core of the signal transduction pathway networks involved in regulating cell growth, development and division (40). Data from our recent study implied that the ERK signaling pathway might play a key role in IL-17A-induced endothelial injury and vascular smooth muscle cell activation (10). In the current study, we found that tanshinone IIA decreased IL-17A-induced phospho-ERK in a time- and dose-dependent manner. These results indicate that tanshinone IIA might affect IL-17A-induced proliferation, collagen synthesis and migration of SSc patient-derived DVSMCs via inhibition of the ERK signaling pathway.
In summary, our results provide preliminary evidence that tanshinone IIA exerts an inhibitory effect on IL-17A-induced proliferation, collagen synthesis and migration of SSc patient-derived DVSMCs. This inhibitory effect may be dependent on the ERK MAPK signaling pathway. These collective findings potentially explain why Salvia miltiorrhiza clearly relieves symptoms in SSc patients in the clinic. These findings suggest that tanshinone IIA might serve as a promising therapeutic agent for SSc patients. However, the current results require validation with further studies.
AUTHOR CONTRIBUTIONSLi M conceived and designed the experiments. Liu M performed the experiments. Liu M and Ji Yang analyzed the data. Liu M and Yang J wrote the paper. The authors have participated sufficiently in the work to take public responsibility for appropriate portions of the content.
This work was supported by grants from the National Natural Science Foundation of China (No. 30872274, 81000693 and 81472874) and the Shanghai Natural Science Foundation of China (No. 15ZR1406500).
No potential conflict of interest was reported.