The determination of thyroglobulin levels by immunoassay and imaging studies is subject to interference by antithyroglobulin antibodies in up to 30% of cases, suggesting a need to find alternative methods for the follow-up of a significant number of thyroid cancer patients.
ObjectivesAssess the sensitivity, specificity, and predictive values of thyroglobulin messenger RNA levels measured by quantitative Real Time-PCR (qRT-PCR) in the blood of patients followed for differentiated thyroid cancer.
MethodsThis is a prospective study of Tg-mRNA levels measured with qRT-PCR. A peripheral blood sample was taken in patients with excellent response (69) and with structural incomplete response to treatment (23). Results were analysed using the Unity Real-Time program and expressed as fg/μg RNA. A Receiver Operating Characteristic curve was constructed to assess Tg-mRNA cut-off values.
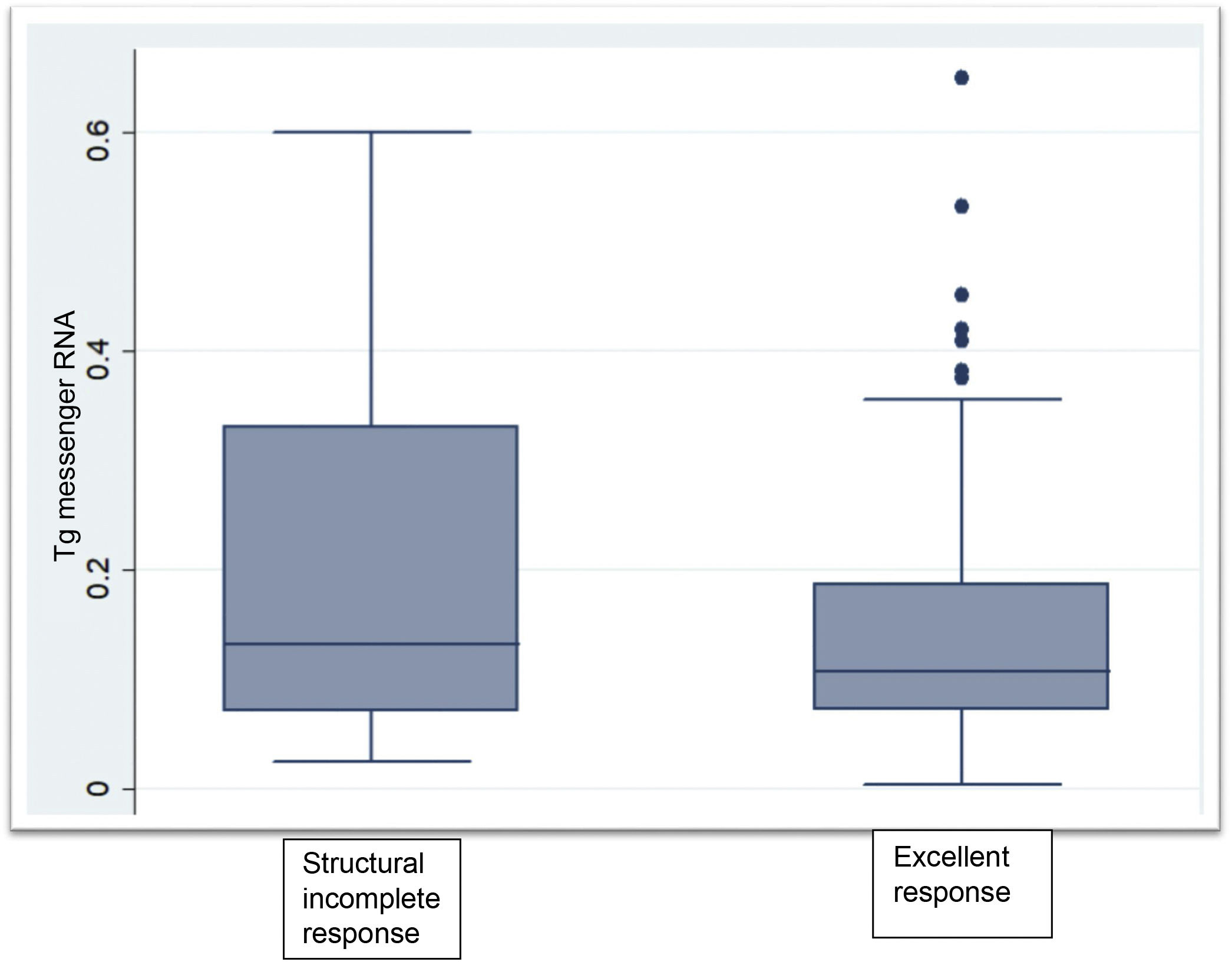
ResultsTg-mRNA levels were not significantly different between the group with excellent response [0.10 fg/μg RNA (0.08−0.17)] and the group with incomplete structural response [0.133 fg/μg RNA (0.07−0.33)] (P < .06). Test sensitivity was 69.6%, specificity was 59.4%, negative predictive value was 85.4% and positive predictive value 36.4%
ConclusionsOur experience shows that this technique could be useful as a rule-out test in selected cases, but its low sensitivity and specificity preclude its usefulness as a first-line test.
La determinación de tiroglobulina por métodos inmuno radiométricos presenta interferencia por anticuerpos antitiroglobulina hasta en el 30% de los casos, sugiriendo la necesidad de encontrar métodos alternativos para el seguimiento de un número significativo de pacientes con cáncer de tiroides.
ObjetivosEvaluar la sensibilidad, especificidad y valores predictivos de los niveles de ARN mensajero de la tiroglobulina medidos con la técnica de PCR cuantitativa en tiempo real (qRT-PCR) en sangre periférica de pacientes en seguimiento por cáncer diferenciado de tiroides.
MétodosEstudio prospectivo de los niveles de ARNm de Tg (ARNm Tg) determinados por qRT-PCR cuantitativa. Se extrajo una muestra de sangre periférica en pacientes con respuesta excelente (69), y con respuesta estructural incompleta al tratamiento(23). Los resultados fueron analizados por el programa Unity Real-Time y expresados en fg/μg ARN. Se realizó una curva ROC para evaluar el punto de corte de niveles de ARNm Tg.
ResultadosLos niveles de ARNm Tg no fueron significativamente diferentes entre el grupo con respuesta excelente [0,10 fg/μg ARN (0,08–0,17)] y el grupo respuesta estructural incompleta [0,133 fg/μg ARN (0,07–0,33)] (P < .06). La sensibilidad fue 69.6%, la especificidad 59.4%, el valor predictivo negativo 85.4% y el valor predictivo positivo 36.4%.
ConclusionesNuestra experiencia muestra que esta técnica tendría utilidad como prueba de exclusión en casos seleccionados, pero sus características no permiten su utilización como una prueba de primera línea.
Thyroid cancer has shown a marked increase in global incidence over the past 20 years.1 According to data from the United States National Institutes of Health (NIH), it is the cancer with the fifth highest incidence in females, with an incidence of 23.1 per 100,000 population per year,2 representing a significant public health problem. This increase in incidence is tied to differentiated thyroid cancer, since the incidences of the other types of thyroid cancer, such as medullary thyroid cancer and anaplastic thyroid cancer, have remained stable. The Argentinian population has also seen this increased incidence of thyroid cancer.3,4
Follow-up of thyroid cancer, once initial treatment with surgery and radioiodine ablation in selected cases is completed, is based on neck ultrasound and measurement of serum thyroglobulin (Tg) levels. At present, under the follow-up paradigm suggested by the latest national and international recommendations, follow-up conforms to categories of risk of recurrence, which in turn determine subsequent monitoring and treatment behaviours. Determinations of Tg, with or without thyroid stimulating hormone (TSH), are an essential piece of this diagnostic/therapeutic algorithm.5,6
Tg is a 660-kDa glycoprotein synthesised by the rough endoplasmic reticulum of thyroid cells and its functions are iodine storage and thyroid hormone production.7 Part of the Tg stored in the colloid is internalised and follows an alternative pathway to lysosomal proteolysis that releases thyroid hormones from Tg for secretion. Through binding to megalin, this alternative pathway transports Tg to the basolateral membrane of thyroid cells; from there, it enters the general circulation.8 In patients who have undergone thyroidectomy for thyroid cancer, Tg measurement is used as a tumour marker, since its levels in that context are correlated to the mass of functioning pathological tissue.9 However, its excellent prognostic value is compromised in 20%–30% of patients with thyroglobulin antibodies (Tg Ab), as in, for example, patients with concomitant Hashimoto's thyroiditis. The presence of Tg Ab interferes with the measurement of Tg by immunometric methods such as immunoradiometric assay (IRMA), yielding falsely low or undetectable Tg levels.10 Therefore, the use of Tg levels measured by immunometric methods presents this limitation in the follow-up of a significant number of patients with thyroid cancer.
Our objective was to evaluate the detection and prognostic value of measuring Tg messenger ribonucleic acid (Tg mRNA) levels as an alternative to measuring Tg levels by immunometric methods in the follow-up of patients having undergone surgery for thyroid cancer.
Specific objectivesTo evaluate the clinical sensitivity and specificity of Tg mRNA levels measured by quantitative real-time polymerase chain reaction (qRT-PCR) testing in peripheral blood from patients in follow-up for differentiated thyroid cancer having undergone radioiodine ablation. An exploratory descriptive study of Tg mRNA levels in patients not having undergone radioiodine ablation with no structural disease on imaging or nuclear medicine studies.
MethodsLaboratory techniquesDetermination of Tg mRNABlood extraction: Blood extraction was performed by venipuncture. A total of 12 mL of blood were extracted in tubes with ethylenediaminetetraacetic acid (EDTA). Mononuclear cells were separated by gradient centrifugation with Ficoll-Paque Plus (GE Healthcare) according to the manufacturer's recommendations. The blood was diluted with an equal volume of normal saline, placed over 15 mL of Ficoll and centrifuged at 400G for 40 min; next, the layer corresponding to the mononuclear cells was separated. These cells were washed with normal saline and sedimented by centrifugation for RNA extraction.
mRNA extraction: mRNA extraction was performed using the RiboPure-Blood Kit (Invitrogen) according to the manufacturer's recommendations. Briefly, the cells obtained in the previous step were lysed by adding 800 μl of lysis solution and 50 μl of sodium acetate solution to the pellet and mixing with a vortex mixer to complete the lysis. For extraction, 500 μl of acid-phenol:chloroform were added and the mixture was incubated for 5 min at room temperature, then centrifuged; after that, the aqueous phase was recovered and 600 μl of 100% ethanol were added to it. Purification was done by passing the sample through the kit's purification columns, centrifuging for 10 s and washing the column with 700 μl of wash solutions.
Elution of the RNA from the column: The RNA was eluted with 50 μl of the elution solution, preheated to 75 °C.
Processing with DNase: 2.5 μl of 20X DNase buffer + 0.5 μl DNase I were added to the RNA and incubated for 30 min at 37 °C. Finally, 10 μl of DNase Inactivation Reagent were added and the mixture was mixed with a vortex mixer, incubated for 2 min at room temperature and centrifuged for 1 min at maximum speed; then, the supernatant was transferred into a new tube.
Reverse transcription: Reverse transcription was performed with the ImProm-II Reverse Transcription System reagent (Promega) using 1 μg of total RNA according to the manufacturer's recommendations.
Real-time PCR reaction: Absolute quantification was performed using a calibration curve prepared by amplification of Tg mRNA based on total RNA from thyroid tissue obtained from a surgical procedure. The PCR product was purified, quantified and analysed by sequencing. Each RT-PCR reaction was performed in duplicate, from 5 μl of complementary DNA (cDNA) using Mezcla Real (Biodynamics) with SYBR Green according to the manufacturer's recommendations. The LightCycler 96 real-time PCR system (Roche) was used. The following primers were used for Tg: Tg forward 1F: CTATCGACGGCTCCTTCTTG and Tg antisense 1R:nCCAGTTGCCACTCACCTCTC. These primers generate a fragment of 116 base pairs that comprise exons 40 and 41 of Tg cDNA.11 The results were analysed using the Unity Real Time software programme and expressed in fg/μg of RNA. RNA samples were processed without reverse transcription in the RT reaction to be used as controls for detecting contamination with DNA. No contamination was found.
Hormone measurementsTSH and Tg Ab were determined in plasma and Tg was determined in serum, without suspending treatment with thyroid hormone (unstimulated TSH). The samples were centrifuged and processed in automated equipment. Hormone levels were measured by chemiluminescence: TSH (ABBOTT Architect, reference range [RR] 0.35–4.94 µIU/mL), functional sensitivity (FS) 0.01 IU/mL, coefficient of variation (%CV): less than 10%; Tg Ab (ABBOTT Architect, RR 0.2–4.1 IU/mL), FS 0.31 IU/mL, %CV: less than 10%. Tg levels were measured with an Immulite 2000 system (Siemens). The RR for this Tg assay is: up to 2.0 ng/mL (athyreotic with elevated TSH) or 0.7–84.0 ng/mL (non-athyreotic). The FS of the assay is 0.5 ng/mL and the %CV is 10%.
Tg levels were classified for analysis into three categories: 1) levels less than or equal to 0.5 ng/mL; 2) levels greater than 0.5 and less than 1 ng/mL, and 3) levels greater than or equal to 1 ng/mL. Given that the sensitivity of our method for measuring Tg levels was lower than that used in the American Thyroid Association (ATA) classification, we did a second evaluation of laboratory results and imaging studies prospectively in 31 patients having undergone ablation in order to determine whether they were possible allocation errors, in particular possible cases incorrectly included in the group having undergone ablation with an excellent response. Antibodies were considered positive when their levels exceeded 4.1 IU/mL.
PatientsA search for patients of both sexes over 18 years of age with thyroid cancer was conducted in pathology registries, and physicians from the Endocrinology Department of the Hospital Italiano de Buenos Aires and the Instituto de Oncología Ángel H. Roffo with patients in follow-up for thyroid cancer were asked to invite their patients to participate. The inclusion criteria were: having undergone total thyroidectomy at least six months earlier, having had at least one full evaluation of their disease stage after surgery and having complete follow-up data so that they could be appropriately categorised. Levels of risk of postoperative recurrence were determined according to a modified version of the risks of recurrence proposed in the latest ATA guidelines6 (Appendix B, Supplementary data).
To establish the groups to be compared, patients were classified by treatment response accorded to modified ATA criteria.6 1) Excellent response: Tg less than or equal to 0.5 ng/mL and negative imaging studies. This group also included patients with no evidence of structural disease with positive Tg Ab who had been in follow-up long enough to rule out persistent disease. 2) Structural incomplete response: disease detected on imaging studies and confirmed by pathology where applicable, regardless of Tg levels. 3) Biochemical incomplete response: patients with Tg levels greater than or equal to 1 ng/mL or with Tg Ab levels on an upward curve, but without structural disease on imaging studies. 4) Indeterminate response: patients with Tg levels greater than 0.5 ng/mL but less than 1 ng/mL or nonspecific findings on imaging studies.
The analysis included only patients who had received an ablative or therapeutic dose with radioiodine in the postoperative period, and at the time of recruitment had an excellent response or structural incomplete response, such that the predictive accuracy of Tg mRNA levels could be compared using reliable true positives (patients with structural incomplete response) and true negatives (patients with an excellent response). Data from patients with biochemical incomplete response or indeterminate response are presented in Appendix B (Supplementary data).
Those who met the inclusion criteria were later contacted by telephone by the principal investigator to arrange an appointment to explain the protocol to them and obtain their written informed consent. The sample was collected in that same appointment or a later one, depending on which was more convenient for the patient.
All patients recruited for evaluation of Tg mRNA levels as a parameter for diagnosis and follow-up had undergone radioiodine ablation (primary endpoint). In addition, a patient group with an excellent response not having undergone radioiodine ablation (secondary endpoint) was evaluated separately.
The protocol was approved by the Hospital Italiano de Buenos Aires Independent Ethics Committee for Research Protocols (number 2828).
The procedures were performed in patients after they had granted their written informed consent.
Statistical analysisThis was a cross-sectional observational study. Continuous variables were expressed in terms of median and interquartile range (IQR) or range, depending on their distribution, while categorical variables were reported in terms of absolute number and percentage of total cases. To compare the characteristics of the different groups, the chi-squared and analysis of variance (ANOVA) statistical tests were used. Comparisons between medians for the groups having undergone ablation with an excellent response or structural incomplete response were made using the Mann–Whitney U test where applicable. A P value <.05 was considered statistically significant.
A receiver operating characteristic (ROC) curve was built, considering patients who had received radioiodine and were free from structural disease to be true negatives and considering patients who had received radioiodine and had confirmed structural disease to be true positives.
Statistical calculations were performed with the Stata software programme, version 13.0.
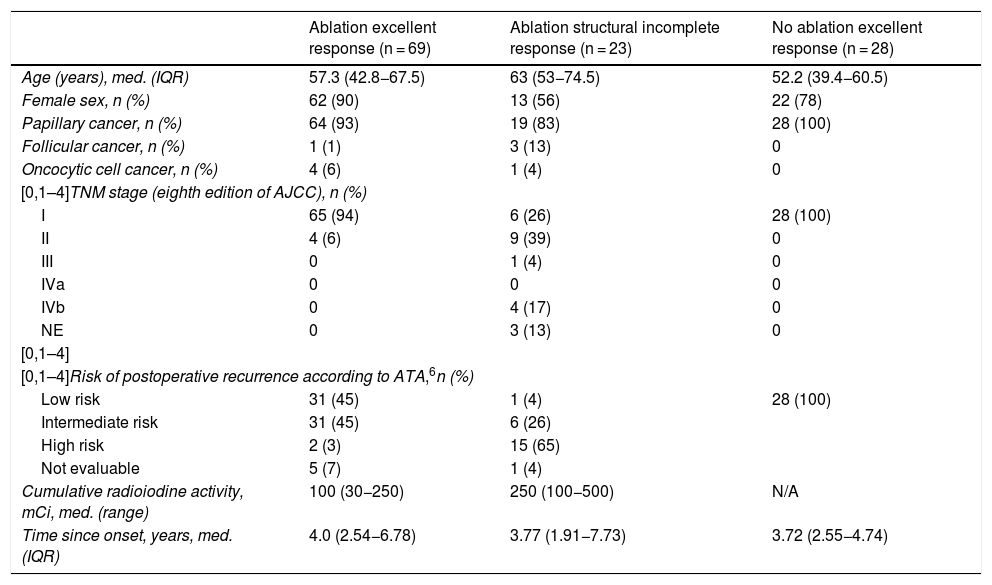
ResultsWe recruited 120 patients: 97 women and 23 men, with a mean age of 54.8 (43.3–66.6) years. All had undergone total thyroidectomy, and 92 had received radioiodine ablation. The predominant histological type was papillary thyroid cancer (n = 111, 92%), 50 (45%) of them with the classic variant, and 99 (82%) were in stage I according to the eighth edition of the American Joint Committee on Cancer (AJCC) cancer staging manual.12 Half of the patients (60; 50%) belonged to a group with a low risk of recurrence, and most showed an excellent response to treatment (97; 75.8%) according to ATA criteria (Table 1). Within the group that had received radioiodine ablation, 69 showed an excellent response to treatment. This group included four patients who had Tg levels of 0.5 ng/mL, as this value was at the limit of quantitation of the method, and showed no evidence of structural disease in follow-up (30, 45, 82 and 109 months of follow-up). These patients were included in the group of 31 patients who underwent another extraction with a median of 8.46 (7.53–11.06) months after the first evaluation and were found to have undetectable Tg levels in the second extraction. None of the patients who underwent additional evaluation of treatment response had to change groups due to results inconsistent with their allocation in the study. Twenty-three patients had structural incomplete response; 16 of them had distant metastases. Cumulative radioiodine activity was significantly higher in the group with structural incomplete response (P < .001). Four of the patients with distant metastatic disease were receiving treatment with tyrosine kinase inhibitors (one with sorafenib and three with lenvatinib) at the time of the extraction. The group with an excellent response not having undergone ablation included 28 patients.
Characteristics of patients by disease stage.
| Ablation excellent response (n = 69) | Ablation structural incomplete response (n = 23) | No ablation excellent response (n = 28) | |
|---|---|---|---|
| Age (years), med. (IQR) | 57.3 (42.8−67.5) | 63 (53−74.5) | 52.2 (39.4−60.5) |
| Female sex, n (%) | 62 (90) | 13 (56) | 22 (78) |
| Papillary cancer, n (%) | 64 (93) | 19 (83) | 28 (100) |
| Follicular cancer, n (%) | 1 (1) | 3 (13) | 0 |
| Oncocytic cell cancer, n (%) | 4 (6) | 1 (4) | 0 |
| [0,1–4]TNM stage (eighth edition of AJCC), n (%) | |||
| I | 65 (94) | 6 (26) | 28 (100) |
| II | 4 (6) | 9 (39) | 0 |
| III | 0 | 1 (4) | 0 |
| IVa | 0 | 0 | 0 |
| IVb | 0 | 4 (17) | 0 |
| NE | 0 | 3 (13) | 0 |
| [0,1–4] | |||
| [0,1–4]Risk of postoperative recurrence according to ATA,6n (%) | |||
| Low risk | 31 (45) | 1 (4) | 28 (100) |
| Intermediate risk | 31 (45) | 6 (26) | |
| High risk | 2 (3) | 15 (65) | |
| Not evaluable | 5 (7) | 1 (4) | |
| Cumulative radioiodine activity, mCi, med. (range) | 100 (30−250) | 250 (100−500) | N/A |
| Time since onset, years, med. (IQR) | 4.0 (2.54−6.78) | 3.77 (1.91−7.73) | 3.72 (2.55−4.74) |
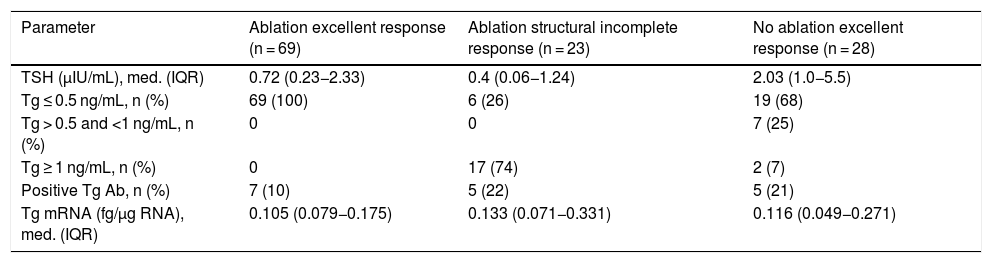
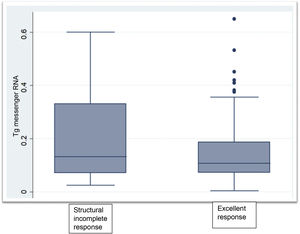
Clinical chemistry characteristics are shown in Table 2. TSH levels in the group not having undergone ablation were significantly higher than in the groups having undergone ablation (P < .002). The proportion of patients with Tg levels less than or equal to 0.5 ng/mL was significantly higher in the disease-free group having undergone ablation (P < .001). Two patients within the group not having undergone ablation had Tg levels greater than 1 ng/mL but less than 2 ng/mL. Tg mRNA levels did not significantly differ between the group with no evidence of disease and the group with persistent structural disease (P < .06). The group that had not received radioiodine ablation showed similar levels to the groups that had (Fig. 1).
Clinical chemistry characteristics of patients by disease stage.
| Parameter | Ablation excellent response (n = 69) | Ablation structural incomplete response (n = 23) | No ablation excellent response (n = 28) |
|---|---|---|---|
| TSH (μIU/mL), med. (IQR) | 0.72 (0.23−2.33) | 0.4 (0.06−1.24) | 2.03 (1.0−5.5) |
| Tg ≤ 0.5 ng/mL, n (%) | 69 (100) | 6 (26) | 19 (68) |
| Tg > 0.5 and <1 ng/mL, n (%) | 0 | 0 | 7 (25) |
| Tg ≥ 1 ng/mL, n (%) | 0 | 17 (74) | 2 (7) |
| Positive Tg Ab, n (%) | 7 (10) | 5 (22) | 5 (21) |
| Tg mRNA (fg/μg RNA), med. (IQR) | 0.105 (0.079−0.175) | 0.133 (0.071−0.331) | 0.116 (0.049−0.271) |
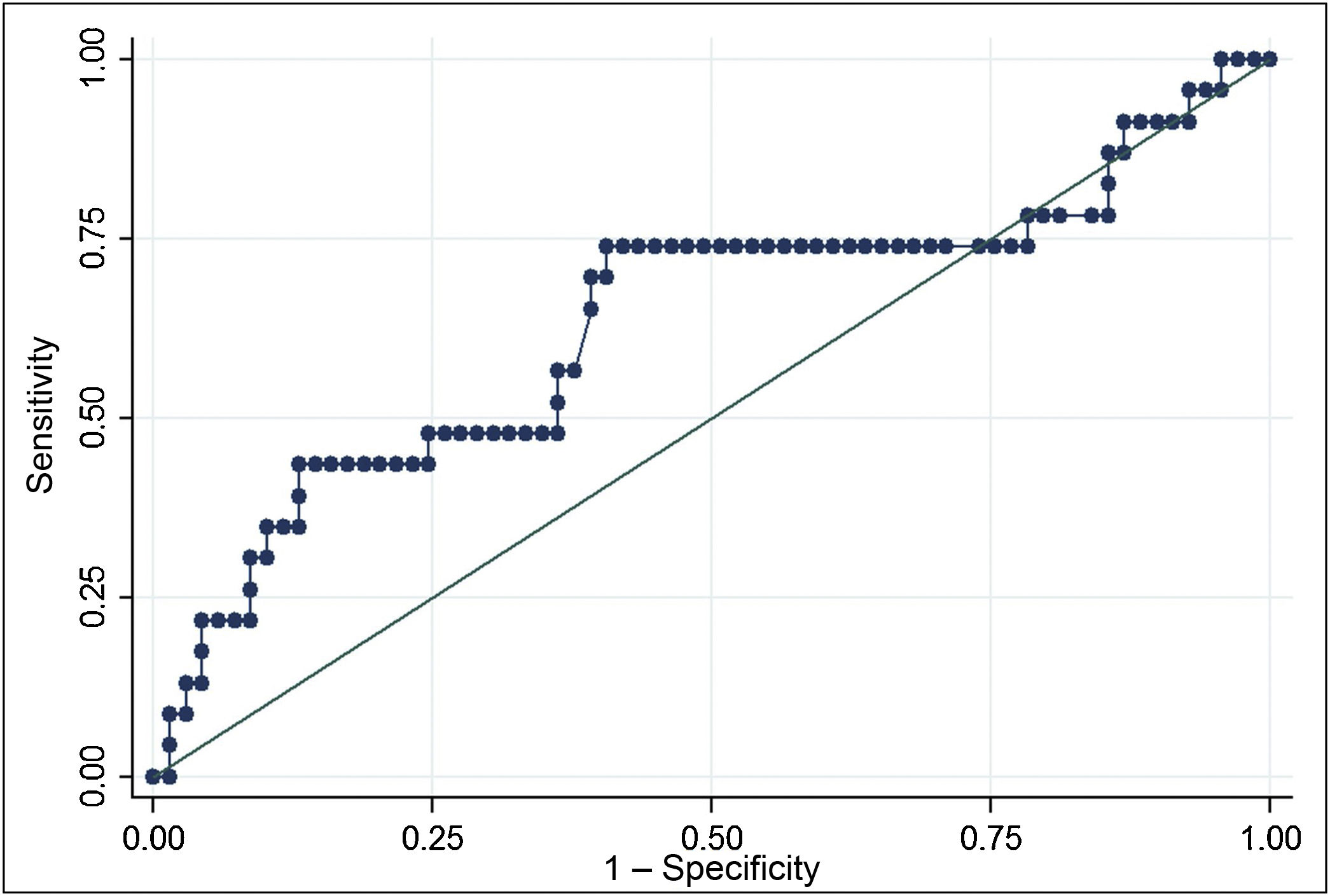
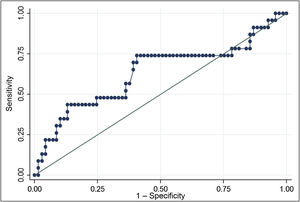
The ROC curve for Tg mRNA levels yielded an area under the curve of 0.6320 (0.48−0.78). The analysis demonstrated that Tg mRNA levels greater than 0.116 fg/μg Tg mRNA were associated with a higher risk of persistent structural disease (OR 3.35; 95% CI 1.22–9.19), with a sensitivity of 69.6% and a specificity of 59.4%. In a population with a prevalence of cases with persistent structural disease of 25%, positive predictive value was 36.4% and negative predictive value was 85.4% (Fig. 2).
DiscussionOur study evaluated the sensitivity, specificity and predictive values of Tg mRNA levels measured by qRT-PCR for the first time in a local population of patients with differentiated thyroid cancer. Our experience showed that this technique would be of limited use in the follow-up of patients with differentiated thyroid cancer, since Tg mRNA levels were similar in patients with an excellent response to treatment and in patients with confirmed structural incomplete response, with low sensitivity and specificity for detecting persistent structural disease. However, its high negative predictive value of 85% suggests that it could be a useful rule-out test in selected cases.
Routine follow-up of patients with thyroid carcinoma involves measurement of blood Tg levels by immunometric methods and neck ultrasound,6 less commonly, a whole-body radioiodine scan is also included. A disadvantage of this strategy is that Tg measurement has methodological interferences, including Tg Ab.9 The most commonly used immunometric methods yield values that are too low for disease staging in the presence of Tg Ab.10 For this reason, antibodies at stable or decreasing levels place a patient in an indeterminate stage of their disease. This happens in around 8%–29% of patients, and 15%–20% of these patients develop structural disease in the course of their follow-up.13 Other methods for measuring Tg without interference due to antibodies, such as mass spectrometry, have been tested, but have not demonstrated advantages in this context.14,15
The hypothesis of the usefulness of Tg mRNA levels in the follow-up of patients with thyroid cancer is based on detection of circulating thyroid cells in patients without thyroid disease, patients with benign disease and patients with thyroid cancer.16 The first assays, using qualitative RT-PCR, showed suitable sensitivity but unfortunately low specificity.17 Quantitative measurement techniques were then developed, but yielded disparate results. Ringel et al. reported a sensitivity of 84% and a specificity of 62% when they amplified a fragment of 87 base pairs between base pairs 262 and 348 of Tg cDNA.18 A subsequent publication from the same group reported better accuracy (a higher proportion of patients correctly identified as disease free-or having persistent disease) compared to Tg levels measured by immunoassay (84% versus 75%, respectively).19 These results could not be replicated by other authors. Takano et al. found similar Tg mRNA levels in patients with thyroid cancer without metastases and with confirmed metastases; in their experience, serum Tg levels measured by immunometric methods were a better disease marker than Tg mRNA levels.20 Span et al., using the same primers as Ringel et al., also found no differences in Tg mRNA levels between patients with and without metastatic thyroid carcinoma, whether they were stratified by radioiodine uptake on scintigraphy or by serum Tg levels measured by immunoradiometric methods.21 All these studies used radioiodine uptake on scintigraphy or serum Tg levels measured with assays that are now considered not to be very sensitive (FS > 1 ng/mL) as a gold standard for identifying persistent disease. Tg Ab were also evaluated with methods that were not very sensitive (haemagglutination), casting doubt on the capacity to identify Tg levels that were too low due to interference on the part of antibodies in the group of patients considered “disease-free”. Elisei et al., by contrast, used serum Tg levels measured by ultrasensitive methods (corresponding FSs 0.9 ng/mL and 0.03 ng/mL to confirm the absence of disease) and radioiodine uptake on scintigraphy to stratify patients. With this methodology, sensitivity was 82.3%, specificity 24.2%, positive predictive value was 65.6% and negative predictive value was 47.3%.22 In 2010, Boldarine et al., using a novel strategy, generated and validated a pair of primers that did not include alternative splicing sites. In their study, they achieved a Tg mRNA cut-off point that distinguished between the disease-free population and the population with persistent structural disease with a sensitivity of 94.7% and a specificity of 95.8%.18 However, in our experience, the same pair of primers did not achieve a cut-off point exceeding 90% sensitivity and/or specificity. Many authors have agreed that differences between the different assays can be explained by technical differences.17,18 One of the most significant differences between our experience and that of Boldarine et al. was the comparatively lower mass of Tg mRNA in our assay. This could be attributed to a lower rate of recovery of circulating tumour cells. Hypothetically, uneven loss across groups could contribute to decreases in sensitivity and specificity.
Our study had a population of patients with thyroid cancer who were well identified and staged according to current disease-staging concepts.6 Almost all previously published studies based patient stratification on Tg levels with lower sensitivities than currently recommended (radioimmunoassays [RIAs] or immunoradiometric assays with sensitivities of 1 ng/mL), and many used a whole-body scan with 2−5 mCi of radioiodine as a gold standard for determining the presence of distant metastases.18–22
Thus, patients considered disease-free might actually have low-volume persistent structural disease that can only be detected by higher-sensitivity methods such as neck ultrasound, which is the method of choice at present.6 Detection of Tg mRNA in patients considered disease-free (38%–75.8%)18,22 appears to support this hypothesis, with the resulting loss of specificity. Our patients were categorised according to the results of their neck ultrasound performed by expert operators, other diagnostic methods when indicated and Tg levels with an FS of 0.5 ng/mL, yielding a more suitably identified disease-free population. Despite this, 40% of our disease-free patients in the group having undergone ablation had circulating Tg mRNA levels above the cut-off point. It is possible that the lower FS of our Tg assay resulted in the inclusion of patients who would have been considered to have indeterminate response with a more sensitive method due to having Tg levels greater than 0.2 ng/mL, 15%–20% of whom progress to structural incomplete response. Low-volume structural persistence that could not be detected by imaging methods in the excellent response group might have contributed to the relatively high Tg mRNA levels in this group. However, when we performed repeat determinations of Tg levels and imaging studies in a group of patients, including patients with detectable Tg levels in the excellent response group, the results were consistent and there was no need to change the allocation groups. Alternative hypotheses include illegitimate transcription in non-thyroid cells in the blood, interfering with detection of Tg mRNA originating in circulating thyroid cells.22
To date, no method for clinical use enables evaluation of Tg levels in the presence of Tg Ab. Measurement of alternative parameters such as TSH receptor mRNA23 and BRAF V600E has been tested,24 and more recently a great deal of interest in measuring microRNA has arisen.25,26 As yet, none of these methods has proven to be a good replacement for Tg levels measured by immunoradiometric methods. Could measurement of Tg mRNA play a role in the follow-up of patients with Tg Ab? Under these circumstances, Tg mRNA levels could be useful as a rule-out test, considering their high negative predictive value and the fact that the prevalence of recurrences in the population with indeterminate response is around 20%, which is very close to the 25% found in our study. Nevertheless, this hypothesis should be confirmed in a prospective study of patients with indeterminate response since our study was not designed for this purpose.
ConclusionsMeasurement of Tg mRNA levels by qRT-PCR lacks sufficient sensitivity and specificity to be used routinely as a replacement for Tg levels measured by immunoradiometric methods. However, it could have a place as a rule-out test in the follow-up of specific patients, such as those with positive Tg Ab. This hypothesis should be confirmed in a prospective study.
FundingThis study was funded by the Fondo para la Investigación Científica y Tecnológica [Fund for Research in Science and Technology] (FONCYT) of the Agencia Nacional de Promoción Científica y Tecnológica [Argentinian National Agency for the Promotion of Science and Technology]. Ministry of Science, Technology and Productive Innovation of the Republic of Argentina.
Proyectos de Investigación Científica y Tecnológica Orientados [Science- and Technology-Oriented Research Projects] (2016) grant ID number: 011. (ID: PICTO 2016-011).
The agency was involved in the initial evaluation and follow-up of the project only.
Conflicts of interestNone of the authors has any conflicts of interest in relation to this research study.
Please cite this article as: Russo-Picasso MF, Serra MP, Viale ML, Puga MC, Terrasa S, Kozak AE, et al. Evaluación de la utilidad de medir los niveles de ARN mensajero de tiroglobulina por PCR cuantitativa en tiempo real para el seguimiento de pacientes con cáncer diferenciado de tiroides. Endocrinol Diabetes Nutr. 2021;68:680–688.