Cerebral sparganosis is a rare parasitic disease of the central nervous system that is caused by sparganum. Cerebral sparganosis occurs worldwide, but it is found more frequently in China, Japan, and Southeastern Asia (1–2). Sparganum, the second-stage larva of Spirometra mansoni, can produce chronic active inflammation through slow migration in the brain (1). They can also parasitize other sites of the human body, such as the subcutaneous tissue, body cavities and eyes, and survive for 5-20 years (3).
In this paper, we report a case of cerebral sparganosis, including the magnetic resonance imaging (MRI) characteristics of local migration and the surgical finding of a live worm. A new pathological finding, a tunnel-like structure, was observed and most likely represented a sparganum migration path.
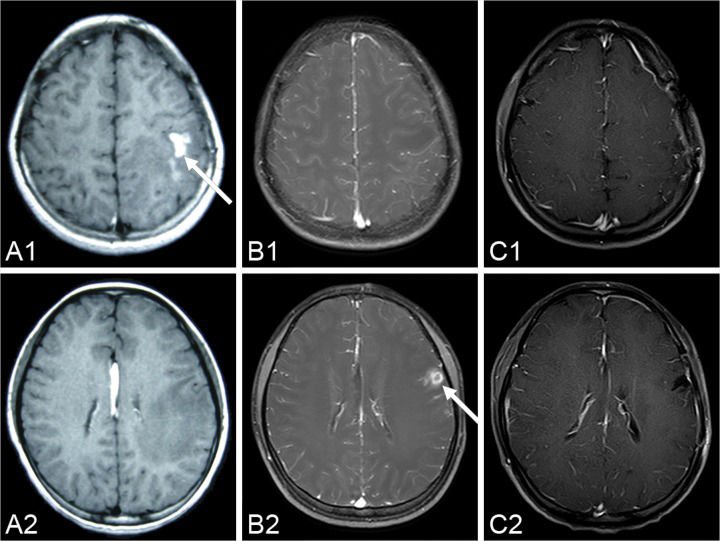
CASE DESCRIPTIONA fifteen-year-old girl suffered from facial and upper limb numbness on her right side one year ago. She was previously diagnosed with encephalitis at the Department of Neurology until she suffered a generalized tonic–clonic seizure six months later. MRI showed a contrast-enhanced lesion with an irregular shape located in the left frontal lobe (Figure 1 A1 and A2). The patient was advised to undergo further treatment in the Department of Neurosurgery. However, because the seizure was acutely controlled, she was noncompliant with treatment. One week before admission, the seizure became uncontrollable. An MRI revealed a contrast-enhanced lesion with an irregular shape (Figure 1 B1 and B2); however, local migration of the lesion was observed.
A series of pre-operational MR images demonstrates the local migration of an irregular, enhanced lesion (arrow) in the left hemisphere (A1-A2) six months before the operation; (B1-B2) three days before the operation. The lesion disappears in the post-operation MR images over the two-year follow-up period (C1-C2) 26 months after the operation.
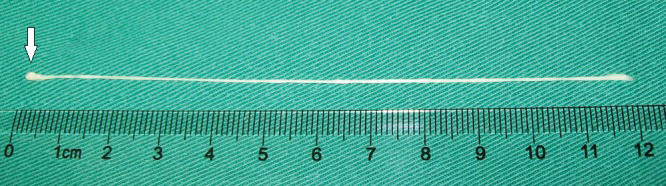
After admission, the patient's serum was tested by ELISA, which was positive for sparganum. The patient mentioned that she had eaten inadequately cooked frog flesh. Therefore, we hypothesized that the lesion was a live tapeworm. An operation was performed, and a light yellow lesion was identified in the posterior portion of the superior temporal gyrus with a live worm inside it. The worm was milky white, approximately 12cm long and could twist its body under stimulus (Figure 2).
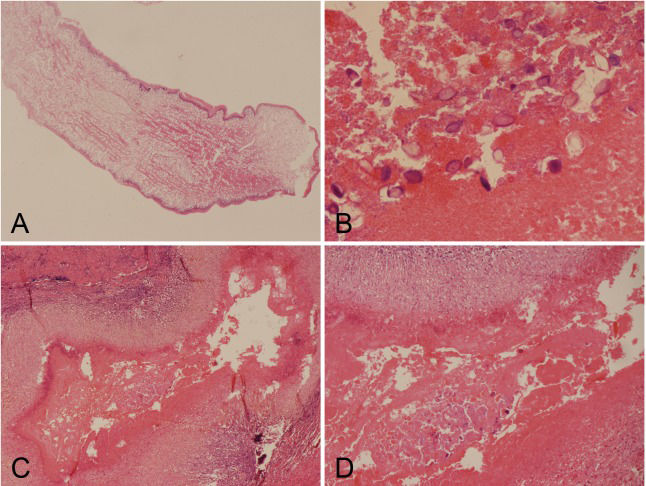
The histology of the larval cestode showed a brush border and eosinophilic smooth muscle fibers (Figure 3A). Some portions of the parasite body contained calcareous bodies (Figure 3B). A new tunnel-like structure with infiltrating plasma cells and eosinophils was identified in the tissue (Figure 3C). Under higher power, calcareous bodies were also observed within the tunnel (Figure 3D). Based on the pathological findings, the patient history and serum test results, a final diagnosis of cerebral sparganosis was made. During a follow-up period of more than two years, the patient's symptoms disappeared. No enhanced lesion was found on MRI at 26 months after the operation (Figure 1 C1 and C2).
Histological findings of the larval cestode and lesion. (A) Photomicrograph of the live larval showing a brush border and eosinophilic smooth muscle fibers (H&E 4X). (B) Photomicrograph showing calcareous bodies (H&E 40X). (C) A tunnel-like structure surrounded by plasma cells and eosinophils. (H&E 10X). (D) Calcareous bodies were observed within the tunnel. (H&E 20X).
The clinical manifestations of cerebral sparganosis include seizure, hemiparesis, and headache with a chronic course, in which seizure is the most common symptom (1,4). In our case, the patient first presented with sensation disorders on her right side and later with seizure. Serum ELISA is sensitive against sparganum and was essential evidence in our patient's diagnosis.
The characteristics of cerebral sparganosis on MRI include the following: A) degeneration of the cerebral white matter with edema and ventricle dilation; B) a contrast-enhanced lesion with an irregular shape, a string-of-beads shape or tunneling signs; and C) local or contralateral migration of the lesion or a change of the shape in follow-up MRI studies (1–2),. Migration may suggest a live tapeworm (1), which was observed and confirmed in this case. Other infectious granulomas, primary or secondary neoplasms, chronic infarctions, and degenerative diseases should be excluded from the diagnosis (4). Rengarajan et al. (9) indicated that cerebral sparganosis mimicked a tuberculoma on imaging, which should be included in the differential diagnosis. The patient in this case was previously diagnosed with encephalitis, which should also be included in the differential diagnosis.
The main pathologic findings in cerebral sparganosis include larval cestodes with a brush border and eosinophilic smooth muscle fibers and granulomas with calcareous bodies surrounded by plasma cells and eosinophils (1,6–8),. In our case, a new tunnel-like structure with infiltrating plasma cells and eosinophils was found in addition to the previously mentioned structures. Calcareous bodies were also observed within the tunnel under higher power. This tunnel-like structure was most likely the migration path of the sparganum, suggesting that migration phenomena may be significant histological evidence.
Neurocysticercosis is the most common parasitic disease of the human central nervous system, and it should be considered as a very important differential diagnosis in cases such as the present one. Similar to cerebral sparganosis, neurocysticercosis typically develops following oral ingestion of the parasite, and seizures and headache are the most commonly encountered symptoms (11). However, several factors can differentiate between the two infections: A) there are some highly suggestive radiological findings for neurocysticercosis, such as cyst structures (containing a scolex), enhancing lesions, and parenchymal calcifications (12); B) the detection of Taenia solium antigens or anti-T. solium antibodies in serum or cerebrospinal fluid can suggest the presence of neurocysticercosis 13; and C) cysticerci in different stages that are associated with inflammatory responses can be found upon microscopic examination.
Surgical resection of the lesion, including the tapeworm and the granuloma, is considered to be an effective treatment for cerebral sparganosis. Patients with total lesion excision can achieve good long-term outcomes (1). Many researchers (1–2), recommend surgery for patients with a live worm and signs of migration on MR images because this can prevent further damage caused by disease progression. Drug therapy, such as praziquantel, has a limited effect because live tapeworms are not affected (1) In this case, the operation was performed because local migration that was suggestive of a live worm was observed on serial MR images. The patient's symptoms disappeared, and recurrence was not observed over a follow-up period of more than two years.
In conclusion, the observed migration was an important radiological sign for preoperative diagnosis, identification of tapeworm survival, and judgment of surgical indication. The tunnel-like structure demonstrated by pathology might be an important diagnostic clue for cerebral sparganosis.
AUTHOR CONTRIBUTIONSWang P drafted the manuscript, reviewed the literature, and participated in the operation. Su XY made the histopathologic diagnosis. Mao Q and Liu YH performed the operation and revised the manuscript.
No potential conflict of interest was reported.