During coronary artery bypass graft (CABG) surgery, the saphenous vein is sutured through its proximal segment to the aorta. Intimal hyperplasia is one of the possible causes of graft occlusion. Notably, blood turbulence can induce wall shear stress that may also play an important role in this process.
OBJECTIVEWe propose a new technique for performing proximal anastomosis to avoid CABG failure.
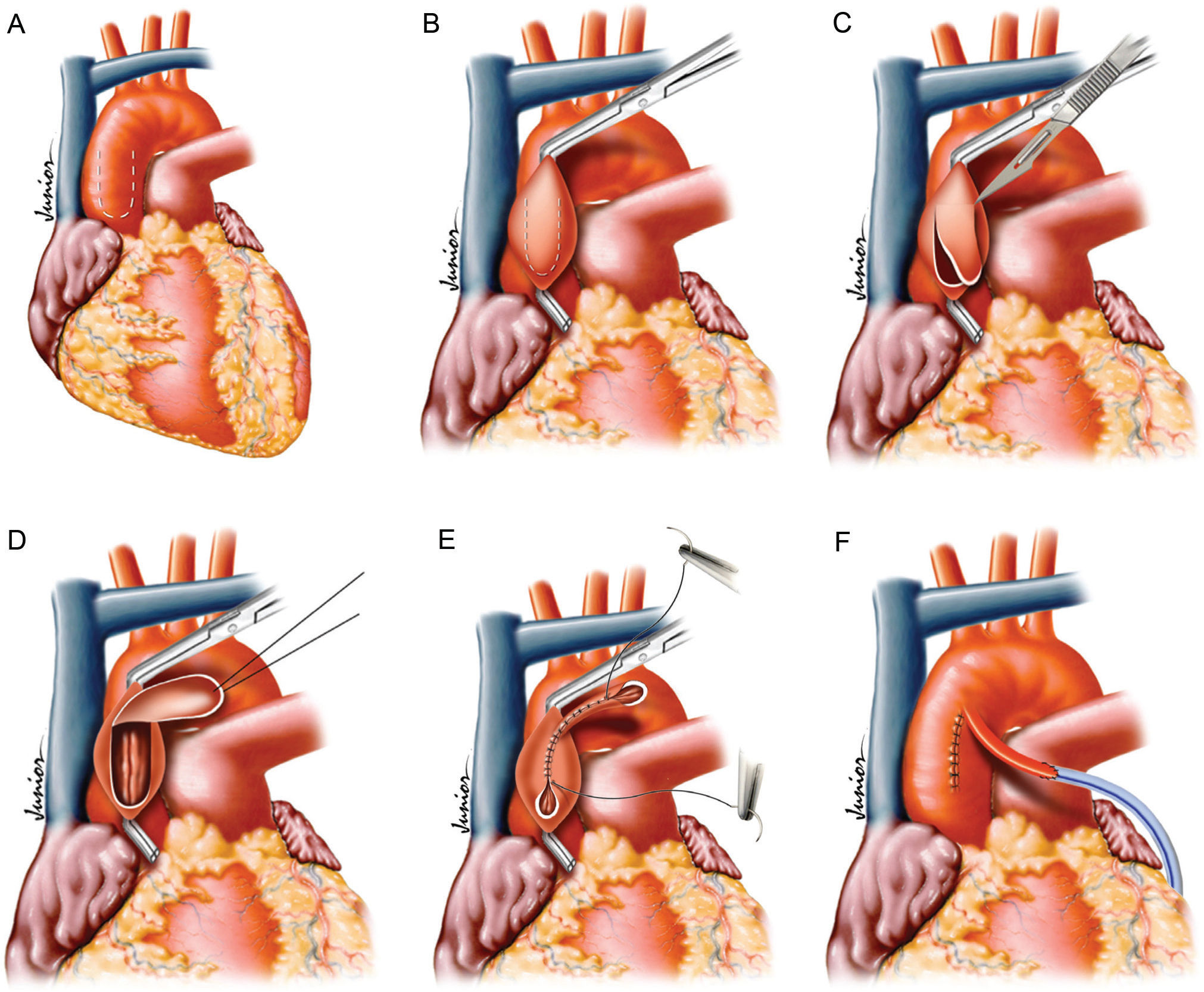
METHODAn 80 kg pig was subjected to open heart surgery. Four stitches were placed in the anterior ascending aorta, which formed a 2 cm by 4 cm patch. This patch was isolated through the application of a tangential clamp that was oriented parallel to the axis of the aorta. After releasing the patch, which was held to the aorta through its cranial end pedicle, the rims were sutured to each other creating a conduit with a length of 4 cm and an internal diameter of 4 mm. The rest of the aortotomy was closed by placing a direct suture between its rims.
RESULTThis novel technique created an “in situ” aortic wall graft that was 4 cm long and characterized as being of uniform 4 mm caliber.
Coronary artery bypass graft (CABG) using the left internal thoracic artery (LITA) to the left anterior descending artery (LADA) is the gold standard for multi-vessel coronary disease procedures. The second graft is usually implanted with its proximal segment to the aorta. However, approximately 20% of the saphenous veins present some degree of stenosis during the first year after surgery.1 Recent studies have demonstrated that wall shear stress is maximized at the proximal anastomosis, probably due to turbulence.2,3 This elevated wall shear stress may be related to the occurrence of intimal hyperplasia that leads to late graft failure.4–6
In order to avoid graft failure, we propose a new technique for performing the proximal anastomosis procedure such that the high wall shear stress will be localized at an “in situ” aortic wall graft that may exhibit improved pressure tolerance. Our approach may help avoid proximal intimal hyperplasia.
MATERIAL AND METHODSThis research was approved by the Ethics Committee of the Jundiaí School of Medicine. An 80 kg pig was subjected to general anesthesia with mechanical ventilation. Access to the heart was through sternotomy and pericardial opening. Four stitches were placed in the anterior ascending aorta to create a 2 cm by 4 cm patch (Figure 1A). This patch was isolated using a tangential clamp that was positioned parallel to the axis of the aorta (Figure 1B). After releasing the patch, which was held to the aorta through its cranial end pedicle (Figures 1C and 1D), the rims of the patch were sutured to each other, creating a conduit of length 4 cm and with an internal diameter of 4 mm (Figure 1E). The rest of the aortotomy was closed by applying a direct suture between it’s rims. In addition, a PTFE tube that simulated a saphenous conduit was sutured to the distal end of the conduit (Figure 1F).
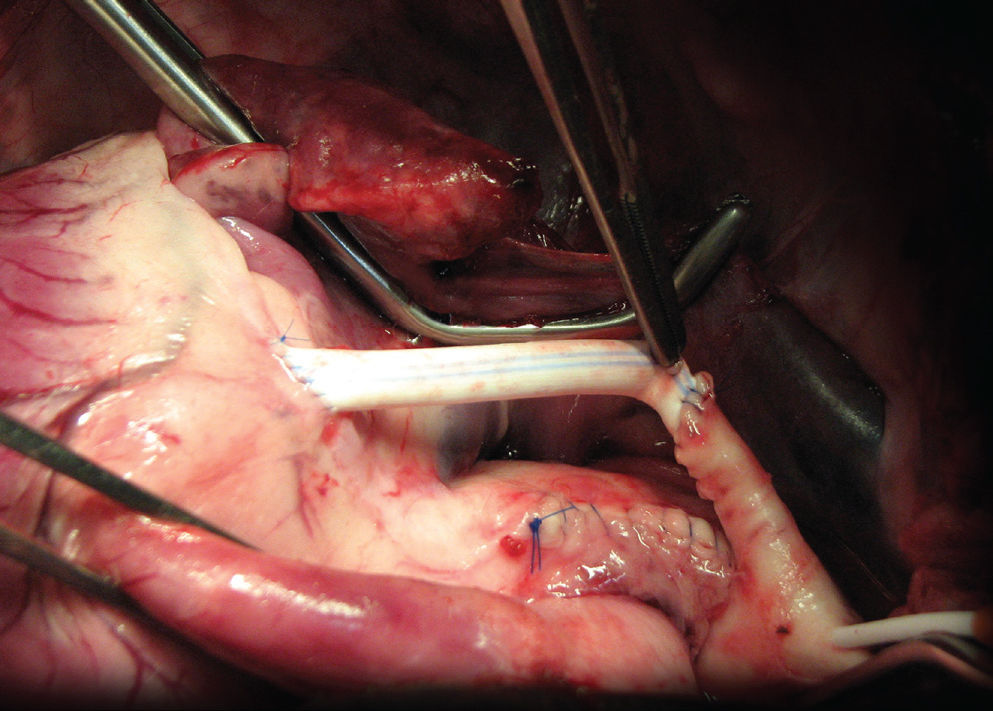
ResultsThis technique resulted in the creation of a proximal aortic wall graft 4 cm long with an internal diameter of 4 mm. This graft possessed a distal end with an opening similar to the usual aortic site for proximal anastomosis (Figure 2). The graft exhibited sufficient mobility and was appropriate for either the right or left coronary arteries. The graft allowed for the implantation of the composite graft without further aortic clamping.
In our experimental model, a PTFE tube was implanted towards the distal end of the aortic graft (simulating a saphenous graft) in order to create a composite graft with a homogenous diameter (Figure 3).
DISCUSSIONThe aortic wall graft is an easy technique to perform because once the aortic patch is obtained, both conduit creation and the aortic closure only require linear sutures.
During standard anastomosis, the high wall shear stresses associated with the proximal anastomosis may lead to intimal hyperplasia and graft failure.3,6 With this proposed technique, the high wall shear stress will be localized to the “in situ” aortic wall graft. This may offer better pressure tolerance and will thus be better equipped to avoid intimal hyperplasia.
Because the composite graft to be implanted in the end of the aortic graft will be of similar diameter, there will be little turbulence and consequently low wall shear stress. We expect that intimal hyperplasia would be unlikely to occur under these conditions.
Several studies have demonstrated the benefits of complete arterial revascularization.7–11 This technique can add an additional 4 cm to a radial artery graft, which in turn allows it to reach the circumflex and the right coronary regions through sequential anastomosis. In addition, this should not increase the chances of morbidity because the aortic manipulation consists of a couple of linear sutures.
The potential benefits of this technique include a possible reduction in the graft failure rate due to intimal hyperplasia, which avoids the recurrence of symptoms and future operations.
In some cases, where the patient presents with some limitation in the graft (previous bilateral saphenectomy, redo CABG, or low quality saphenous graft), this technique may allow for a complete revascularization of the surgical area. Moreover, in circumstances when the graft size is underestimated, this technique can overcome the problem.
CONCLUSIONTo the best of our knowledge, this technique has never been described. Our results suggest that it allows for a proximal “in situ” aortic wall graft of excellent quality. Further work is required to determine its safety and reliability. As described above, this procedure appears to be a simple and straightforward approach that adds an extra 4 cm onto the composite graft.