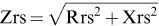Recent work has suggested that within-breath respiratory impedance measurements performed using the forced oscillation technique may help to noninvasively evaluate respiratory mechanics. We investigated the influence of airway obstruction on the within-breath forced oscillation technique in smokers and chronic obstructive pulmonary disease patients and evaluated the contribution of this analysis to the diagnosis of chronic obstructive pulmonary disease.
METHODS:Twenty healthy individuals and 20 smokers were assessed. The study also included 74 patients with stable chronic obstructive pulmonary disease. We evaluated the mean respiratory impedance (Zm) as well as values for the inspiration (Zi) and expiration cycles (Ze) at the beginning of inspiration (Zbi) and expiration (Zbe), respectively. The peak-to-peak impedance (Zpp=Zbe-Zbi) and the respiratory cycle dependence (ΔZrs=Ze-Zi) were also analyzed. The diagnostic utility was evaluated by investigating the sensitivity, the specificity and the area under the receiver operating characteristic curve. ClinicalTrials.gov: NCT01888705.
RESULTS:Airway obstruction increased the within-breath respiratory impedance parameters that were significantly correlated with the spirometric indices of airway obstruction (R=−0.65, p<0.0001). In contrast to the control subjects and the smokers, the chronic obstructive pulmonary disease patients presented significant expiratory-inspiratory differences (p<0.002). The adverse effects of moderate airway obstruction were detected based on the Zpp with an accuracy of 83%. Additionally, abnormal effects in severe and very severe patients were detected based on the Zm, Zi, Ze, Zbe, Zpp and ΔZrs with a high degree of accuracy (>90%).
CONCLUSIONS:We conclude the following: (1) chronic obstructive pulmonary disease introduces higher respiratory cycle dependence, (2) this increase is proportional to airway obstruction, and (3) the within-breath forced oscillation technique may provide novel parameters that facilitate the diagnosis of respiratory abnormalities in chronic obstructive pulmonary disease.
The mechanical changes due to chronic obstructive pulmonary disease (COPD) are associated with a progressive increase in airflow obstruction 1, which is usually evaluated by spirometric tests. However, these tests are highly dependent on patient cooperation and effort, which may be a limitation in older people and in patients in the advanced stages of the disease 2.
The forced oscillation technique (FOT) offers a simple and detailed approach to investigating the mechanical properties of the respiratory system 3–5. In practice, sinusoidal excitations are superimposed on spontaneous breathing at the airway opening using a loudspeaker. The resulting oscillations in airflow and pressure are recorded and used to estimate the mechanical impedance of the respiratory system. These features make this technique potentially suitable for the routine evaluation of respiratory function in COPD 6.
An interesting characteristic of this method is its excellent time resolution, allowing for the analysis of changes within the respiratory cycles. This is an important advantage for pathophysiological research, as it provides a detailed characterization of the patient's respiratory mechanics. In recent years, strong evidence has emerged supporting the utility of within-breath FOT (WbFOT) measurements in several contexts. This method has been successfully applied to undertake difficult studies, including studies on sleep disorders 7,8 and studies evaluating pediatric 9 and elderly 10 subjects. A detailed analysis of the expiratory flow limitation (EFL) 11,12 and the response to salbutamol 13 in COPD patients was also recently performed. Previous studies have obtained promising results using the WbFOT to evaluate abnormal respiratory mechanics in asthma patients 14–16, smokers 17, COPD patients 18 and interstitial lung disease patients 19. In particular, a recent study from our group provided evidence that WbFOT measurements may improve our understanding of COPD pathophysiology and simplify the diagnosis of respiratory alterations in COPD 20. However, this preliminary study was limited to evaluating patients in the severe stages of the disease. To the best of our knowledge, there are no data in the literature concerning the influence of airway obstruction on the within-breath respiratory impedance of COPD patients.
In this context, the objectives of the present study were (1) to compare the respiratory mechanics of normal subjects and COPD patients, with an emphasis on the differences between the phases of the respiratory cycle, and (2) to evaluate the contribution of this analysis to COPD diagnosis.
METHODSStudy designThe present work was a controlled cross-sectional study developed at the State University of Rio de Janeiro. The examinations included spirometry and FOT measurements. The Research Ethics Committee of the State University of Rio de Janeiro approved this study. The work was carried out in accordance with the Declaration of Helsinki and has been registered at ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT01888705). The objectives were explained to all of the participants, and their written consent was obtained before their inclusion in the study.
SubjectsThis study involved 114 volunteers, including 20 never-smoking controls with normal spirometric evaluations and without a previous history of cardiac disease. Twenty smokers presenting a normal respiratory response to the spirometric exam constituted the “normal exam” (NE) group. The study also included 74 patients with stable COPD who were classified according to the GOLD criteria as having mild (Group I, n=14), moderate (Group II, n=20), severe (Group III, n=20), or very severe (Group IV, n=20) obstruction 1. The eligibility criteria for COPD included a history of smoking more than ten packs of cigarettes per year; a ratio of the forced expiratory volume in the first second (FEV1) to the forced vital capacity (FVC) of less than 0.7; no respiratory infections in the previous three weeks; an absence of other respiratory diseases; and an absence of extrathoracic comorbidities, including cardiovascular diseases, malignant diseases, and chest deformities.
Study protocolThe subjects were informed of the need to suspend the use of bronchodilators during the 12 h that preceded the tests. The examination sequence was carried out as follows: evaluation of clinical history; collection of anthropometric measurements (age, body weight and height) and risk factors associated with the disease; testing of FOT impedance; and finally, gathering of the spirometric measurements.
SpirometryFlow-volume curves were obtained using a bellows spirometer (Vitatrace VT 130 SL model; Pro Médico Ind. Ltd., Rio de Janeiro, Brazil) to assess the FEV1, the FVC, the forced expiratory flow (FEF) between 25% and 75% of the FVC, the FEF/FVC ratio and the FEV1/FVC ratio. These exams were performed according to the ATS-ERS guidelines for spirometry 21. All of the spirometric parameters were assessed as absolute and percentage values relative to the values predicted for the Brazilian population 22.
Within-breath respiratory impedance measurementsRespiratory system impedance (Zrs) was measured at 5 Hz using a device developed in our laboratory 8,23. During the measurements, the instrument applies a low-pressure (2.0 cmH2O peak-to-peak amplitude) sinusoidal signal to the subject's respiratory system, which remains under spontaneous ventilation. The instrument allows the evaluation of the Zrs from signals coming from a pressure transducer and a pneumotachograph placed close to the individual's mouth. The resulting pressure (P) and airflow (V) signals are used to obtain the within-breath impedance module (Zrs=P/V) 23. This parameter is traditionally used in WbFOT measurements 2,18,20,24 and is interpreted as the total mechanical load of the respiratory system 3–5, including the respiratory resistive (Rrs) and reactive (Xrs) effects observed at 5 Hz, as described by Equation 1. Notably, the Xrs reflects the elastic properties of the respiratory system at 5 Hz.
The within-breath input impedance is usually determined using fast Fourier transforms 4,18, cross-correlation or least-squares techniques 5. These methods allow measurements with limited time resolutions, typically of 0.2 s (11) or 0.25 s (15). The present study used an analog signal-processing circuit that allowed for a continuous real-time calculation of the WbFOT 23. This method helps to improve discrimination between the phases of the respiratory cycle as well as to derive precise measurements at specific points of interest.
The resulting impedance and flow signals (f ≈ 0.2 Hz) were low-pass filtered at 5 Hz using analog filters (Butterworth, 4th order) to remove the external noise (60 Hz) and were digitized with 12-bit resolution at a sampling rate of 16 Hz. The instrument was calibrated using a reference mechanical load, resulting in measurement errors of <0.5%.
The following secondary parameters were used to characterize the changes in the respiratory mechanics during the different phases of the respiratory cycle:
- •
The mean respiratory impedance (Zm), calculated for the complete exam;
- •
The mean impedance during the inspiration cycles (Zi);
- •
The mean impedance during the expiration cycles (Ze);
- •
The mean impedance at the beginning of inspiration (Zbi);
- •
The mean impedance at the beginning of expiration (Zbe);
- •
The peak-to-peak impedance (Zpp), which is the difference between the Zbe and the Zbi;
- •
The mean impedance change (ΔZrs), which is the difference between the Ze and the Zi.
These measurements lasted 120 s and were obtained with the subjects seated and spontaneously breathing while holding their cheeks and the floor of their mouth with their hands to avoid the shunt effect of the upper airways. The data acquisition commenced once the subject was comfortable using the mouthpiece with a good mouth seal. The study subjects were encouraged to breathe in a regular manner, to avoid swallowing and to maintain a tight mouthpiece seal. The recordings were deemed acceptable if the airflow and frequency appeared stable, with no obvious leaks or glottal closures, as determined by visual inspection of the airflow and impedance traces. Measurements with distortions due to artifacts such as coughs or sneezes were also discarded. Whenever the impedance time series was not considered adequate, the maneuver was not considered valid and was repeated. If the correct maneuvers could not be performed, the subjects were excluded from the study. The FOT exams were carried out first, and the delay between the FOT and the spirometric exams was less than 30 min.
Sample size and statistical analysisTo estimate the sample size, a pilot study on a group of 27 subjects (13 subjects with COPD and 14 controls) was conducted using a protocol identical to that described above. Based on these preliminary results, the sample size was calculated based on the difference between the means, assuming type I and type II errors of 1%. The minimum calculated value for this study was 12 individuals for each group.
Initially, the sample characteristics were evaluated using the Shapiro-Wilk test. Depending on these characteristics, we then used the independent t-test or the Mann-Whitney U test to assess between-group differences and a paired-t test and one-way ANOVA to perform intra-group comparisons. Differences were considered statistically significant when p<0.05.
To measure the overall agreement between the variables related to the spirometry and respiratory impedance, we calculated Spearman's rank correlation coefficient for the whole group of studied volunteers. The correlations were classified as follows 26:
- •
Small or no correlation: correlation between 0 and 0.25 (or −0.25);
- •
Reasonable correlation: between 0.25 and 0.50 (or −0.25 to −0.50);
- •
Moderate to good correlation: between 0.50 and 0.75 (or −0.50 to −0.75);
- •
Very good to excellent correlation: correlation greater than 0.75 (or −0.75).
We used the area under the receiver operating characteristic curve (AUC) to evaluate the ability of the within-breath FOT indices to distinguish the patients from the control subjects. The interpretation of these results followed previous work, in which an AUC>0.80 was usually considered as adequate for clinical use 27,28. More specifically, the curves with AUCs between 0.50 and 0.70 indicate low diagnostic accuracy, AUCs between 0.70 and 0.90 indicate moderate diagnostic accuracy, and AUCs between 0.90 and 1.00 indicate high diagnostic accuracy 27,28. The optimal cut-off point was chosen to balance the highest values of sensitivity and specificity.
The analyses described in this section were performed using MedCalc® 12.3 (MedCalc Software, Mariakerke, Belgium) and STATISTICA® 5.0 for Windows (StatSoft Inc., Tulsa, OK, USA).
RESULTSAnthropometric and spirometric resultsThe anthropometric and spirometric characteristics of the patients and healthy control subjects are presented in Table 1. The clinical characteristics are also described in this table. There were no significant differences in height among the groups, but there were significant group differences for age, weight and BMI. As observed in Table 1, the patients with COPD had significant reductions in all of the studied spirometric parameters (p<0.001).
Biometric and spirometric characteristics of the studied groups (mean±SD).The right column shows comparisons of the six groups/comparisons between adjacent groups; dashes indicate significant differences.
| Control(A) | NE(B) | Mild(C) | Moderate(D) | Severe(E) | Very Severe(F) | ANOVA / Between groups | |
|---|---|---|---|---|---|---|---|
| Age (years) | 68.3±8.3 | 54.5±8.8 | 61.4±11.5 | 65.1±10.6 | 70.7±10.4 | 69.3±8.9 | p<0.001 / A-B,C,D,E,F,A |
| Mass (kg) | 73.5±12.3 | 77.8±16.9 | 70.1±11.2 | 69.6±12.6 | 61.2±12.3 | 60±15.4 | p<0.001 / A,B,C,D,E,F-A |
| Height (m) | 1.62± 0.1 | 1.64±0.1 | 10.63±0.1 | 1.63±0.1 | 1.59±0.1 | 1.61±0.1 | ns |
| BMI | 27.8±4.4 | 28.4±4.2 | 26.1±3.2 | 26.1±5.3 | 24.1±4.3 | 23.2±6.2 | p<0.01 / A,B,C,D-E,F-A |
| FEV1 (L) | 2.3±0.6 | 2.6±0.8 | 20.4±0.5 | 1.9±0.4 | 0.93±0.22 | 0.94±1.2 | p<0.001 / A,B,C,D-E,F-A |
| FEV1 (%) | 96.4±15.2 | 90.3±10.5 | 88±7.7 | 67.5±7.4 | 39.3±5.4 | 24.8±3.3 | p<0.001 / A,B,C-D-E,F-A |
| FVC (L) | 2.9±0.6 | 3.3±0.9 | 30.6±0.8 | 3.0±0.9 | 2.4±1.2 | 1.9±0.7 | p<0.001 / A,B,C,D,E-F-A |
| FVC (%) | 92.7±15.7 | 93.2±11.9 | 104.6±0.3 | 84.8±16.6 | 77.9±28.5 | 55.7±14.1 | p<0.001 / A,B-C,D-E-F-A |
| FEV1/FVC | 81.3±5.3 | 78.4±4.5 | 66.9±3.3 | 64.4±12.5 | 42.5±11.2 | 38.4±9.6 | p<0.001 / A,B-C,D-E,F-A |
| FEF25-75 (L) | 2.4±0.9 | 2.4±1.0 | 1.4±0.4 | 1.1±0.5 | 0.3±0.1 | 0.2±0.05 | p<0.001 / A.B-C,D-E,F-A |
| FEF25–75 (%) | 99.4±25.8 | 88±23.3 | 53.4±14.2 | 41±19.2 | 12.2±3.9 | 8.2±1.5 | p<0.001 / A,B-C,D-E,F-A |
| Smoking habit, current/former/never | 0/0/20 | 13/7/0 | 6/14/0 | 11/8/1 | 6/12/2 | 5/12/3 | - |
| Pack-years | - | 42.9±30.1 | 50.2±38.5 | 58.0±50.5 | 60.0±42.9 | 76.3±62.4 | - |
| Patient on medication (%) | - | 15.8 | 57.1 | 60.0 | 95.0 | 100.0 | - |
| Dyspnea (yes/no) | - | 12/8 | 9/5 | 11/9 | 18/2 | 20/0 | - |
| Cough (%) | - | 15.0 | 14.3 | 25.0 | 45.0 | 40.0 | - |
| Secretion (%) | - | 10.0 | 7.1 | 5.0 | 40.0 | 35.0 | - |
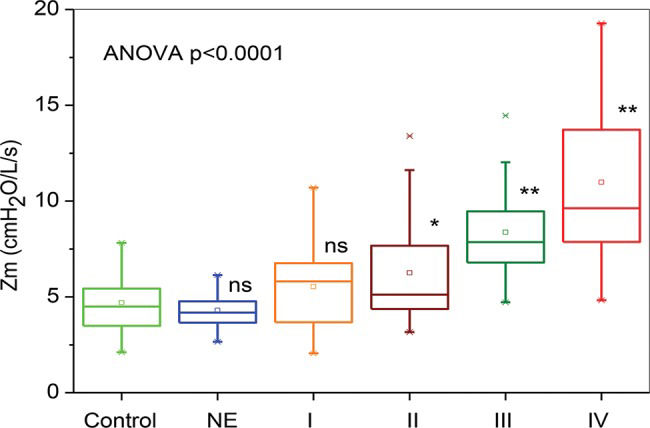
Figure 1 depicts the results of the whole-breath analysis for the groups classified according to spirometry. The Zm increased with airway obstruction (ANOVA p<0.0001). The moderate, severe, and very severe groups presented increased Zm values in comparison with the controls (p<0.01).
Respiratory impedance values throughout the respiratory cycle in healthy subjects; in smokers with normal spirometry; and in chronic obstructive pulmonary disease patients with mild (I), moderate (II), severe (III) or very severe (IV) airway obstruction. The top and bottom of the box plots represent the 25th- and 75th-percentile values, respectively. Additionally, the circles represent the mean values, the bars across the boxes represent the median values, and the whiskers outside the boxes represent the 5th- and 95th-percentile values. Differences relative to the controls: *p<0.01; **p<0.0001.
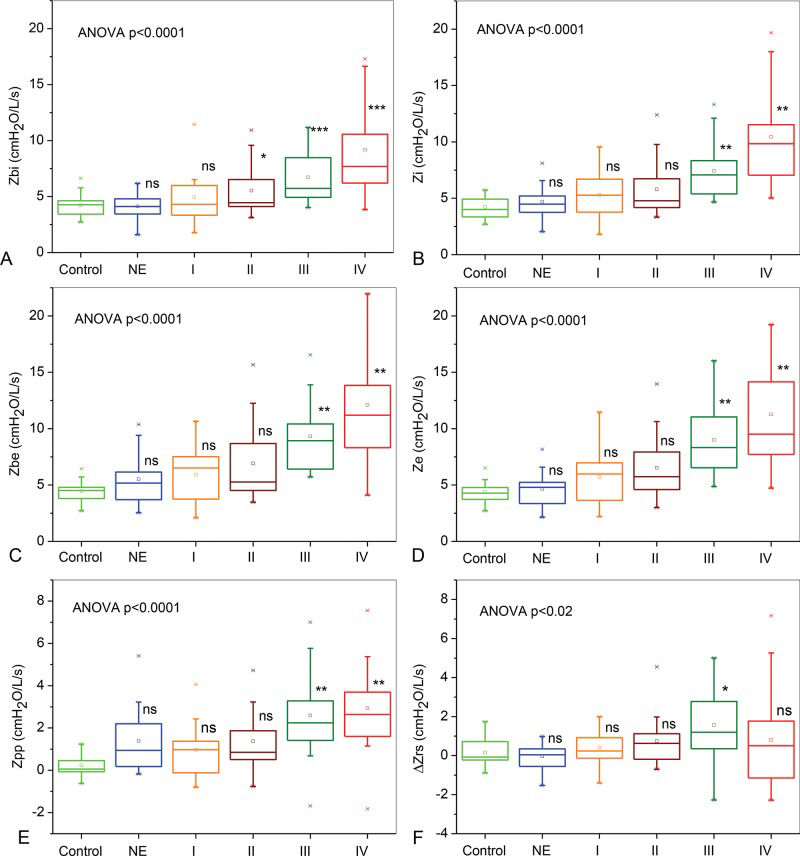
The respiratory impedance measurements increased with airway obstruction in the COPD patients (Figure 2: Zbi, Zi, Zbe and Ze; ANOVA p<0.0001). Similar comparisons revealed that the Zpp and ΔZrs also increased with airway obstruction (Figure 2E, ANOVA p<0.0001, and Figure 2F, ANOVA p<0.02, respectively).
Comparisons among the mean impedances at the beginning of inspiration (Zbi, A), during the inspiratory phase (Zi, B), at the beginning of expiration (Zbe, C), and during the expiratory phase (Ze, C), in addition to the peak-to-peak impedance (Zpp=Zbe-Zbi; D) and the respiratory cycle dependence (ΔZrs=Ze-Zi; F). The top and bottom of the box plots represent the 25th- and 75th-percentile values, respectively. Additionally, the circles represent the mean values, the bars across the boxes represent the median values, and the whiskers outside the boxes represent the 5th- and 95th-percentile values. Differences relative to the controls: *p<0.05, **p<0.004.
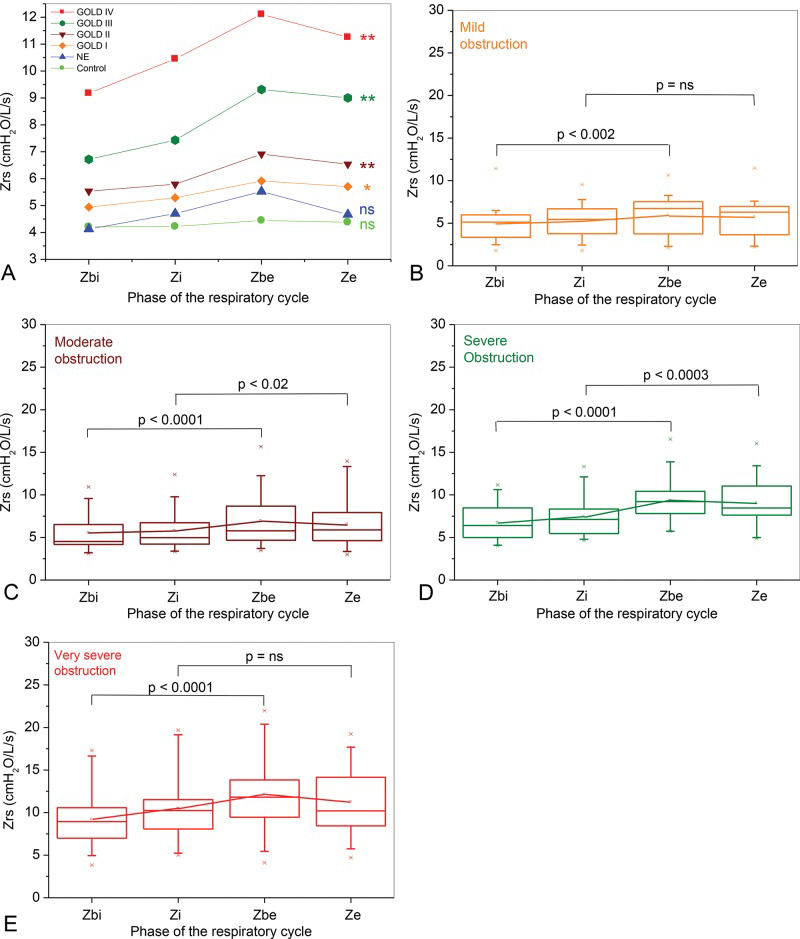
Figure 3 shows the influence of airway obstruction on respiratory impedance along the ventilation cycle in the studied subjects. Respiratory impedance did not change significantly throughout the respiratory cycle in the control and the NE groups (Figure 3A; ANOVA p=ns). In contrast, the impedance in the mild COPD patients showed significant increases from the beginning of inspiration to the expiratory phase (Figure 3A; ANOVA p<0.002). The mild COPD patients presented significantly higher Zbe values compared with the Zbi values (Figure 3B, p<0.002), whereas the Ze was not significantly higher compared with the Zi. Comparisons with the more advanced COPD patients showed more pronounced changes than in the mild COPD patients. Additionally, the Zrs significantly increased from the beginning of the inspiratory phase to the end of the expiratory phase in the moderate, severe and very severe patients (Figures 3A, C, D, and E; ANOVA p<0.0001).
Correlations between within-breath impedance and spirometryThe associations between these variables are described in Table 2. The Zm, Zi, Ze, Zbi and Zbe showed statistically significant (p<0.0001) moderate to good inverse correlations with all of the spirometric parameters, with the exception of the FVC. Significant moderate to good and reasonable inverse correlations were also observed among the spirometric parameters and the Zpp. Meanwhile, the ΔZrs presented small and reasonable inverse correlations with nearly all of the spirometric parameters and was not correlated with the FVC.
Correlation coefficient for and significance level of the association between respiratory impedance and pulmonary function parameters.
| FEV1 (L) | FEV1 (%) | FVC (L) | FVC (%) | FEV1/FVC (%) | FEF25–75% (L) | FEF25–75% (%) | FEF/FVC (%) | |
|---|---|---|---|---|---|---|---|---|
| Zm (cmH2O/L/s) | ||||||||
| R | −0.66 | −0.68 | −0.48 | −0.47 | −0.64 | −0.66 | −0.67 | −0.63 |
| p | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Zi (cmH2O/L/s) | ||||||||
| R | −0.63 | −0.65 | −0.46 | −0.45 | −0.54 | −0.62 | −0.63 | −0.59 |
| p | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Ze (cmH2O/L/s) | ||||||||
| R | −0.66 | −0.68 | −0.47 | −0.47 | −0.65 | −0.67 | −0.67 | −0.64 |
| p | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Zbi (cmH2O/L/s) | ||||||||
| R | −0.60 | −0.62 | −0.45 | −0.42 | −0.58 | −0.59 | −0.60 | −0.57 |
| p | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Zbe (cmH2O/L/s) | ||||||||
| R | −0.63 | −0.65 | −0.44 | −0.46 | −0.62 | −0.63 | −0.64 | −0.61 |
| p | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Zpp (cmH2O/L/s) | ||||||||
| R | −0.49 | −0.53 | −0.32 | −0.38 | −0.50 | −0.47 | −0.52 | −0.47 |
| p | 0.0001 | 0.0001 | 0.003 | 0.0005 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| ΔZrs (cmH2O/L/s) | ||||||||
| R | −0.22 | −0.24 | −0.07 | −0.08 | −0.32 | −0.33 | −0.33 | −0.34 |
| p | 0.02 | 0.01 | 0.45 | 0.37 | 0.0005 | 0.0003 | 0.0003 | 0.0001 |
Table 3 shows the values for the area under the curve (AUC), the sensitivity (Se), and the specificity (Sp) for the optimal cut-off points obtained for the studied FOT parameters. The Zpp performed adequately in the patients with moderate obstruction, whereas five of the six studied parameters showed high performance in those with severe obstruction. In patients with very severe obstruction, all of the studied parameters had a high diagnostic accuracy.
Sensitivity, specificity, and area under the curve values for the optimal cut-off points obtained using receiver operating characteristic curves.
| Sensibility (Se) | Specificity (Sp) | Area under the curve (AUC) | Cut-off point (cmH2O/L/s) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NE | I | II | III | IV | NE | I | II | III | IV | NE | I | II | III | IV | NE | I | II | III | IV | |
| Zm | 0.65 | 0.64 | 0.70 | 0.95 | 1.00 | 0.55 | 0.85 | 0.6 | 0.85 | 0.80 | 0.60 | 0.68 | 0.75 | 0.97 | 0.98 | 4.325 | 4.837 | 4.550 | 4.837 | 4.785 |
| Zi | 0.60 | 0.64 | 0.65 | 0.85 | 1.00 | 0.55 | 0.85 | 0.65 | 0.85 | 0.80 | 0.58 | 0.66 | 0.72 | 0.93 | 0.98 | 4.251 | 5.058 | 4.608 | 5.058 | 4.967 |
| Ze | 0.60 | 0.64 | 0.80 | 0.90 | 0.95 | 0.65 | 0.75 | 0.65 | 0.85 | 0.95 | 0.58 | 0.67 | 0.76 | 0.97 | 0.98 | 4.724 | 4.781 | 4.563 | 5.075 | 5.4805 |
| Zbi | 0.65 | 0.57 | 0.55 | 0.80 | 0.95 | 0.45 | 0.50 | 0.65 | 0.85 | 0.85 | 0.51 | 0.58 | 0.67 | 0.86 | 0.96 | 4.145 | 4.274 | 4.452 | 4.667 | 4.667 |
| Zbe | 0.60 | 0.64 | 0.75 | 1.00 | 0.95 | 0.65 | 0.80 | 0.65 | 0.95 | 0.85 | 0.64 | 0.68 | 0.77 | 0.99 | 0.96 | 4.610 | 4.839 | 4.610 | 5.714 | 5.034 |
| Zpp | 0.70 | 0.64 | 0.80 | 0.90 | 0.95 | 0.65 | 0.80 | 0.80 | 0.90 | 0.95 | 0.76 | 0.70 | 0.83 | 0.94 | 0.95 | 0.381 | 0.491 | 0.491 | 0.810 | 1.025 |
| ΔZrs | 0.45 | 0.64 | 0.65 | 0.65 | 0.65 | 0.50 | 0.65 | 0.70 | 0.80 | 0.65 | 0.52 | 0.60 | 0.64 | 0.80 | 0.59 | 0.008 | 0.038 | 0.105 | 0.748 | 0.038 |
Previous studies have compared WbFOT measurements between groups of controls and COPD patients and have observed clear modifications 2,15,18,20,29,30. These findings raised two questions: (1) Is the effect of increasing the degree of airway obstruction in COPD adequately described by WbFOT measurements? (2) Is the WbFOT able to contribute to the clinical diagnosis of COPD? Nearly all of the cited studies used impulse oscillation systems 2,15,18,29,30, which differ from the classical FOT, including in terms of the signal applied at the subject's mouth and the data processing 3,31. An additional limitation is that these studies did not evaluate the diagnostic use of the WbFOT 2,15,18,29,30. Thus, to answer these questions, the present study investigated the possibility of detecting changes in respiratory mechanics in progressive airway obstruction in COPD patients using the classical FOT. It was shown that the WbFOT method reveals changes in the inspiratory and expiratory impedance that are significantly correlated with the spirometric indices of airway obstruction. COPD was also found to introduce higher respiratory cycle dependence that is proportional to the airway obstruction. This study thus confirms the clinical potential of the within-breath impedance analysis for the diagnosis of respiratory modifications related to COPD.
The individuals in the group of COPD patients had a lower body mass and BMI than those in the control group (Table 1). This difference may be easily explained considering the clinical condition of these patients, who presented with advanced COPD. As expected, the alterations of routine lung function parameters in the COPD patients were in agreement with the presence of airway obstruction (1), showing significant reductions in the spirometric results.
In agreement with previous studies 19,29,32, we observed small mean respiratory impedance values in normal subjects and increasing mean respiratory impedance values in COPD patients (Figure 1). COPD is characterized by the presence of airway wall inflammation and mucus hypersecretion, which result in airway obstruction 1,6. These phenomena may explain the increase in the Zm observed in Figure 1. The respiratory system impedance module is associated with the resistive and reactive properties of the entire respiratory system, including the lung and chest wall 3,4. At lower frequencies, such as the frequency used in this study (5 Hz), the reactive properties are dominated by the elastic properties of the respiratory system. In the present study, the COPD patients showed decreased dynamic compliance due to the difficulty encountered by the oscillatory signals emitted by the FOT when crossing segments of the bronchial tree. This difficulty is associated with increased small airway resistance in regions usually denominated as “choke points”, as proposed by Dellacà et al. 11. The formation of choke points prevents the oscillatory signal from penetrating further into the lung, thus decreasing the apparent volume and hence the lung compliance. These features result in more negative reactance values and a consequent increase in the impedance module (Equation 1). COPD progression is also associated with peribronchial fibrosis and consequent airway tissue remodeling. This reduced airway compliance may also introduce a more negative Xrs, contributing to a higher Zrs (Equation 1). Additionally, the deformation of the thoracic wall associated with lung hyperinflation in COPD introduces an important restrictive factor into the interaction between the lung and the thoracic wall. This feature may also help to explain the increase in the Zm observed in the COPD subjects (Figure 1). Accordingly, the increase in the Zm in the studied COPD subjects could have been associated with the progressive increase in central and peripheral airway resistance as well as with a reduction in the compliance of the respiratory system. Thus, the increase in the Zm (Figure 1) describes the increase in the elastic and resistive respiratory work associated with the progression of COPD. These results are consistent with those recently obtained by Paredi et al. 29 and Shinke et al. 17 for groups with predominantly moderate obstruction. The findings are also in line with those reported by Mori et al. 15 and Sugiyama et al. 19 for groups of COPD patients presenting mainly moderate to severe obstruction and by Kubota and collaborators (2) for patients presenting severe or very severe obstruction.
The behavior of the Zrs values throughout the respiratory cycle was different between the control subjects and the COPD patients (Figures 2 and 3). In particular, in the control subjects, the Zrs values were approximately constant from the beginning of inspiration to expiration (Figure 2 and 3A). Recent studies 19,29,32 have also reported minimal changes in the impedance values between inspiration and expiration in normal individuals. In line with these results, we observed only non-significant changes along the respiratory cycle in healthy subjects and in smokers (Figure 3A).
In contrast, the COPD patients with mild obstruction presented more pronounced changes along the respiratory cycle (Figures 3A and B), showing increased values from the beginning of inspiration to expiration (p<0.002). Even more pronounced changes were observed in the patients with moderate, severe or very severe obstruction (Figures 3A, C, D, and E; p<0.0001). In close agreement with the results described in Figure 3, Sugiyama et al. 19 and Shinke et al. 17 recently observed increased expiratory impedance values in groups of COPD patients presenting mainly moderate to severe or moderate obstruction. Yamauchi and collaborators also reported expiratory impedance values higher than the inspiratory values in patients with mild or moderate obstruction 30. Patients with COPD may exhibit EFL at rest, and previous studies have shown that the within-breath respiratory impedance is sensitive to the presence of EFL in COPD 11–13. Under normal conditions, the low-frequency reactance measurements reflect the elastic properties of the entire respiratory system. However, if EFL is present, the oscillatory signal cannot pass through the choke point and reach the alveoli, and the reactance reflects the mechanical properties of the airways proximal to the choke point 11, which are much stiffer than those in the periphery. As a result, the reactance increases (becomes more negative). Because the Zrs increases with the Xrs, as described by Equation 1, the presence of the EFL may explain the higher Zrs values observed during expiration in COPD patients (Figures 2 and 3). In this context, the lack of a difference between Ze and Zi values observed in mild COPD patients (Figure 3B) may be explained by these patients′ negligible flow limitation. Notably, the expiratory measurements were higher than the inspiratory measurements in the patients with moderate or severe obstruction (Figures 3C and D). In the very severe patients (Figure 3E), however, the Ze was not significantly higher than the Zi.
The Zrs significantly increased with airway obstruction in all phases of the ventilatory cycle (Figures 2A, B, C, and D). Previous studies from our group showed higher inspiratory and expiratory impedance values when comparing asthmatics and healthy subjects using a similar methodology 14. Given that the progression of COPD is associated with peribronchial fibrosis and consequent airway tissue remodeling, this reduced airway compliance may introduce a more negative Xrs, thereby contributing to the increased impedance values (Equation 1). In close agreement with this theory, the Zbi and Zbe increased significantly with airway obstruction (Figures 2A and C).
In COPD, the change in the tidal volume operating point favors lung hyperinflation, reducing the efficiency of the diaphragm as a pump and inducing the use of accessory muscles. In the present study, the recruitment of accessory muscles in the COPD subjects due to impaired diaphragmatic mechanics may be one possible contributing factor to the increase in respiratory impedance. Because the respiratory impedance measurements include the influence of the chest wall, we believe that abnormal accessory muscle contraction during inspiration may have contributed to the progressive increase in impedance during inspiration in our patients (Figure 2B). Other aspects may also contribute to the elevated Zi values, including reduced homogeneity of the time constants in the lung 6,11,33. Thus, the increase in the Zi may have been related to increases in the respiratory resistive and elastic work presented by these individuals. Moreover, the increase observed during expiration (Figure 2D) may be explained, at least in part, by the presence of the EFL and active expiratory efforts.
Figures 2E and F show that the control subjects exhibited small ΔZrs and Zpp values, which is consistent with physiological principles and previous studies 2,10,19. In contrast, the COPD subjects showed significant increases in respiratory cycle dependency (ΔZrs and Zpp). This result is in line with previous studies that also reported increased expiratory-inspiratory differences in patients with COPD compared with healthy subjects 18,19.
The current study shows that all within-breath impedance measurements were associated with airway obstruction (Table 2). The best associations among the impedance and spirometric parameters were obtained with the FEV1 (%), Zm and Ze (R=−0.68, p<0.0001). These moderate to good correlations suggest that these parameters strongly reflect the alterations in the central airways. The association with FEF25−75% (R=−0.67, p<0.0001) also suggests the influence of a smaller peripheral obstruction. The Ze, Zbi and Zbe values follow a similar pattern of moderate to good correlation with the spirometric parameters (Table 2).
Another significant finding in this study was the inverse correlation of the Zpp with the FEV1 (%) and FEV1/FVC (Table 2, R=−0.53 and R=−0.50, respectively), which provides evidence of a relationship between the Zpp and COPD severity. The greatest correlations observed between the ΔZrs and the spirometric parameters were exhibited by the FEF25−75 and FEF/FVC (%) (Table 2, R=−0.33 and R=−0.32, respectively). Although weak, these associations were significant, suggesting that the ΔZrs is related to changes in the peripheral airways.
These findings provide additional evidence of a relationship between the within-breath analysis of respiratory impedance and airway obstruction in COPD. Overall, the results of the present study indicate a moderate to good relationship (Table 2). These findings may be at least partially attributed to the methodological differences between the tests; spontaneous respiration is used in the WbFOT analysis, whereas spirometry employs forced maneuvers. These differences may also be explained by the fact that the WbFOT analysis and spirometry values provide information on different characteristics of the respiratory system. Whereas the impedance module describes its resistive and elastic properties, the spirometric parameters are related to the airflow volumes and flows. Therefore, these methods provide complementary information on different characteristics of the respiratory system.
The WbFOT analysis was not sufficiently sensitive to detect the respiratory system changes in the smokers and the mild COPD patients (Figure 4). A previous ROC analysis showed similar AUC values in smokers 17. In the present study, the Zpp reached acceptable values for diagnostic use (AUC>0.80) in the moderate COPD patients. In the severe and very severe COPD patients, high diagnostic accuracy was observed (AUC>0.90). Under these conditions, the Zbe was most suitable for correctly identifying the effects of COPD in the severe patients, with a sensitivity of 100% and a specificity of 95%, whereas in the very severe patients, the Ze was most suitable, with sensitivity and specificity values of 95%. These promising results are consistent with physiological principles (1) and suggest that the Zrs observed in the different phases of the respiratory cycle may be useful in the diagnosis of COPD.
Our study has certain limitations. First, the presence of a shunt may induce changes in respiratory impedance that can mask the physiological and pathophysiological information 3. To minimize these errors, the participants were asked to firmly hold their cheeks during the tests.
Second the relationships between within-breath impedance and dyspnea, exercise capacity, and hyperinflation at the different stages of COPD are also of interest but were not addressed in the present study. This study specifically evaluated the respiratory impedance module. The evaluation of resistive and reactive parameters may complement such measurements, providing additional information associated with respiratory cycle dependence and the EFL, among other phenomena.
We believe that studies focusing on groups of COPD patients with clearly characterized pulmonary emphysema or chronic bronchitis could contribute to a more detailed understanding of the changes in within-breath impedance and should thus be performed in the future.
The correlations between the WbFOT and spirometric parameters were generally moderate to good, indicating that the FOT may provide new and complementary information on respiratory mechanics. Use of the clinical criteria as a gold standard may also demonstrate the added value of the WbFOT over conventional spirometry. We believe that this hypothesis should be evaluated in further studies.
In conclusion, COPD introduces higher respiratory cycle dependence that is proportional to airway obstruction. WbFOT parameters provide information in addition to that provided by spirometric measurements and can adequately detect alterations in the respiratory mechanics in moderate, severe and very severe COPD patients. These results are in close agreement with physiological principles 1–6, supporting and adding new information to the results reported previously 2,15,18,29 and suggesting that WbFOT parameters appear to be good quantitative indicators of COPD severity. Considering that the WbFOT is easy to perform, it may be a promising tool to facilitate the diagnosis of respiratory abnormalities in patients with COPD.
AUTHOR CONTRIBUTIONSSilva KK conducted the measurements for this study, analyzed the data, and drafted the manuscript. Faria AC analyzed the data and drafted the manuscript. Lopes AJ provided data and subject identification and participated in the data analysis process. Melo PL mentored Silva KK, organized the study and helped to draft the manuscript. All authors have read and approved this manuscript.
The authors would like to thank JG Noronha Filho, FR Ventromilli, C Lara, and all of the volunteers who participated in the present study. The Brazilian Council for Scientific and Technological Development (CNPq) and the Rio de Janeiro State Research Supporting Foundation (FAPERJ) supported this study.