Pituitary carcinomas are rare and difficult to manage despite intensive and multimodal treatment. Temozolomide, an oral chemotherapy agent approved for the treatment of malignant gliomas, has been shown to be effective in aggressive pituitary tumors and pituitary carcinomas.
We report the case of a 66-year-old male with a prolactin-secreting pituitary carcinoma and his response to treatment with temozolomide. At the age of 58 years, after attending another center complaining of headache, the patient was diagnosed with a non-functioning pituitary macroadenoma, 22×19mm in size. One month later, he attended the emergency room of our hospital reporting disabling headache, diplopia due to right sixth cranial nerve palsy, and bitemporal hemianopsia. MRI revealed an increase in adenoma size to 33×26mm. The tumor had eroded the floor of the sella turcica, contacted with the optic chiasm on the right side and with the proximal tract of the right optic nerve, and invaded the cavernous sinus on the same side.
There was also a spontaneous hypersignal area in T1 that did not change after gadolinium injection and appeared to correspond to methemoglobin. Pituitary apoplexy was suspected, and the patient was therefore admitted to the neurosurgery department and transsphenoidal surgery was performed, which allowed for partial tumor resection. At the histological study, the surgical specimen was found to be positive for prolactin at immunohistochemistry and to have a Ki 67 of 15% and weak positivity for p53. After surgery, serial laboratory tests performed during his hospital stay showed prolactin levels > 500ng/mL (4–15.2), which led to treatment with cabergoline being started.
One month later, the patient was readmitted to hospital for headache. MRI performed at that time showed a residual tumor 33×33mm in size, with significant occupation of the suprasellar cistern and compression of the optic chiasm and brainstem. Because of this, analgesia was adjusted and the cabergoline dose was increased, but doses > 2mg weekly could not be reached due to gastrointestinal intolerance.
Three months after cabergoline was started, the patient was readmitted for disabling headache and underwent partial resection with a frontotemporal approach, followed by 3D conformal external radiotherapy, using a photon beam linear accelerator, until a total dose of 5040cGY was reached. One month after radiotherapy, due to poor tolerability of cabergoline, the patient was switched to bromocriptine, with a good biochemical and radiographic response (Table 1). One year after radiotherapy, prolactin levels started to increase, and the size of the pituitary lesion was found to be increased by approximately 20%. Due to transaminase elevation, a whole body computed tomography was performed, which showed metastases in the liver, lymph nodes, lung, and vertebras. A biopsy of liver metastases showed cell proliferation of moderate size, with oval or polygonal cells having round nuclei and dense eosinophil cytoplasm and expressing immunoreactivity to prolactin, chromogranin, and P53, but with no CK20 or CK7 expression in hepatocytes. These findings supported the diagnosis of a malignant prolactinoma.
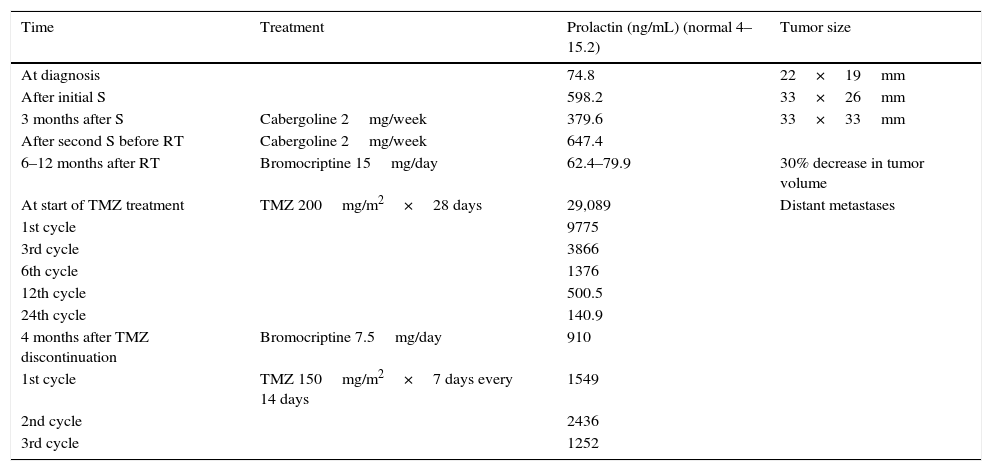
Change over time in biochemical and radiographic variables with the different treatment modalities.
| Time | Treatment | Prolactin (ng/mL) (normal 4–15.2) | Tumor size |
|---|---|---|---|
| At diagnosis | 74.8 | 22×19mm | |
| After initial S | 598.2 | 33×26mm | |
| 3 months after S | Cabergoline 2mg/week | 379.6 | 33×33mm |
| After second S before RT | Cabergoline 2mg/week | 647.4 | |
| 6–12 months after RT | Bromocriptine 15mg/day | 62.4–79.9 | 30% decrease in tumor volume |
| At start of TMZ treatment | TMZ 200mg/m2×28 days | 29,089 | Distant metastases |
| 1st cycle | 9775 | ||
| 3rd cycle | 3866 | ||
| 6th cycle | 1376 | ||
| 12th cycle | 500.5 | ||
| 24th cycle | 140.9 | ||
| 4 months after TMZ discontinuation | Bromocriptine 7.5mg/day | 910 | |
| 1st cycle | TMZ 150mg/m2×7 days every 14 days | 1549 | |
| 2nd cycle | 2436 | ||
| 3rd cycle | 1252 |
S: surgery; RT: radiotherapy; TMZ: temozolomide.
Treatment was started with temozolomide 200mg/m2 for 5 days every 28 days and radiotherapy to vertebral lesions. Prolactin levels decreased from the first cycle, and the radiographic stability of the primary lesion and metastases was confirmed after the third cycle. Treatment was maintained for 24 cycles, causing no significant side effects except hyperglycemia, which required insulin therapy.
Four months after temozolomide discontinuation, prolactin levels started to gradually increase. Ten months after the last cycle was completed, treatment was restarted with more intense temozolomide doses (150mg/m2 for 7 days every 14 days) due to metastatic progression. The decrease in prolactin levels was less marked, and the progression of metastases and the growth of the primary tumor were noted. The patient's overall condition impaired from ECOG 1 to ECOG 4. It was therefore decided to discontinue treatment and to ensure the greatest possible well-being with palliative measures. The patient died three months after the discontinuation of temozolomide treatment.
The identification of adenomas with more aggressive or metastasizing potential represents a considerable challenge. Some biomarkers, especially Ki 67, may be helpful,1,2 but are not always available or may not be consistent. The clinical course is therefore the most helpful tool, despite the fact that latency from the diagnosis of a pituitary tumor and the diagnosis of its metastases ranges from 4 months to 18 years. Survival after the detection of metastases is usually less than 4 years. Conventional chemotherapy is of little value in this clinical context, and temozolomide appears to be a useful alternative, although the dosage to be used in tumors of this type has not been clearly established.3–5 It appears reasonable to start treatment with 200mg/m2 for 5 days every 28 days, and if a response is seen after the third cycle, the dosage should be maintained for at least 12 cycles, regardless of the status of the enzyme methylguanine-DNA methyltransferase (MGMT) and the genes encoding for the proteins that repair DNA replication errors (MLH1, MSH2, MSH6). The highest response rates, up to 50% in a first cycle and even greater when6 radiotherapy is also given, are seen in prolactinomas and corticotropinomass. Tumor progression is often seen after treatment discontinuation, with a progression-free period of 8–18 months. A second treatment often results in tumor progression despite a good response after the initial administration.7,8 The high recurrence rate after a second treatment confirms the need for additional, more effective therapies. It could be used with capecitabine, for example.6,9 It should be determined whether the latency period is longer in patients given concomitant radiotherapy. In most cases, including the one reported here, temozolomide had been started after all other measures had failed.
Until clinical guidelines for the management of pituitary carcinomas are available, our experience suggests that a second temozolomide cycle is not effective. Thus, it appears more adequate to look for alternative therapies once tumor progression occurs.
Please cite this article as: Bilbao I, Egaña N, García C, Olaizola I. Fracaso de un segundo ciclo de tratamiento con temozolamida en un paciente afectado de carcinoma hipofisario productor de prolactina. Endocrinol Diabetes Nutr. 2017;64:564–566.