To investigate the effect of elemene on the radiosensitivity of A549 cells and its possible molecular mechanism.
METHODS:Apoptosis of A549 cells was detected by flow cytometry and fluorescence microscopy. The effect of double-strand break (DSB) damage repair in A549 cells was evaluated using the neutral comet assay. Protein expression levels were detected using western blotting, and the correlation between protein levels was analyzed.
RESULTS:Elemene exhibited a radiosensitizing effect on A549 cells. The level of apoptosis induced by elemene combined with radiation was significantly greater (p<0.01) than that elicited by either radiation or elemene alone. Following radiation and subsequent repair for 24 h, the tail intensity of A549 cells treated with a combination of elemene and radiation was greater than that of cells treated with either elemene or radiation alone (p<0.01). This result indicates that elemene inhibits cellular DSB repair. Both elemene combined with radiation and radiation alone decreased the protein expression of DNA-PKcs and Bcl-2 compared to elemene alone (p<0.01), while p53 protein expression was increased (p<0.01). A negative correlation was observed between DNA-PKcs and p53 expression (r=-0.569, p=0.040), while a positive correlation was found between DNA-PKcs and Bcl-2 expression (r=0.755, p=0.012).
CONCLUSIONS:Elemene exhibits a radiosensitizing effect on A549 cells, and its underlying molecular mechanism of action may be related to the downregulation of DNA-PKcs gene expression.
Lung cancer is one of the most common malignant tumors, and radiotherapy is the prevailing method of choice for treatment 1, especially for middle- to late-stage lung cancer. Radiation works by damaging the DNA of cancerous cells and altering apoptosis-related genes or proteins, leading to cell death. Improving the radiosensitivity of tumor cells is a significant factor that would improve the efficacy of radiotherapy.
DNA-dependent protein kinase (DNA-PK) is an important enzyme that participates in DNA damage repair and has become the main target of radiation sensitivity interventions 2–4. The catalytic subunit (cs) of DNA-PK affects cellular radiosensitivity by regulating the phosphorylation of DNA damage repair-related proteins 5. Thus, inhibition of DNA-PKcs gene expression can block DNA double-strand break (DSB) repair and improve cellular radiosensitivity.
Cellular apoptosis is the core characteristic of radiotherapy, and regulation of this process thus plays an important role in cellular radiosensitivity 6,7. Previous studies have shown that apoptosis-related genes, such as phosphoprotein (p53), p16, B-cell lymphoma-2 (Bcl-2), and erythroblastic leukemia viral oncogene homolog 2 (erbB-2), are associated with tumor radiosensitivity 8,9, especially p53 and Bcl-2. It has also been reported that elemene interacts with the frontier orbitals of DNA bases to form complexes between DNA molecules. Specifically, Jiang et al. 10 showed that elemene increases the radiosensitivity of A549 cells, the mechanism for which may be related to the upregulation of p53, downregulation of Bcl-2, and induction of cellular apoptosis.
Elemene, which is extracted from Zingiberaceae plants (Curcuma aromatica Salisb.), is a non-cytotoxic antitumor compound that can improve the radiosensitivity of tumor cells 11. Results of an in vitro study showed that elemene increased the radiosensitivity of renal carcinoma cells, tongue squamous cancer cells, and non-small cell lung cancer cells 10,12,13. Animal experiments further showed that elemene exhibited radiotherapy-sensitizing effects in many types of tumor cells, such as transplanted murine U14 tumors, kidney cancer GRC-1 cells, and tongue squamous carcinoma Tca-8113 cells 13–15. In addition, beta elemene enhances A549 cell radiosensitivity through enhancement of DNA damage and suppression of DNA repair 16.
In the current study, A549 cells were irradiated following elemene treatment, and the changes in expression of the apoptosis-related genes Bcl-2 and p53 as well as the double-stranded DNA damage repair-related gene DNA-PKcs were evaluated. These experiments were conducted to further understand elemene's molecular mechanism of action in enhancing radiation sensitivity of A549 cells.
MATERIALS AND METHODSCell cultureThe human lung adenocarcinoma A549 cell line was purchased from the Chinese Academy of Medical Sciences (CAMS) Cell Center and passaged at the Second Affiliated Hospital of Dalian Medical University Center Laboratory. The cells were cultured in RPMI 1640 medium containing 10% inactivated fetal bovine serum (FBS) at 37°C under an atmosphere of 5% CO2 and saturated humidity. The cells were subcultured when they reached the exponential phase.
Reagents and instrumentsElemene (0.1 g/20 mL), which was obtained from DaLian JinGang Pharmaceutical Co. Ltd. (China), was dissolved in RPMI 1640 medium to final working concentrations of 10 and 20 µg/mL before use. RPMI 1640 medium was obtained from Gibco (USA); FBS was obtained from TianJin TBD Biotechnology Company (China); p53 and Bcl-2 antibodies were obtained from Santa Cruz (USA, 1:1,000); and DNA-PKcs antibody (1:2,000), anti-human β-actin mouse monoclonal antibody and histone H1 internuclear internal reference antibodies (1:200) were obtained from Neomarker (USA). The Jim-X half-dry transfer electrophoresis apparatus was obtained from DaLian JingMai Biotechnology Co. Ltd. (China). The flow cytometer was purchased from the Gene Company (USA). The CK2 type inverted microscope was obtained from Olympus (Japan). The BX51 type fluorescent microscope was also obtained from Olympus (Japan).
Irradiation conditionsCell irradiation was performed using the Varian 2300C/D medical linear accelerator (Varian Companies, USA) with a coverage field of 20 cm × 20 cm. The culture dish was placed in the radiation field above 1.5 cm of organic glass. Cells with irradiated with 6 MV X-ray irradiation at a dosage rate of 300 cGy/min, a rack angle of 180°, and source-to-surface distance (SSD) of 100 cm.
Clonogenic assayLogarithmic-growth phase cells were inoculated in a 60-mm culture dish. After adherence, the cells in the drug and combined irradiation groups were cultured in the presence of 10 or 20 μg/mL elemene and seeded in culture plates at 100 cells/well for 24 h. The cells were exposed to 0, 2, 4, 6, 8, and 10 Gy of irradiation and cultured for another 14 days. The number of cell clones viewed under a low magnification microscope was 50. The plating efficiency (PE) was calculated relative to the control group (0 Gy), and the survival fraction (SF) of each group was calculated as follows: SER=control group (D0, Dq)/experimental group (D0, Dq).
Morphological assessment of apoptosisThe cells were treated as follows: the control group received RPMI 1640 medium; the radiation group received a radiation dose of 4 Gy; the drug group was treated with 10 or 20 µg/mL elemene; and the drug plus radiation group was treated with 10 or 20 µg/mL elemene followed by a radiation dose of 4 Gy. Following incubation with elemene, the exponentially growing cells were irradiated as described above. Samples of 3 × 105 cells were then collected from each group, treated with pancreatic enzyme digesting cells, rinsed twice with PBS, and centrifuged at 1,000 rpm for 5 min. The SF was determined using the following equation: SF=colony number/(plating cell number × PE).
The dose survival curve was fitted using the linear-quadratic (LQ) function model S=e-(αd+βd2) 17 for calculating radiobiological parameters, including the sensitivity enhancement ratio (SER), SER of the mean lethal dose (D0) SERDq, and SER of the quasi-threshold dose (Dq) SERD0. Nuclear morphology was examined using fluorescence microscopy following Hoechst 33342 staining (final concentration 8 mg/mL) for 15 min at 37°C. Imaging was performed using an Olympus BX-51 fluorescent microscope with appropriate filter cubes. The excitation and emission wavelengths were 350 nm 460 nm, respectively.
Apoptosis standardNormal cells demonstrated uniform dispersion of low-density fluorescence, while apoptotic cells showed high-density fluorescence, characterized by a bright blue hue.
Assessment of apoptosisThe cells used were grouped and treated as specified above. Then, samples of 3 × 105 cells were collected from each group, treated with pancreatic enzyme digesting cells, rinsed twice with PBS, and centrifuged at 1,000 rpm for 5 min. The cells were treated with 100 μL 2% Triton X-100 for 20 min, rinsed twice with PBS, and centrifuged at 1,000 rpm for 5 min. Next, 200 μL of DNA-Prep LPR reagent (Beckman-Coulter Ltd) was added for 20 min, and the cells were rinsed twice with PBS, followed by centrifugation at 1,000 rpm for 5 min. The cells were resuspended in PBS and 50 µg/mL propidium iodide (PI) reagent containing 480 μL of PBS, 5 μL of PI (5 mg /mL), and 5 μL of RNase (10 mg /mL), and 10 μL of Triton X-100 (10%) was added 30 s later. Single-cell suspensions were analyzed by flow cytometry to determine the cellular apoptosis rate.
Neutral comet assayA549 cells were irradiated in the absence or presence of elemene (10 or 20 µg/mL) and assayed immediately after radiation or returned to the incubator for 24 h to permit repair. The comet assays were performed immediately after incubation with elemene, irradiation or combination treatment. Tumor cells were grown on glass microscope slides using a standard protocol 18. The slides were submerged in lysing solution containing 30 mM ethylenediaminetetraacetic acid (EDTA) and 0.5% sodium dodecyl sulfate (SDS, pH 8.3) for 1.5 h at 37°C. Following lysis, the slides were rinsed three times in Tris-borate-EDTA (TBE) buffer consisting of 90 mM Tris, 90 mM boric acid, and 2 mM EDTA, pH 8.5, and stored overnight in TBE buffer at 4°C. Slides were transferred to an electrophoresis unit with TBE buffer and electrophoresed at 1 V/cm for 20 min. Following electrophoresis, the slides were neutralized with 0.4 M Tris buffer (pH 7.5) and stained with ethidium bromide (20 µg/mL). Finally, the slides were viewed using an Olympus BX‐51 fluorescent microscope (excitation filter 549 nm, barrier filter 590 nm). Images of 50 randomly selected cells from each slide were analyzed with Comet Assay Software Project casp‐1.2.2 (University of Wroclaw, Poland). The tail moment was used as a parameter to assess DNA damage. The assay was completed three separate times, and 50 cells were evaluated per experiment.
Western blot assayWestern blotting was performed to detect the expression levels of DNA-PKcs, p53, and Bcl-2. The cells were grouped and treated as specified above. After 24 h, western blot analysis was performed using cytosolic fractions as previously described 19. Equal amounts of cytosolic protein were separated on 8–12% SDS polyacrylamide denaturing gels and transferred to nitrocellulose membranes. The membranes were blocked in TBS-Tween (TBS-T, 10 mM Tris-HCl, pH 7.4; 150 mM NaCl; and 0.1% Tween-20) with 5% non-fat milk for 2 h and incubated with specific primary antibodies overnight at 4°C. Finally, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies at 37°C for 2 h and assayed using an enhanced chemiluminescence plus detection system.
Statistical analysisData were analyzed using the statistical package for the social sciences (SPSS) v13.0 software. Data are expressed as the mean ± standard deviation (SD). The statistical significance of differences between groups was determined by one-way analysis of variance (ANOVA), followed by post hoc analysis using the least significant difference (LSD) for multiple comparisons. The Spearman test was used for the correlation analysis of the relationships between the expression levels of genes. The level of significance was set at p<0.05 and p<0.01 for all statistical analysis.
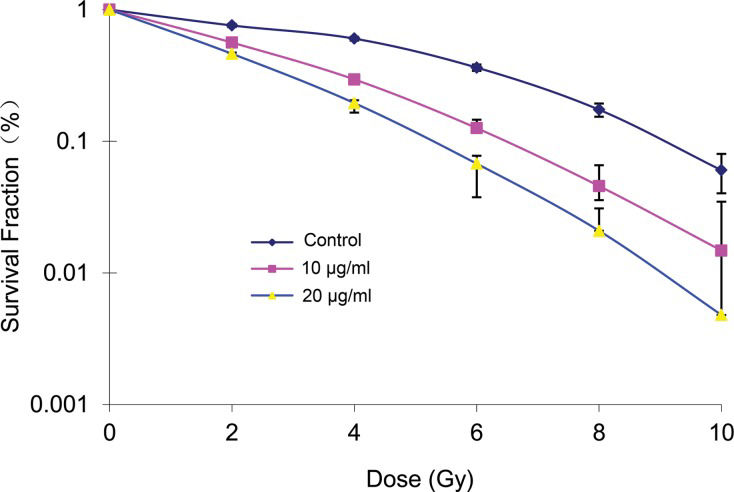
RESULTSEffects of elemene on cell radiosensitivityThe survival fraction of A549 cells decreased following treatment with different doses of radiation and the same concentration of elemene. Conversely, the A549 cell survival fraction decreased in the groups treated with the same dose of radiation in combination with increasing concentrations of elemene (Table 1). Following treatment with 10 or 20 μg/mL elemene, the A549 cell survival curve shifted to the left, the shoulder area was diminished, and the steepness of the curve increased (Figure 1). Based on the cell survival curve, the radiobiological parameters and radiosensitization ratio were obtained and are listed in Table 1. These data show that, compared with the control group, the SERD0 and SERDq values for the 10 and 20 μg/mL elemene treatment groups were greater than 1. Furthermore, the ratio gradually increased with an increasing drug concentration (Table 2).
Survival fraction (%).
| Group | 0 Gy | 2 Gy | 4 Gy | 6 Gy | 8 Gy | 10 Gy |
|---|---|---|---|---|---|---|
| Control | 100 | 84.1±13.2 | 70.2±10.5 | 66.3±1.9 | 47.3±2.5 | 26.0±1.3 |
| 10 μg/ml | 97.7±20.2 | 76.2±10.4 | 49.5±6.4 | 22.5±2.3 | 7.5±1.8 | 3.4±0.2 |
| 20 μg/ml | 95.4±18.8 | 63.6±7.5 | 30.4±3.0 | 5.7±1.1 | 2.7±0.6 | 0.7±0.1 |
Cells were incubated with 10 or 20 μg/mL concentrations of elemene for 24 h and were then irradiated with 0, 2, 4, 6, 8, and 10 Gy and cultured for another 14 days. The number of cells forming more than 50 clones was counted under the inverted microscope to calculate the cloning efficiency (CE): CE (%)=(Clone formation average of treatment group/inoculated cell number) × 100%. Survival fraction (SF)=(irritated group CE/non-irradiated group PE) × 100%. The experiment was repeated 3 times to calculate the average.
Radiation parameters for a single-hit multi-target model.
| Group | D0 (Gy) | Dq (Gy) | SF2 (%) | SERD0 | SERDq |
|---|---|---|---|---|---|
| Control | 2.54±0.24 | 2.68±0.25 | 84.6±20.9 | - | - |
| 10 μg/mL | 1.64±0.15 | 1.87±0.22 | 56.3±14.9 | 1.54±0.20 | 1.43±0.15 |
| 20 μg/mL | 1.55±0.13 | 1.53±0.11 | 43.2±10.7 | 1.63±0.32 | 1.75±0.19 |
SPSS17.0 was used to calculate survival parameters including SF2 (surviving fraction of 2 Gy), D0 (mean lethal dose or final slope), and Dq (quasi-thres-hold dose). SER (sensitization enhancement ratio). SER D0 (Dq)=control group D0 (Dq)/drug group D0 (Dq). Three parallel samples were set at each radiation dosage.
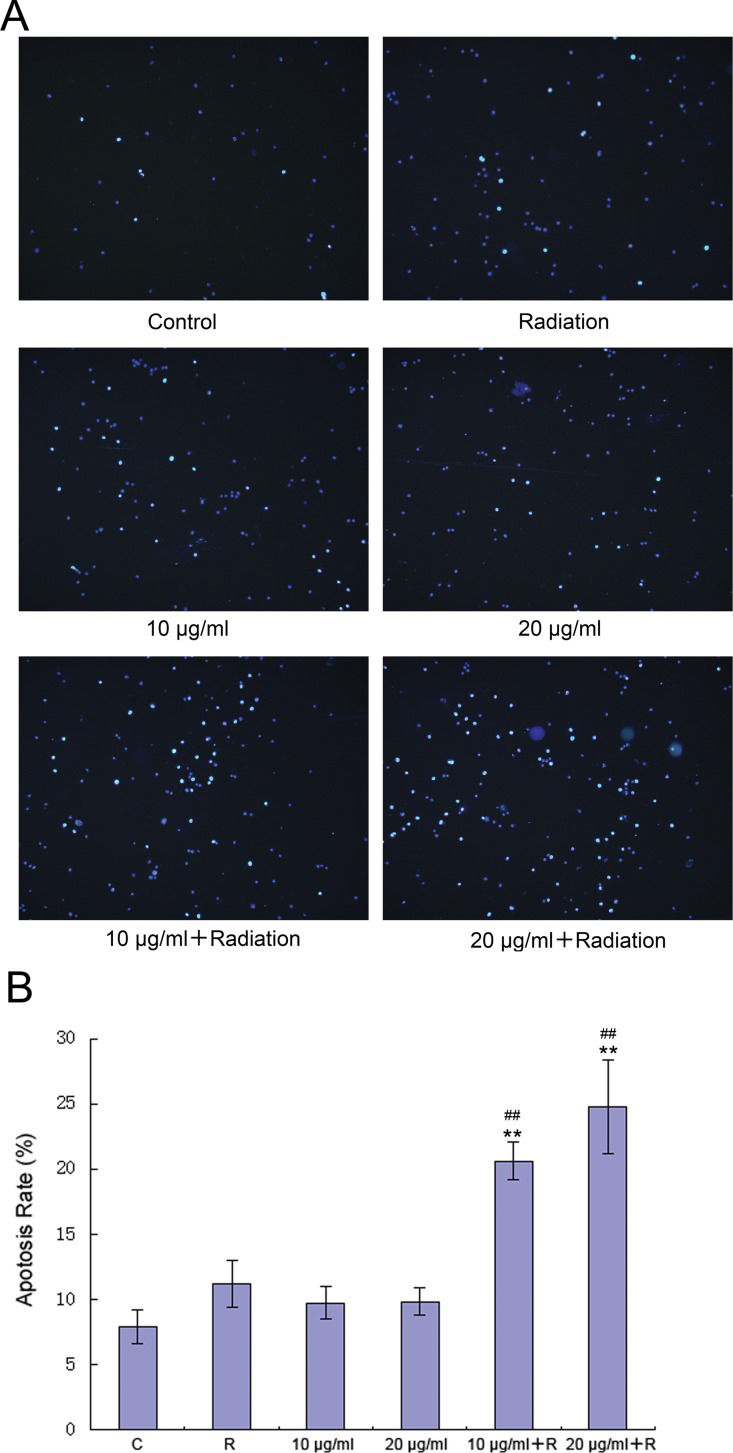
Fluorescence microscopy showed that compared with the control group, the groups treated with radiation alone and elemene alone contained more apoptotic cells (Figure 2). Furthermore, significantly higher apoptotic levels were observed in the groups treated with radiation and elemene at 10 or 20 μg/mL (Figure 2A). Flow cytometry revealed that compared with the control group, the apoptosis rate of the group treated with radiation alone appeared to increase, but this was not found to be statistically significant (p>0.05). The apoptosis rate of the group treated with elemene alone did not change (p>0.05), while the apoptosis rate of the elemene plus radiation group increased significantly (p<0.01). The rate of apoptosis increased with increasing concentrations of elemene (Figure 2B).
Elemene induces apoptosis in A549 cells. Cells were incubated with 10 or 20 μg/mL elemene for 24 h, followed by irradiation with 4 Gy X-rays and washing with PBS. (A) Cells were imaged with an Olympus BX-51 fluorescent microscope using appropriate filter cubes. (B) Chromatin condensation was analyzed by fluorescence microscopy after DNA staining with Hoechst 3334. The results are expressed as the mean ± SD of three independent experiments, n=3, **p<0.01 compared to control, ##p<0.01 compared to radiation alone.
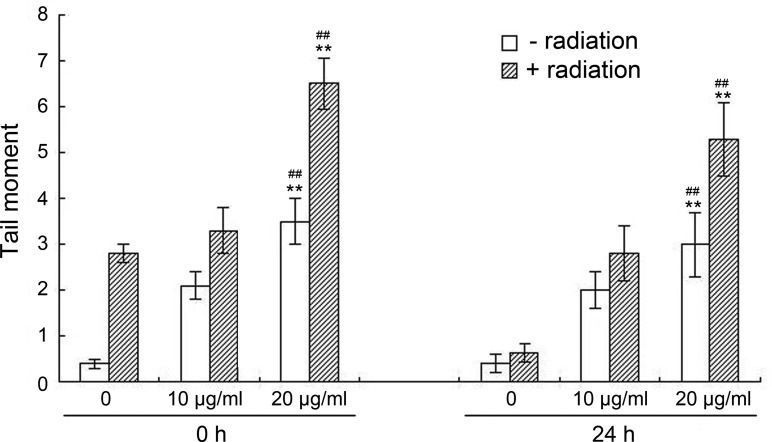
Figure 3 (0 h) shows that both elemene and irradiation alone increased the tail intensity in A549 cells, and the degree of DSB was augmented as the elemene concentration increased. In particular, the results showed a higher number of DSB in the combination group than the irradiation alone and elemene alone groups (p<0.01). As shown in Figure 3 (24 h), following incubation for 24 h, the tail intensity in the irradiation alone group returned to background levels, while there was a significant increase in the amount of remaining tail intensity in the combination group compared with the irradiation alone and elemene alone groups (p<0.01).
Influence of elemene on radiation-induced DSB. A549 cells were irradiated with 4 Gy X-rays in the absence or presence of elemene (10 or 20 μg/mL) and assayed immediately after radiation or returned to the incubator for 24 h to permit repair. Comet assays were performed immediately after incubation with elemene treatment, irradiation, or combination treatment. Columns represent the means of the tail moments from three independent experiments, and the bars represent the SD, **p<0.01 vs. control, ##p<0.01 compared to radiation alone.
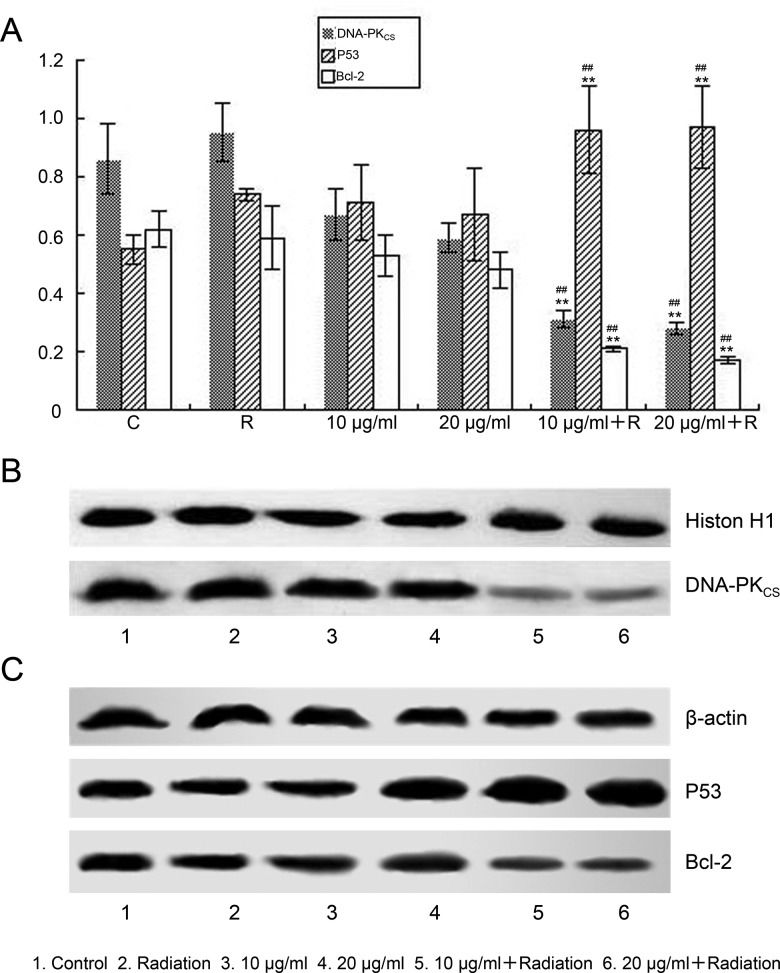
The results shown in Figure 4A revealed that in the 10 and 20 μg/mL combined treatment groups, a significant decrease in the protein expression of DNA-PKcs (p<0.01, Figure 4B) and Bcl-2 (p<0.01, Figure 4C) was observed, while the p53 protein expression level was significantly increased (p<0.01, Figure 4C).
Western blot analysis of the protein levels of Bcl-2, p53 and DNA-PKcs. A549 cells were irradiated with 4 Gy X-rays following treatment with 10 or 20 μg/mL elemene for 24 h. Proteins were extracted and separated by SDS-PAGE. (A) Levels of Bcl-2, p53 and DNA-PKcs were quantitated by densitometry, and the ratios of the three proteins are displayed. Values represent the mean ± SD, n=3, **p<0.01 compared to the control, ##p<0.01 compared to radiation alone. Elemene inhibited the protein expression of (B) DNA-PKcs and (C) Bcl-2 and promoted the protein expression of p53. Key: 1, Control; 2, irradiation; 3, 10 μg/mL; 4, 20 μg/mL; 5, 10 μg/mL + irradiation; 6, 20 μg/mL + irradiation.
Spearman correlation analysis showed that DNA-PKcs and p53 protein expression was negatively correlated (r=-0.569, p<0.05), while DNA-PKcs expression was positively correlated with Bcl-2 protein expression (r=0.755, p<0.05).
DISCUSSIONBasic research in radiation biology has shown that radiation therapy works mainly by damaging tumor cell DNA and altering the expression of apoptosis-related genes and proteins. The radiosensitivity of tumor cells relates to their capacity to repair DSB via the related genes DNA-PKcs, Ku70/80, and ataxia telangiectasia mutated (ATM). Other genes known to be involved in radiosensitivity and responsible for apoptosis regulation include p53, Bcl-2, c-myc proto-oncogene (c-myc), and survivin 9. Beta elemene, which is the active component of elemene, has recently been demonstrated to enhance the radiosensitivity of human cancer cell lines in vitro and in an animal tumor model in vivo16,20. In particular, beta elemene was found to enhance radiosensitivity by influencing the cell cycle distribution of gastric cancer MKN28 cells, and the mechanisms responsible for this effect include the induction of G2/M phase arrest, inhibition of sublethal damage repair, and induction of cell apoptosis, which enhances the killing effects of radioactive rays 21. The results of the current study show that the SERD0 and SERDq values of A549 cells exposed to a low concentration of cytotoxic elemene were greater than 1. In addition, elemene enhanced the sensitivity of A549 cells to radiotherapy. Cellular apoptosis is fundamental to radiotherapy, and its regulatory mechanism plays an important role in cellular radiosensitivity. Apoptosis-related genes such as p53 and Bcl-2 have important regulatory functions in the progression of rapid apoptosis induced by radiation therapy. For instance, a previous study showed that the levels of the antiapoptotic genes Bcl-2 and Bcl-xl in A549 cells decreased, while p53 expression and the production of exosomes increased, following elemene treatment 22. This result demonstrates that both p53 and Bcl-2 have important regulatory actions in cervical cancer cell apoptosis induced by radiation. A number of experimental studies have further shown that elemene is involved in regulating the expression of Bax, c-myc, p53, poly (ADP-ribose) polymerase (PARP), survivin, and livin as well as inducing tumor cell apoptosis 23–26. Our results showed that, compared with the exposure alone group, the group that received elemene combined with irradiation exhibited increased p53 gene expression and significantly decreased Bcl-2 gene expression, and the expression of both genes was significantly correlated. Furthermore, elemene was shown to regulate expression of the apoptosis-related genes Bcl-2 and p53 and induce A549 cell apoptosis, thereby increasing cell radiosensitivity.
Interestingly, when Bcl-2 and p53 gene expression was significantly altered, DNA-PKcs protein expression was significantly decreased in the combined treatment group. This result indicates that elemene is also involved in regulating DNA damage repair pathways. Protein kinase activation leads to the phosphorylation of downstream DNA repair proteins, which initiate DNA chain fracture repair 27, and the relationship between DNA-PKcs and radiotherapy sensitivity has been a topic of significant research in recent years. It is well established that inhibiting tumor cell expression of DNA-PKcs increases radiation sensitivity. Panet al. 28 studied the relationship between DNA-PKcs expression and radiation sensitivity in non-small cell lung cancer cell lines, and in adenocarcinomas and large cell carcinomas, DNA-PKcs was shown to be an important component regulating cellular radiosensitivity. This result indicates that DNA-PKcs may be predictive of non-small cell lung cancer cell radiosensitivity. Zou et al. 29 silenced the DNA-PKcs gene of human mammary epithelial cells (MCF10F) using small interfering RNA (siRNA) technology. Simultaneously, the expression of DNA repair-related proteins, such as DNA-PKcs, Ku80, ATM, and p53, was decreased in these cells, while their sensitivity increased with low doses of radiation. Small molecule inhibitors of DNA-PKcs were also shown to enhance radiation sensitivity of cervical cancer cells 30. Our experimental results showed that elemene inhibited DNA-PKcs expression in A549 cells, reduced DNA damage repair, and increased cellular radiosensitivity.
DNA-PKcs is a protein with a wide range of functions and is involved in DNA damage repair, apoptosis, and V(D)J recombination 31. Yu et al. 32 found that in non-small cell lung cancer, high expression of DNA-PKcs increased the activity of the DNA damage repair system. In addition, apoptosis inhibition caused by mutant p53 and Bcl-2 expression exhibited a combined effect, which may explain the development of resistance to radiotherapy in small-cell lung cancer. Daido et al. 33 indicated that following exposure to low doses of radiation, human malignant glioma M059J cells that lack DNA-PKcs underwent massive autophagic cell death that was significantly increased after exposure to DNA-PKcs inhibitors. Furthermore, DNA-PKcs inhibitors exert radiotherapy-sensitizing effects on glioma cells by enhancing type II programmed cell death. Lee et al. 34 found that p53-inducible gene 3 (PIG3) is involved in apoptosis caused by p53 activation and that this molecule can regulate DNA-PKcs expression. Moreover, knockdown of PIG3 was shown to decrease the level of DNA-PKcs in cells. Our study further addressed the correlation between DNA-PKcs, Bcl-2, and p53 expression, and the results showed that DNA-PKcs expression was significantly positively correlated with that of Bcl-2 (r=0.755, p<0.05) and significantly negatively correlated with p53 (r=0.569, p<0.05). We further showed that DNA-PKcs was closely related to apoptosis and that elemene increased apoptosis of A549 cells and strengthened cellular radiosensitivity by inhibiting DNA-PKcs expression.
In summary, elemene exhibits radiotherapy-sensitizing effects on lung adenocarcinoma A549 cells, and its mechanism of action involves the upregulation of p53 and downregulation of Bcl-2 to promote cell apoptosis, as well as the downregulation of DNA-PKcs to inhibit DSB repair. However, the specific mechanism of action of elemene requires further elucidation.
AUTHOR CONTRIBUTIONSZou K, Zhang Z and Zou L designed the research study and wrote the paper. Zou K and Liu C performed the research. Zou K and Zhang Z analyzed the data.
This study was supported by grants from the National Natural Science Foundation of China (No. 81473452).
No potential conflict of interest was reported.