Thymosin beta 4 (Tβ4) is a ubiquitous peptide that plays pivotal roles in the cytoskeletal system and in cell differentiation. Recently, a role for Tβ4 has been proposed in experimental and human carcinogenesis, including gastrointestinal cancer. This study was aimed at evaluating the relationship between Tβ4 immunoreactivity and the initial steps of carcinogenesis.
METHODSIn total, 60 intestinal biopsies, including 10 hyperplastic polyps, 10 sessile serrated adenomas/polyps, 15 colorectal adenomas with low-grade dysplasia, 15 adenomas with high-grade dysplasia, 15 adenocarcinomas and 10 samples of normal colon mucosa, were analyzed for Tβ4 expression by immunohistochemistry.
RESULTSWeak cytoplasmic reactivity for Tβ4 was detected in the normal colon mucosa. No reactivity for Tβ4 was found in hyperplastic and sessile serrated polyps/adenomas. Tβ4 expression was observed in 10/15 colorectal adenocarcinomas. In adenomas with low-grade dysplasia, Tβ4 immunoreactivity was mainly detected in dysplastic glands but was absent in hyperplastic glands. Tβ4 immunoreactivity was characterized by spot-like perinuclear staining. In high-grade dysplastic polyps, immunostaining for Tβ4 appeared diffuse throughout the entire cytoplasm of dysplastic cells. Spot-like perinuclear reactivity was detected in adenocarcinoma tumor cells.
CONCLUSIONSOur study shows for the first time that Tβ4 is expressed during different steps of colon carcinogenesis. The shift of Tβ4 immunolocalization from low-grade to high-grade dysplastic glands suggests a role for Tβ4 in colorectal carcinogenesis. However, the real meaning of Tβ4 reactivity in dysplastic intestinal epithelium remains unknown.
Thymosin beta 4 (Tβ4), a peptide named after its first detection in the calf thymus (1), is a member of the β-thymosins, a versatile actin-binding protein family (2). Tβ4 has traditionally been associated with a role as a regulator of actin polymerization in living cells (3). Tβ4 is thought to be involved in many critical biological processes, including angiogenesis (4), wound healing (5), the inflammatory response (6) and cell migration (7). Tβ4 may also stimulate the AKT pathway, resulting in a strong anti-apoptotic effect on human cells (7), and it has recently been documented to play an essential role in cardioprotection after myocardial infarction (8) and in the protection of gingival fibroblasts from apoptosis induced by TNF-α (9). Recently, Tβ4 activity was implicated in experimental and human carcinogenesis. This concept is mainly based on the observation that Tβ4 may facilitate tumor cell motility and induce intra- and peritumoral angiogenesis (10). Tβ4 has been recently documented in breast cancer and in a few cases of colorectal cancer (11). Possible pro-metastatic (12) and pro-angiogenic activity has been hypothesized for Tβ4 (13), which has encouraged the development of Tβ4 inhibitors as anti-cancer drugs (14). Recently, Tβ4 immunoreactivity was detected by our group in the vast majority of colon carcinomas, where it showed a patchy distribution and well-differentiated areas that were significantly more reactive than less-differentiated tumor zones. Moreover, the localization of Tβ4 changed during cancer progression, moving from the cell membrane to the Golgi apparatus (15).
On the basis of these data, this study was aimed at analyzing the expression of Tβ4 in the initial steps of colorectal carcinogenesis. We analyzed Tβ4 expression by immunohistochemistry in hyperplastic polyps and colorectal adenomas with different degrees of dysplasia to shed light on the relationship between Tβ4 expression and colon cancer insurgence and progression.
MATERIALS AND METHODSThe study included archival paraffin-embedded colorectal biopsies obtained from 75 patients who underwent colonoscopy and biopsy. The cohort included six groups: normal colon mucosa (10 samples), hyperplastic polyps (10 samples), sessile serrated adenomas/polyps (10 samples), adenomas with low-grade dysplasia (15 samples), adenomas with high-grade dysplasia (15 samples) and adenocarcinomas (15 samples). Dysplasia was graded according to the degree of nuclear atypia and glandular architectural changes (16).
Paraffin sections were immunostained with anti-Tβ4 antibodies using the labeled streptavidin-biotin complex system (LSAB2, Dako) in a Dako Autostainer (DakoCytomation, Carpinteria, CA, USA). Briefly, slides were deparaffinized and rehydrated, and endogenous peroxidase activity was quenched (30 min) by 0.3% hydrogen peroxide in methanol. The slides were then subjected to heat-induced antigen retrieval by steaming unstained sections in Target Retrieval Solution (Dako TRS pH 6.1) for 30 min. Next, the slides were incubated with 10% normal goat serum in phosphate-buffered saline (PBS) for 60 min to block non-specific binding, followed by incubation (60 min at room temperature) with a monoclonal anti-Thymosin Beta 4 antibody (Bachem-Peninsula Lab, San Carlos, CA, USA), diluted 1:100 in the blocking solution. Slides were extensively washed with PBS containing 0.01% Triton X-100 and incubated with a secondary reagent (En Vision kit) according to the manufacturer's (Dako, Glostrup, Denmark) instructions. Diaminobenzidine (DAB) was used as a chromogen. After additional washes, the color was developed using AEC reagent (Dako), and the sections were counterstained with Mayer's hematoxylin and mounted. Sections of reactive lymph nodes with Tβ4-immunoreactive histiocytes were utilized as positive controls. For negative controls, the same procedure was applied, omitting the primary antibody. All cases were independently analyzed by two pathologists specialized in gastrointestinal pathology (SN, GF).
RESULTSIn this study, Tβ4 expression was always restricted to the cytoplasm of normal and dysplastic cells. No nuclear reactivity was detected in intestinal cells. Four main patterns of immunostaining for Tβ4 were observed, as follows: a) granular cytoplasmic staining, localized to the entire cytoplasm or to the apical regions of the enterocytes and possibly representative of the localization of the peptide in vesicles; b) spot-like perinuclear immunostaining, possibly representative of Tβ4 localization in the trans-Golgi network, c) homogeneous cytoplasmic staining, probably marking the actin-binding activity of the peptide, and d) intraluminal granular reactivity. No significant differences regarding Tβ4 immunoreactivity were detected within the first five groups, whereas inter-individual differences were only found for the intensity of immunostaining. Marked individual differences were observed in cases of colon cancer, in which Tβ4 was expressed in 10 of 15 cases.
Normal colon mucosaAll specimens of human colonic mucosa showed cytoplasmic expression of Tβ4. Immunoreactivity for Tβ4 was detected in the superficial enterocytes and in crypt cells. Immunoreactivity was consistently restricted to the cytoplasm and appeared as homogeneous staining. Tβ4 was also found in granular deposits in the intestinal lumen that were dispersed in the mucous secretions.
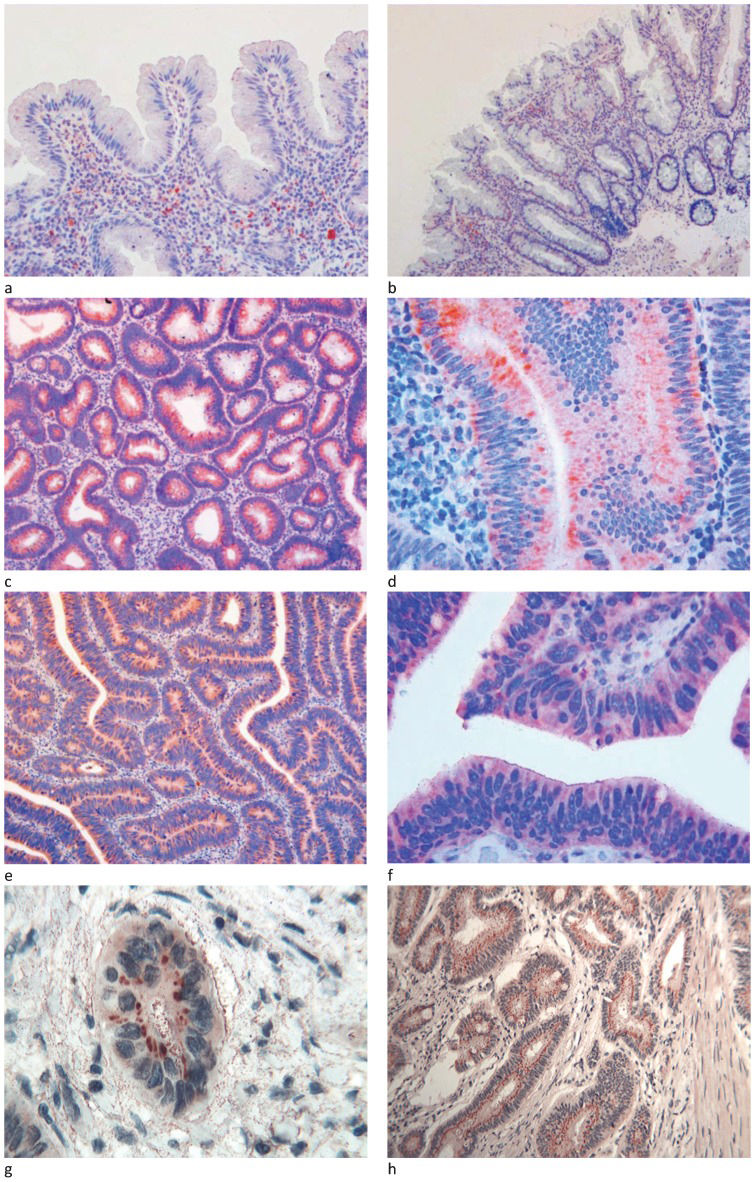
Hyperplastic polyps and sessile serrated polyps/adenomasHyperplastic glands and sessile serrated polyps/adenomas did not show any significant reactivity for Tβ4. Intense and granular cytoplasmic positivity was occasionally detected in scattered stromal mast cells (Figures 1A and 1B).
Hyperplastic and serrated hyperplastic polyps: A), B) No immunoreactivity for Tβ4 was observed in hyperplastic and serrated hyperplastic glands of the polyps analyzed. Scattered mast cells showed an intense and granular positivity for Tβ4 (OM X250 and X 100). Adenoma with low-grade dysplasia: C) at low magnification, the diffuse cytoplasmic immunoreactivity for Tβ4 was restricted to the dysplastic crypt epithelium (OM x100); D) at higher magnification, three main patterns of reactivity for Tβ4 were observed in the dysplastic glands: perinuclear spots, cytoplasmic granules and homogeneous cytoplasmic immunostaining (OM x400); adenoma with high-grade dysplasia: E) at low magnification, intense cytoplasmic immunoreactivity for Tβ4 was observed in the cytoplasm of dysplastic cells (OMX100); F) at higher magnification, diffuse and homogeneous Tβ4 immunoreactivity was observed in the cytoplasm of dysplastic glands (OMx400); adenocarcinoma: G) at higher magnification, peri-nuclear spot-like reactivity for Tβ4 was observed in the cytoplasm of the neoplastic nest (OMx400); H) perinuclear spot-like reactivity for Tβ4 was observed in the cytoplasm of the infiltrating tumor glands. Fine granular immunoreactivity was detected in the stroma surrounding the infiltrating tumor glands (OMx250).
In adenomas with low-grade dysplasia, Tβ4 reactivity observed at low magnification appeared to be homogeneously present in all of the observed dysplastic glands (Figure 1C). No notable staining for Tβ4 was detected in hyperplastic gland cells. At higher magnification, three main patterns of reactivity were observed in dysplastic glands: i) a punctate granular pattern observed in the entire cytoplasm; ii) a band-like pattern occupying the apical zone of the proliferating enterocytes; and iii) spot-like perinuclear reactivity (Figure 1D).
Adenomas with high-grade dysplasiaWhen we studied Tβ4 expression in colon adenomas with high-grade dysplasia, we found significant differences from low-grade dysplastic adenomas. Immunoreactivity for Tβ4 appeared to be more diffuse throughout the cytoplasm of dysplastic cells (Figure 1E). In tubulo-villous adenomas, Tβ4 was mainly expressed in the cytoplasm of superficial dysplastic epithelial cells (Figure 1F).
AdenocarcinomaImmunoreactivity for Tβ4 was detected in 10 out of 15 adenocarcinomas immunostained for the peptide. The most frequent immunohistochemical pattern was characterized by the accumulation of Tβ4 in the perinuclear area, giving rise to spot-like reactivity highly suggestive of localization to the trans-Golgi Network (Figure 1G). Granular discrete reactivity for Tβ4 was also detected in the stroma in close proximity to the glands with infiltrating tumors (Figure 1H).
DISCUSSIONThis study comprehensively investigated the Tβ4 protein expression pattern in human colorectal polyps and adenomas, showing relevant changes in Tβ4 localization in enterocytes during different steps of colon carcinogenesis. In previous studies, Tβ4 was found to be expressed in cultured colon carcinoma cells (17). Subsequent data showed that Tβ4 overexpression was associated with a more malignant phenotype and with enhanced tumor cell invasion, which suggests that Tβ4 expression is associated with tumor aggressiveness and poor survival (18). A study investigating Tβ4 expression in a limited number of human colon cancers reported highly variable immunostaining for Tβ4 in tumor cells and in endothelial and stromal cells (10). A recent study from our group showed strong Tβ4 cytoplasmic immunostaining in the tumor cells of the vast majority of colorectal adenocarcinomas (15). In this study, Tβ4 expression was found even in adenomas with low-grade dysplasia, representing the earliest stage of colorectal tumorigenesis. Further increases in Tβ4 expression paralleled the progression through distinct pathological stages, namely low-grade dysplasia and high-grade dysplasia. These findings clearly indicate that Tβ4 plays a role in colorectal carcinogenesis, starting from the early stages of adenomatous polyps and continuing through the progression towards colorectal cancer. It remains unclear whether Tβ4 staining could serve as a biomarker to distinguish colorectal adenomas that are more likely to progress to adenocarcinoma from Tβ4-negative polyps with no tendency toward cancer progression; long-term follow up and a large cohort of patients are required.
The absence of immunoreactivity for Tβ4 in sessile serrated polyps/adenomas is an interesting finding of our study. This finding suggests that Tβ4 could play a different role in the “serrated” pathway of colorectal neoplasia. From a practical point of view, the absence of immunoreactivity for Tβ4 in serrated polyps/adenomas might suggest the use of anti-Tβ4 antibodies in complex cases in which the differential diagnosis between low-grade adenoma and serrated polyp/adenoma is uncertain, based exclusively upon morphological criteria.
Colon cancer progression has also been associated with modifications in Tβ4 subcellular localization. In normal human colon mucosa, Tβ4 has a homogeneously diffuse staining pattern in the cytoplasm of superficial enterocytes and in the crypt epithelium. In hyperplastic polyps, we observed a complete disappearance of immunoreactivity for Tβ4. Interestingly, in low-grade dysplastic glands, we observed a preferential localization of Tβ4 to the trans-Golgi network, evidenced by perinuclear spot reactivity. The same immunostaining pattern for Tβ4 was previously reported in well-differentiated adenocarcinomas (15). In contrast, in high-grade adenomas, the pattern of Tβ4 immunolocalization changed to a cytoplasmic diffuse pattern. Although we were unable to attribute a certain function to Tβ4, particularly to its translocation to the Golgi apparatus and then back to the cytoplasm during colon carcinogenesis, we speculate that the diffuse cytoplasmic reactivity observed in normal mucosa could reflect the primary physiological role of Tβ4, i.e., the maintenance of actin cytoskeleton integrity, on which cell morphogenesis and motility depend (19). The translocation of Tβ4 from cytoplasmic vesicles to the trans-Golgi network may suggest a new function for this peptide in low-grade dysplastic cells; however, to the best of our knowledge, this function remains unclear. Translocation from the cell membrane to the trans-Golgi network has previously been reported in liver cells for ATP7B, a copper-transporting P-type ATPase. The localization of ATP7B in the Golgi apparatus reflects a synthetic role in the incorporation of copper atoms in cuproenzymes; under copper stress, ATP7B is relocated to the cell membrane and utilized in the excretion of excess copper (20). On the basis of these data, Tβ4 may play diverse roles in normal, dysplastic and tumor cells. Herein, we reported the preferential localization of Tβ4 in the Golgi apparatus in dysplastic and neoplastic cells, which may suggest a role for Tβ4 in the secretory pathways of dysplastic enterocytes and in colon cancer cells. The absence of significant differences at the mRNA level between the normal colon mucosa and tumor tissues was previously reported by our group (15) and may suggest a deregulation of Tβ4 expression at the post-transcriptional level.
Recently, Tβ4 has been shown to be widely expressed in the gastrointestinal tract (21) and in the salivary glands (22) during human embryogenesis, whereas its expression is low or absent in many normal adult tissues. On the basis of the present data, Tβ4 could be added to the list of proteins involved in both the embryogenesis and carcinogenesis of the oral-gastrointestinal tract (23).
In summary, the present study demonstrated that Tβ4 is frequently expressed and usually deregulated in human colon adenomas and is preferentially localized in the trans-Golgi network in low-grade dysplasia, in the whole cytoplasm in high-grade dysplasia, and back to the Golgi apparatus in colon cancer. These findings suggest that Tβ4 translocation occurs during both the initial and the advanced stages of tumor progression in colorectal cancer. Identifying the molecular mechanism underlying this process is a major challenge for future research focusing on Tβ4 in human colon carcinogenesis. The well-known role of Tβ4 in the recruitment and stimulation of adult and progenitor cells to restore their embryonic pluripotency (19), together with its strong expression in all stages of colorectal carcinogenesis, leads us to hypothesize that Tβ4 may drive the development of colorectal adenocarcinoma during multistage carcinogenesis. Further studies at the molecular level are needed to validate these immunohistochemical results with other analytical methods.
AUTHOR CONTRIBUTIONSNemolato S, Fanni G, Cabras T and Restivo A designed the research and wrote the manuscript. Zorcolo L, Gerosa C and Danni F collected and analyzed the data. Di Felice E performed the immunohistochemical analysis. Faa G, Messana I, Castagnola M and Casula G supervised the final manuscript.
We want to thank Ms. Sandra Serra, Ms. Simonetta Paderi and Ms. Rossana Zedda for their technical assistance and Mr. Ignazio Ferru for his administrative support. This work was supported by Fondazione Banco di Sardegna. We gratefully acknowledge the Sardinia Regional Government for financial support (P.O.R. Sardegna F.S.E. Operational Programme of the Autonomous Region of Sardinia, European Social Fund 2007-2013 - Axis IV Human Resources, Objective l.3, Line of Activity l.3.1 “Avviso di chiamata per il finanziamento di Assegni di Ricerca”).
No potential conflict of interest was reported.