The effects of sevoflurane general anesthesia and bupivacaine selective spinal anesthesia on QT dispersion (QTd) and corrected QT (QTc) interval were investigated.
METHODS AND MATERIALS:This prospective, randomized, double-blind study was conducted between July and September 2009 in the Urology and General Surgery operating rooms. Forty ASA I–II patients undergoing noncardiac surgery were randomized into two groups: Group R (n = 20) and Group V (n = 20). In Group R, 5 mg bupivacaine was administered into the spinal space. Anesthesia induction in Group V was established with sevoflurane + 0.1 mg/kg vecuronium using the maximum vital capacity technique. Anesthesia was maintained with 2–3% sevoflurane + 50% N2O/O2 inhalation. All patients were tested with a 24-hour Holter ECG device. QT, QTc, and QTd intervals were measured using 12-lead ECG records at 1 and 3 minutes during preinduction, postinduction, postincision and postextubation periods. Mean arterial pressure (MAP), heart rate and ECG records were measured simultaneously.
RESULTS:None of the patients displayed arrhythmia. There was no significant difference between the groups with regard to QTd values (p>0.05). However, QTc was longer in Group V than in Group R after the induction of anesthesia at 3 minutes, after the intubation at 1 and 3 minutes, and after the incision at 1 and 3 minutes. MAP and heart rate were generally higher in Group V (p<0.05).
CONCLUSION:Although Volatile Induction and Maintenance of Anesthesia (VIMA) with sevoflurane might prolong the QTc interval and did not result in arrhythmia, selective spinal anesthesia with bupivacaine was not associated with alterations in the QT interval or arrhythmia.
The effects of anesthetics on the cardiovascular system have a complicated character, and almost all of the effects lead to dose-related myocardial depression and decreases in heart rate and arterial pressure. In anesthesia practice, individual responses of patients against procedures such as induction, intubation, and surgical stimulation are influenced by many factors, including preoperatively used drugs, anesthesia type, preferred anesthetic agents, and the autonomic nervous system.1,2
Many of the anesthetics used in anesthesia practice interact with the QT interval. Depolarization and repolarization of the myocardium take place within the QT interval. Varying QT intervals have been associated with heterogeneous repolarization and ventricular arrhythmias.3,4 Therefore, prolonged QT intervals may be harmful, at least in cases with myocardial pathologies, necessitating anesthesia methods that do not influence the QT interval.5
QT dispersion (QTd) is defined as the difference between the longest and shortest QT intervals in 12-lead ECG. QTd is particularly recognized as the indirect measurement of the repolarization and has been associated with complex ventricular arrhythmias.3
Several studies investigating the effects of sevoflurane on QT, QTd, and QTc have revealed differing results.6,7 We found no study investigating the effects of selective spinal anesthesia on QT interval and arrhythmia. In the present study, we aimed to investigate the effects of selective spinal anesthesia with bupivacaine and sevoflurane inhalation anesthesia (VIMA) on QT interval, QTd, QTc, QTcd, and arrhythmia.
METHODS AND MATERIALSOur prospective, randomized, double-blind study was conducted between July and September 2009 in the Urology and General Surgery clinics. Following the approval of the ethics committee and collection of informed consent, 40 ASA I–II patients 18–65 years of age who were scheduled to undergo urologic intervention or inguinal hernia surgery were included in the study. Patients with a history of a chronic obstructive lung disease, cardiovascular disease, electrolyte imbalance, diabetes mellitus, chronic alcoholism, idiopathic, congenital, or acquired prolonged QT syndrome, chronic drug use, or a Mallampati score ≥2 were excluded from the study.
Hemodynamic monitoring included mean arterial pressure (MAP), heart rate (HR), and peripheral oxygen saturation (SpO2). In addition, 24-hour Holter ECG was performed on all patients (Delmar Impresario, USA) to maintain a continuous ECG record.
The cases were randomly split into two groups: Group V (n = 20) and Group R (n = 20). The allocation sequence was generated by a table of random numbers. None of the cases received premedication. Anesthesia induction in Group V was established using the maximum vital capacity technique with 8% sevoflurane + 50% N2O/O2. After the achievement of neuromuscular block with 0.1 mg kg−1 vecuronium, endotracheal intubation was performed. Anesthesia was maintained with 2–3% sevoflurane + 50% N2O/O2 inhalation.
In Group R, selective spinal anesthesia was performed by delivering 5 mg 0.5% hyperbaric bupivacaine to the L4–L5 space via the midline approach to the patients in the sitting position. A local anesthetic agent was administered within 20 seconds without aspiration of the CSF, and the patients were kept sitting at a 45° angle until the achievement of T10 anesthesia (maximum 10 minutes). Sensory block was evaluated with the pinprick test, while motor block was assessed with the Bromage scale. Surgery was allowed when the sensory block reached the T10 level.
The study was designed to examine the following: to elevate the crystalloid infusion speed to 15 mg/kg/h when MAP dropped below 30% and persisted at that level for more than 30 seconds; to carry out 5 mg/kg/h colloid infusion upon persistence of the hypotension for 15 minutes; to apply 5 mg ephedrine when the cases failed to normalize after another 15 minutes; to increase the anesthesia depth when MAP increased above 30%; and to administer 0.1 mg nitroglycerine relative to the MAP. We also planned to deliver 0.5 mg atropine when the HR dropped below 50.
We did not use anticholinesterase or opioid therapy in case they would influence the QT and QTc intervals.
Data analyses of both of the groups were performed at the times noted in Table I. MAP, HR, and SpO2 values were recorded. ECG analyses were carried out.
Measurement phases in VIMA and spinal anesthesia.
| Phases | Spinal | VIMA |
|---|---|---|
| Phase 1 (preinduction) | At 0 minutes | At 0 minutes |
| Phase 2 | At 1 minute | Sevoflurane at 1 minute |
| Phase 3 | At 3 minutes | Sevoflurane at 3 minutes |
| Phase 4 | At 4 minutes | Vecuronium at 1 minute |
| Phase 5 | At 6 minutes | Vecuronium at 3 minutes |
| Phase 6 | At 7 minutes | First minute of intubation |
| Phase 7 | At 9 minutes | Third minute of intubation |
| Phase 8 | First minute of incision | First minute of incision |
| Phase 9 | Third minute of incision | Third minute of incision |
| Phase 10 | First minute of surgical end | First minute of extubation |
| Phase 11 | Third minute of surgical end | Third minute of extubation |
ECG records were examined with a Holter monitor by the same cardiologist. The QT interval was analyzed throughout the whole operation. The QT interval was recognized as the duration between the beginning of the QRS complex and the end of the T wave in the ECG. The mean QT interval was calculated manually, and the QT interval measurement was corrected for heart rate using the Bazett formula (12).
QTc(sn): corrected QT interval, QTd(sn): QT dispersion time, QTcd(sn): corrected QT dispersion time, QT max: maximum QT interval, QT min: minimum QT interval.
The study was conducted with the significance level set at alpha = 0.05 and beta = 0.8 power. The study included 40 subjects (n1 = n2 = 20). No patient was excluded from the study. The power, based on the QTc values of the groups, was found to be 0.83.
The data obtained in the present study were evaluated with SPSS version 12 (SPSS, Chicago, IL). We used the chi-square test for dependency between the variables, the Mann-Whitney U test for comparisons between groups, and the Wilcoxon signed-rank test for comparisons within the groups. The Mann-Whitney U test was used for all comparisons of the demographic characteristics with the exception of gender. Gender was compared using the chi-square test. A p value <0.05 was considered significantly significant.
RESULTSNo significant differences in demographic characteristics were found between the groups (Table 2).
Demographic data (mean±SD).
| Group R (n = 20) | Group V (n = 20) | p | |
|---|---|---|---|
| Age (years) | 42.9±11.88 (18–65) | 44.5±10.47 (26–64) | 0.567 |
| Weight (kg) | 80.36±10.35 | 78.40±9.93 | 0.456 |
| Height (cm) | 168.46±8.42 | 167.36±9.23 | 0.632 |
| Gender (F/M) | 12/18 | 14/16 | 0.602 |
| ASA (I/II) | 12/18 | 13/17 | 0.793 |
| Operation length (min.) | 33 (17–64) | 40 (20–76) | 0.088 |
The HR values did not significantly differ within either group. However, the Group V HR values were high after the induction (p: 0.02) (Figure 1).
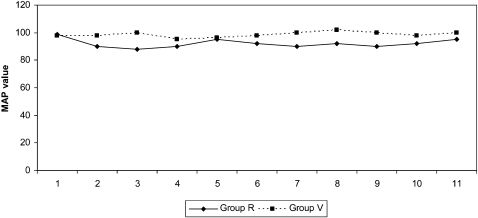
The MAP values were not significantly different within groups, but the Group R MAP values were lower than the Group V values at all measurement time points. However, none of the cases demonstrated a need for therapeutic intervention for blood pressure changes (Figure 2).
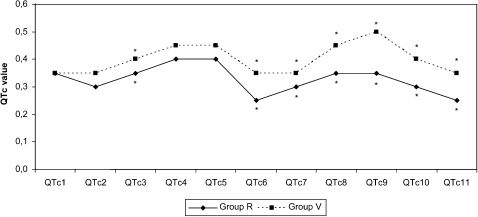
When compared within groups, the QTd, QTc, and QTcd values displayed no significant differences at any of the measured time points. Intergroup comparisons exhibited a high QTd at 3 minutes after extubation in Group V. QTc was significantly higher in Group V than in Group R at 3 minutes after the induction, 1 and 3 minutes after the intubation, and 1 and 3 minutes after the incision (Figures 3 and 4) (p<0.05).
QTcd values were significantly higher in Group V at 1 and 3 minutes after the induction, 1 and 3 minutes after the extubation, and 1 and 3 minutes after the incision (Figure 5).
DISCUSSIONSeveral conditions, such as diabetes mellitus, arrhythmia, ischemic cardiac diseases, pulmonary disease, uremia, electrolyte and acid/base disorders, and prolonged QT syndrome, and the drugs used in their treatment, such as antihypertensives, beta-blockers, antidiabetics, and opioids, are known to influence the QT interval.8
A prolonged QT interval should be considered an important symptom because it can lead to serious ventricular arrhythmias.8 It is not easy to differentiate the underlying cause of changes in QT interval among factors such as delivered pharmaceutical agents, drug interactions, and sympathetic activation occurring upon laryngoscopy and intubation.1
Michaloudis et al. applied VIMA with sevoflurane and isoflurane and found that isoflurane prolonged the QTc interval but did not change QTd or QTcd, whereas sevoflurane did not affect those three parameters; overall, they determined that sevoflurane was a good agent.9 Guler et al. showed that sevoflurane, isoflurane, and halothane prolonged QTd and QTcd in patients who had no congenital heart disease. However, they found no statistically significant difference between the agents and concluded that those anesthetic agents caused myocardial repolarization abnormalities by increasing QTcd. In the same study, prolonged QTcd was found to have the potential to play a role in arrhythmias observed among people receiving anesthesia who had no cardiovascular disease. In light of the fact that those agents can prolong QTcd further, Guler et al. noted that this parameter could be used during the development of new agents.10
Saarnivaara et al.5 reported that changes in ventricular repolarization might cause a prolonged QTc interval in cases where anesthetic agents have induced sympathoadrenal hyperactivity. Prolonged QTc interval has been reported to show autonomic nervous system imbalance in the heart, thereby lowering the ventricular fibrillation and leading to ventricular arrhythmia.11 Abe et al.6 reported ventricular tachycardia development under sevoflurane and N2O anesthesia. A study using the single-breath induction technique with sevoflurane found marked prolongation of and increased development of arrhythmia in the QT interval.12 A study comparing 1 MAC sevoflurane and desflurane reported a prolonged QTc interval and increased QTd upon delivery of each of those two agents.13 Karagoz et al.14 conducted a study to analyze the effects of halothane, isoflurane, and sevoflurane, in which they reported no changes in the QTc interval as a result of 2% sevoflurane and 50% O2 + 50% N2O. In the current study, QTc was higher in Group V than in Group R at every measured time point, and significant differences were identified during times of intense perioperative sympathetic activation, particularly at 3 minutes after the induction and 1 and 3 minutes after the incision (p<0.05). Given the previous finding claiming the presence of a direct relationship between plasma catecholamine levels and QT interval, it is probably no coincidence that QTc was markedly prolonged during times of intense sympathetic activation.15
In the present study, a QTc of 506 ms, recognized as the upper limit of the normal QTc interval, was not reached in any of the cases. Again, no prolonged QTc exceeded the basal value more than 75 ms.
Although some studies have noted that prolonged QTc contributes to the development of cardiac arrhythmias, 5,20 similar to the results of the study of Saarnivaara et al.,5 we did not identify a relationship between the prolonged QTc duration and the development of arrhythmia in the present study. As in our study, this study found that heart rate was generally high during the periods of prolonged QTc, and the authors attributed this to perioperative anxiety and elevated sympathetic activity. In view of the fact that amiodarone, a type III antiarrhythmic agent, prolongs the QT interval while having no influence over QTd or ventricular extrasystole formation, we believe that the electrophysiologic mechanism playing a part in arrhythmia development should have been clearly identified.17
The normal range of QTd is 300–600 ms.18 QTd demonstrates the regional homogeneity of ventricular myocardial cells during repolarization and reflects the physiologic desynchronization of myocardial excitability. A prolonged QTd interval is an important determinant of ventricular arrhythmias and sudden cardiac deaths. Moreover, while some studies note that QTd values longer than 65 ms are important in ventricular tachycardia etiology, there are also studies that describe 70 ms as a normal value. In a detailed study on this subject, QTd was found to provide reliable data on prognosis estimation and foreknowledge of cardiac problems stemming from myocardial repolarization abnormalities. However, the same study recommended that a marked repolarization abnormality be considered in cases with a QTd value above 100 ms.8 In the current study, although QTd was longer in Group V at 3 minutes after the extubation (phase 10), it was not higher than 57 ms in any of the cases. In view of the variation from the initial phases in the VIMA and spinal anesthesia groups, there was no statistically significant difference with regard to the QTc, QTd, and QTcd values.
A multicenter study conducted on 17,000 patients in 2000 investigated the effects of spinal anesthesia on arrhythmia and found that 70.2% of the cases displayed tachycardia, bradycardia or arrhythmia. Most of those arrhythmias were of spontaneous and remitting minor types. Additionally, 30.3% showed sinus arrhythmia, whereas 27.2% and 13.8% exhibited premature beat and bradycardia, respectively.19 Moreover, another prospective spinal anesthesia study on 40,460 patients reported the rate of bradycardia incidence before cardiac arrest as 6.4/10.000. All of the cases that experienced arrest responded to resuscitation. In that study, the formation of varying degrees of cardiac blocks were noted.20 In contrast to that study, our study preferred selective spinal anesthesia, and its influence over arrhythmia was not determined. We believe that the difference between the studies may be due to the different methods applied.
Bupivacaine is an anesthetic agent that is frequently used in current anesthesia practice. Although most of the toxic reactions are associated with high plasma concentrations of bupivacaine, cases of mortality and morbidity have been reported, even at low doses. Systemic complications exhibiting reduced cardiac output and changes in the central nervous system have been observed, even at a 1 µg/ml plasma concentration.21 Hotverd et al. conducted a study showing that bupivacaine at a 2 µg/ml plasma concentration had a negative inotropic effect and prolonged cardiac conduction. That study also implicated bupivacaine in re-entry arrhythmias. Bupivacaine is 4 times more effective than lidocaine and causes cardiovascular and other systemic toxicities 4–17-times more frequently than lidocaine.22 Studies with bupivacaine have reported supraventricular tachycardia, atrioventricular block, early ventricular beats in various forms, QRS changes, and fatal ventricular fibrillation. Moreover, independent of dose, the most common rhythm abnormality in hypoxia and acidosis is bradycardia with a wide QRS complex.
In animal studies, bupivacaine, etidocaine, mepivacaine, and lidocaine delivered as an i.v. bolus and infusion have been reported to cause an increase in the area below the T wave, prolong the QT interval, and induce the formation of a U wave following the T wave; these effects were more significant in the etidocaine and bupivacaine groups. The effects of those agents over the temporal dispersion of the effective refractory period were reported as 37.4 ms in lidocaine, 48.3 ms in mepivacaine, 92.5 ms in etidocaine, and 98.1 ms in bupivacaine. Moreover, a relationship between prolongation of the effective refractory period and ventricular arrhythmia was also noted.22
Decreases in systemic blood pressure can lead to changes in heart rate and cardiac rhythm alterations.2 However, hypotension during anesthesia is described as a reduction of the basal value of arterial pressure below 20%. In the current study, while none of the groups demonstrated changes exceeding that limit in blood pressure or heart rate values, MAP and HR were significantly higher in Group V than in Group R in measurements performed after intubation, incision, and extubation. Lowrie et al. found a considerable increase in HR, SAP, and DAP parallel to the elevations in plasma norepinephrine and epinephrine concentrations. Moreover, they reported a significant rise in epinephrine level and heart rate within the first five minutes of extubation. In the present study, elevation in the heart rate during intubation and extubation in the VIMA group was consistent with the results of the study of Lowrie et al.15
In our study, MAP values showed a significant increase in the VIMA group compared with the spinal group. This result is consistent with the sympathetic discharge results obtained during the intubation and extubation phases in the studies of Tanaka et al. and Lowrie et al.15,24
Selective spinal anesthesia had a greater influence over QT and hemodynamic parameters when compared with the VIMA with sevoflurane alone. If a medication is delivered to block the adrenergic discharge, the cardiac effects of VIMA with sevoflurane can be prevented. Moreover, considering that our study was performed only on ASA I–II patients, we believe that further studies of arrhythmia should be conducted on patients with higher risk and that proper anesthesia protocols should be established. Nevertheless, selective spinal anesthesia has not been compared with VIMA with regard to QTd, QTc, QTcd, and arrhythmia incidence.
In conclusion, although VIMA with sevoflurane might prolong the QTc interval and did not result in arrhythmia, selective spinal anesthesia with bupivacaine was not associated with alterations in the QT interval or arrhythmia.