Deficient or excess iodine intake has effects on human health. Assessment of the prevalence and risk factors in children can therefore support effective prevention or treatment.
MethodA cross-sectional probabilistic study in 631 children aged 5 to 12 years in whom iodine levels were measured in urine and salt samples. Results are reported by type of location and indigenous condition. Association of these variables to urinary iodine levels was assessed using a binary logistic regression.
ResultsMedian urinary iodine level was 278.4μg/L (177.3–360.9, IQR), 13.2% of children assessed had iodine levels <100μg/L, and 41.8% had values ≥300μg/L. Indigenous schoolchildren had the greatest risk of urinary iodine levels <100μg/L (β=2.29, CI 1.1–4.6, p<0.05), while children from urban and non-indigenous localities had a high risk of iodine levels >300μg/L (β=2.2, CI 1.3–3.9, p<0.01, and β=3.8, CI 2.2–6.5, p<0.01 respectively). Median iodine level in salt was 35.9ppm (29.1–42.4 IQR), and there were no differences in iodine levels in salt by type of location or ethnicity.
ConclusionsIn Mexican schoolchildren living in rural and indigenous areas, iodine levels <100μg/L have not been eradicated. There was high prevalence of urinary iodine levels ≥300μg/L in schoolchildren living in urban areas which was associated to high sodium intake from processed food.
El déficit o consumo excesivo de yodo en humanos tiene efectos en la salud, por lo que determinar las prevalencias y los factores de riesgo en los niños ayuda a reorientar las acciones de prevención o tratamiento.
MétodoEstudio transversal probabilístico realizado en 631 infantes de 5-12 años a quienes se les cuantificó yodo en una muestra de orina y de sal; se presentan resultados por tipo de localidad y condición de indigenismo, la asociación de estas variables con intervalos de yoduria fue evaluada con una regresión logística binaria.
ResultadosLa mediana de la concentración de yodo en orina (yoduria) fue de 278,4 μg/L (177,3-360,9, RIC), el 13,2% de los infantes evaluados presentaron yodurias <100μg/L y el 41,8% registraron cifras ≥300μg/L. Los escolares indígenas fueron los que registraron mayor riesgo para yodurias <100μg/L (β= 2,29, 1,1-4,6 IC, p<0,05) e infantes de localidades urbanas y no indígenas riesgos altos para cifras ≥300μg/L (β= 2,2, 1,3-3,9 IC, p< 0,01 y β= 3,8, 2,2-6,5 IC, p<0,01; respectivamente). La mediana de la concentración de yodo en sal fue de 35,9ppm (29,1-42,4, RIC), no se presentaron diferencias en las concentraciones de yodo en sal por tipo de localidad o etnicidad.
ConclusionesEn escolares mexicanos de zonas rurales e indígenas las cifras de yodurias <100μg/L no han sido erradicadas. Se registraron altas prevalencias de cifras elevadas de yoduria en escolares que habitan en localidades urbanas, esto se asoció al consumo de sodio de alimentos procesados.
Iodine deficiency disorders (IDDs) occur from the fetal stage to adulthood; pregnant women with iodine deficiency may experience hypothyroxinemia, and the fetus may suffer congenital anomalies, cretinism or endemic neurocognitive insufficiency.1,2 Infants and adolescents with iodine deficiency may experience subclinical hypothyroidism or goiter, altering their neurological development and intelligence quotient (IQ),3,4 while toxic nodular goiter and hyperthyroidism have been reported in adults.5
In order to prevent IDDs, the World Health Organization (WHO) and other agencies recommended universal salt iodization in 1993. In Mexico there have been regulations in this regard in place since 1963, with salt for human and animal consumption having to be iodinated with 30±10mg/kg of iodine ion (NOM-040-SSA1-1993). Since the year 2000 there have been reports on the nutritional iodine status in the Mexican population. In the 1999 National Nutrition Survey6 8.4%, and in a multinational study7 4.3% of all Mexican children aged 5–12 years had urinary iodine <100μg/l. In October 2016, the elimination of iodine deficiency disorders was declared in Latin America. According to this declaration, in Mexico the median urinary iodine concentration was 297μg/l.8
The WHO recommendation for median urinary iodine levels in a given population is 100–199μg/l, this figure being indicative of adequate iodine intake. Figures ranging from 200–299μg/l in turn indicate consumption above requirements.9 Excessive iodine intake may lead to hyperthyroidism and autoimmune thyroid disease in susceptible adults,10,11 and thyroid volume increments in children.12 Advances in Mexico regarding the eradicating of IDDs require the monitoring of salt iodine levels, as well as ongoing assessment of nutritional iodine status in schoolchildren and pregnant women. The present study examines urinary iodine excretion and iodine in salt in a sample of schoolchildren from rural and urban areas of the state of Hidalgo (Mexico), with a view to assessing the risks associated with low or high concentrations.
Material and methodsStudy population and sampleA descriptive, cross-sectional, analytical study was conducted on a representative sample of children aged 5–12 years from the state of Hidalgo, located in central Mexico (19° 36′–21° 24″ north latitude, 97° 58–99° 54 west latitude), approximately 92km north of Mexico City. According to the 2010 national population census, Hidalgo has 2,858,359 inhabitants, of which 15% are children aged 5–12 years. The children were enrolled in 100 primary schools: general, private, indigenous or community courses of the National Education Development Council in the 2010–2011 school cycle. For the purposes of this study, the statistical power of the sample was calculated by considering that the rural population in Hidalgo represents 52%, with a prevalence of ioduria (<100μg/l) of 15.4% in the rural setting and 5.5% in the urban area.13 With two-tailed contrasting, a significance level of 0.05 and a power of 80%, the required sample size was found to be 137 schoolchildren from the rural setting and 147 schoolchildren from the urban area, in order to identify statistically significant differences. The children were randomly selected from a database of 171,199 records, representing 48% of all schoolchildren enrolled in the 2010–2011 school cycle.
The localities were classified as urban (populations with >2500 inhabitants) or rural (<2500 inhabitants) according to the criteria established by the Instituto Nacional de Estadistica y Geografía (INEGI).14 By interviewing the mother and through direct confirmation by listening to the child, we determined whether the children were speakers or non-speakers of an indigenous language, and the language they spoke was recorded. An informed consent was signed by the parents or guardians of the schoolchildren, and assent was requested from each of the latter. The project was approved by the Ethics Committee of the Institute of Health Sciences of the Autonomous University of the State of Hidalgo (Mexico).
Anthropometric indicatorsThe body weight of the children was measured with a precision of 0.10kg using a scale (SECA®, model 813), and height was recorded with a portable stadiometer with a precision of 1mm (SECA®, model 206). The measurements were taken by trained and standardized staff. We calculated the body mass index (BMI=weight kg/m2), the Z-score of the BMI for age (BMIz) and the Z-score of height for age (HAz), using the WHO growth reference (2007).15
Iodine in urine and saltIn each home, a sample of 30g of salt was obtained from the salt being used at that time to prepare food or to add to cooked dishes. The salt samples were placed in airtight containers and stored under dry conditions until analysis. Iodine concentration in salt was determined using a colorimetric method.16 Specifically, 500mg of salt were dissolved in 25ml of distilled water; 3ml of this solution was mixed with 500μl of HCl 1.0M and 500μl of starch iodine solution (0.42M of KI, 15.4mM of starch, pH 9.0). We used KIO3 to plot the standard curve (20–200μg/dl), and the absorbance of the reaction was measured at a wavelength of 580nm using a spectrophotometer (Agilent model 8453). The samples were measured in duplicate to assess the coefficient of variation (CV), which was required to be no greater than 10%. A salt iodine concentration of <20ppm was considered to be deficient, 20–40ppm optimum, and >40ppm excessive.17
A casual urine sample of 10ml was simultaneously collected from the schoolchildren in a sterile bottle (Monovette® urine tubes). All samples were stored at −35°C until analysis.18 We mixed 250μl of urine with 1ml of ammonium persulfate 1.0M; the mixture was incubated for 60min at 100° C, and then we added 2.5ml of arsenous acid (50.5mM of AsO3 and 0.43M of NaCl dissolved in H2SO4 and distilled water 1:5) and 300μl of ammonium cerium sulfate (75.9mM, dissolved in H2SO4 3.5N); the KIO3 was used to plot the standard curve (20–400μg/l), and the absorbance of the reaction was measured at a wavelength of 405nm using a spectrophotometer (Agilent model 8453). The samples were measured in duplicate to assess the coefficient of variation (CV), which was required to be no greater than 5%. We calculated the percentage of children with ioduria <100μg/l, and distributed them into the following ioduria intervals: 50–99μg/l, 20–49μg/l, <20μg/l, 100–199μg/l, 200–299μg/l and ≥300μg/l.9
Dietary sodium assessmentDiet was assessed using a 7-day consumption frequency questionnaire for the week before the interview, and administered to the mother or person responsible for the feeding of the child. The questionnaire comprised 108 foods or preparations, organized into 14 groups: (1) dairy products, (2) beverages, (3) cereals and derivatives, (4) corn meal preparations, (5) fruit, (6) vegetables, (7) tubers, (8) broth-type preparations, (9) eggs/meat/cold meats, (10) legumes, (11) sugars, sweets and candies, (12) fried food and appetizers, (13) fast food, and (14) fats and oils. To determine the quantity consumed, kitchen utensils were used with standardized measures (grams and milliliters), as well as a manual containing photographs of food in portions, taking as reference the Mexican Equivalent Foods System.19 The quantity and nutrients consumed per day were calculated with the average weekly intake, using the food composition table of the Mexican Equivalent Foods System and the nutritional labels of processed foods. We identified the children that complied with the recommended sodium intake by age groups of 4–8 years (≤1200mg/day) and 9–13 years (≤1500mg/day).20
Statistical analysisData are reported as the mean±standard deviation (SD) or as the median and interquartile range (IQR). The Kolmogorov–Smirnov test was used to assess normal data distribution. The chi-squared test was used to analyze categorical variables; the Mood median test was used to compare the differences between two groups; and two-way analysis of variance (ANOVA) was used in the case of more than two groups with post test Bonferroni correction or Dunnett's T3 test. Binary logistic regression analyses were made to associate the ioduria intervals (dependent variable) to the type of locality and the indigenous condition of the children (independent variables). Age and gender were integrated into the analysis as covariables. Statistical significance was considered for p<0.05. Data analysis was performed using the SPSS version 21 statistical package (SPSS Corp., Chicago, IL, USA).
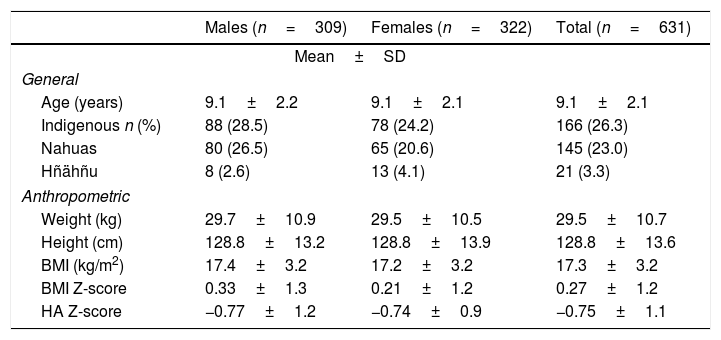
ResultsA total of 631 salt and urine samples were evaluated from children in public (89.4%) and private schools (10.6%) in the state of Hidalgo (Mexico). Seventy-seven percent of the samples were from schoolchildren in rural localities and 73.7% corresponded to non-indigenous schoolchildren. The mean age was 9.1±2.1 years (range 5–13 years), with a gender distribution of 49% males and 51% females. There were no differences in mean Z-scores corresponding to the indicators BMI and HAz between genders. In the case of the BMI the means were positive in all evaluated subjects (0.27±1.2), while in the case of HAz they proved negative (−0.75±1.1) (Table 1). A total of 166 schoolchildren were indigenous (26.3%) and spoke the Nahuatl or Hñähñu languages (Table 1).
General and anthropometric characteristics of Mexican schoolchildren according to gender.
| Males (n=309) | Females (n=322) | Total (n=631) | |
|---|---|---|---|
| Mean±SD | |||
| General | |||
| Age (years) | 9.1±2.2 | 9.1±2.1 | 9.1±2.1 |
| Indigenous n (%) | 88 (28.5) | 78 (24.2) | 166 (26.3) |
| Nahuas | 80 (26.5) | 65 (20.6) | 145 (23.0) |
| Hñähñu | 8 (2.6) | 13 (4.1) | 21 (3.3) |
| Anthropometric | |||
| Weight (kg) | 29.7±10.9 | 29.5±10.5 | 29.5±10.7 |
| Height (cm) | 128.8±13.2 | 128.8±13.9 | 128.8±13.6 |
| BMI (kg/m2) | 17.4±3.2 | 17.2±3.2 | 17.3±3.2 |
| BMI Z-score | 0.33±1.3 | 0.21±1.2 | 0.27±1.2 |
| HA Z-score | −0.77±1.2 | −0.74±0.9 | −0.75±1.1 |
Indigenous: indigenous language speaker; BMI: body mass index; HA: height-for-age indicator.
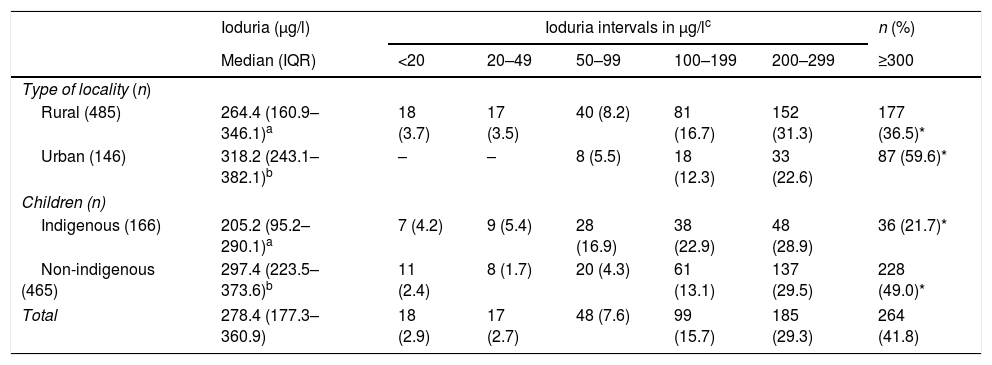
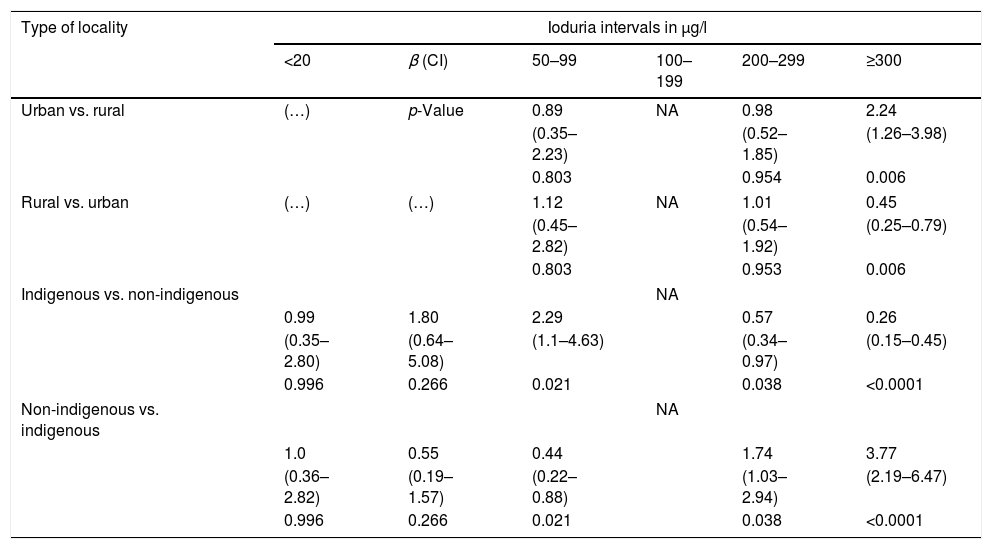
The median iodine concentrations in urine and salt were 278.4μg/l (IQR: 177.3–360.9) and 35.9ppm (IQR: 29.1–42.4), respectively. A total of 13.2% of the evaluated children presented <100μg/l of iodine in urine, with most cases falling within the interval 50–99μg/l (57.5% of the children). Indigenous schoolchildren had a higher prevalence of ioduria <100μg/l (26.5%) than non-indigenous schoolchildren (8.4%) (Table 2), with a logistic regression coefficient (β) for ioduria <100μg/l of 2.29 (CI 1.1–4.63; p=0.021) as compared to non-indigenous schoolchildren (Table 3). Urinary iodine in the ranges of 20–49μg/l and <20μg/l were only observed in schoolchildren from rural locations.
Iodine levels in urine in Mexican schoolchildren according to type of locality and indigenous status.
| Ioduria (μg/l) | Ioduria intervals in μg/lc | n (%) | |||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | <20 | 20–49 | 50–99 | 100–199 | 200–299 | ≥300 | |
| Type of locality (n) | |||||||
| Rural (485) | 264.4 (160.9–346.1)a | 18 (3.7) | 17 (3.5) | 40 (8.2) | 81 (16.7) | 152 (31.3) | 177 (36.5)* |
| Urban (146) | 318.2 (243.1–382.1)b | – | – | 8 (5.5) | 18 (12.3) | 33 (22.6) | 87 (59.6)* |
| Children (n) | |||||||
| Indigenous (166) | 205.2 (95.2–290.1)a | 7 (4.2) | 9 (5.4) | 28 (16.9) | 38 (22.9) | 48 (28.9) | 36 (21.7)* |
| Non-indigenous (465) | 297.4 (223.5–373.6)b | 11 (2.4) | 8 (1.7) | 20 (4.3) | 61 (13.1) | 137 (29.5) | 228 (49.0)* |
| Total | 278.4 (177.3–360.9) | 18 (2.9) | 17 (2.7) | 48 (7.6) | 99 (15.7) | 185 (29.3) | 264 (41.8) |
IQR: interquartile range.
Stratification of the ioduria intervals was consistent with urinary iodine concentration (WHO, UNICEF, ICCIDD). Different letters in the same column indicate p<0.05 for the Mood medians test. Asterisks on the same line represent p<0.001 (*) for the percentages of iodine intake according to the chi-squared test.
Risk of low or high ioduria levels in Mexican schoolchildren according to type of locality and indigenous status.
| Type of locality | Ioduria intervals in μg/l | |||||
|---|---|---|---|---|---|---|
| <20 | β (CI) | 50–99 | 100–199 | 200–299 | ≥300 | |
| Urban vs. rural | (…) | p-Value | 0.89 | NA | 0.98 | 2.24 |
| (0.35–2.23) | (0.52–1.85) | (1.26–3.98) | ||||
| 0.803 | 0.954 | 0.006 | ||||
| Rural vs. urban | (…) | (…) | 1.12 | NA | 1.01 | 0.45 |
| (0.45–2.82) | (0.54–1.92) | (0.25–0.79) | ||||
| 0.803 | 0.953 | 0.006 | ||||
| Indigenous vs. non-indigenous | NA | |||||
| 0.99 | 1.80 | 2.29 | 0.57 | 0.26 | ||
| (0.35–2.80) | (0.64–5.08) | (1.1–4.63) | (0.34–0.97) | (0.15–0.45) | ||
| 0.996 | 0.266 | 0.021 | 0.038 | <0.0001 | ||
| Non-indigenous vs. indigenous | NA | |||||
| 1.0 | 0.55 | 0.44 | 1.74 | 3.77 | ||
| (0.36–2.82) | (0.19–1.57) | (0.22–0.88) | (1.03–2.94) | (2.19–6.47) | ||
| 0.996 | 0.266 | 0.021 | 0.038 | <0.0001 | ||
β: logistic regression coefficient; CI: confidence interval; IS: insufficient sample. In the lines without data (…), the value of β was not calculated, since no children were recorded in this category. The ioduria range of 100–199μg/l constituted the reference group (without risk); the calculation of β is therefore not applicable (NA).
In turn, 41.8% of the urine samples presented concentrations of ≥300μg/l, with differences between the type of locality and indigenous status (Table 2). The β value for these urinary iodine levels in schoolchildren from urban localities was 2.24 (CI 1.23–3.98; p=0.006) and 3.77 (CI 2.19–6.47; p<0.001) for non-indigenous schoolchildren (Table 3).
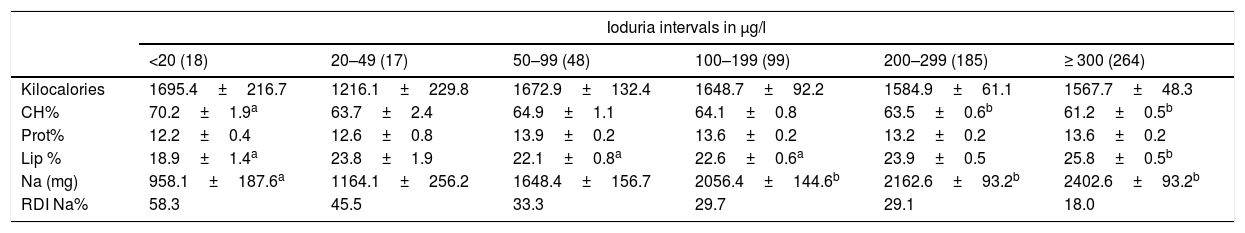
The energy intake among schoolchildren with different urinary iodine ranges was similar, with differences being seen between the proportions of energy-supplying macronutrients, mainly carbohydrates and lipids. The mean sodium levels were significantly higher in schoolchildren with urinary iodine values of >100μg/l, as compared to those with urinary iodine levels of <20μg/l (Table 4). Children with ioduria ≥300μg/l consumed 2.5 times more sodium than children with <20μg/l, and only 18% of these children complied with the recommended daily sodium intake.
Daily energy intake and consumption of energy-supplying foods and sodium in Mexican schoolchildren according to ioduria category.
| Ioduria intervals in μg/l | ||||||
|---|---|---|---|---|---|---|
| <20 (18) | 20–49 (17) | 50–99 (48) | 100–199 (99) | 200–299 (185) | ≥ 300 (264) | |
| Kilocalories | 1695.4±216.7 | 1216.1±229.8 | 1672.9±132.4 | 1648.7±92.2 | 1584.9±61.1 | 1567.7±48.3 |
| CH% | 70.2±1.9a | 63.7±2.4 | 64.9±1.1 | 64.1±0.8 | 63.5±0.6b | 61.2±0.5b |
| Prot% | 12.2±0.4 | 12.6±0.8 | 13.9±0.2 | 13.6±0.2 | 13.2±0.2 | 13.6±0.2 |
| Lip % | 18.9±1.4a | 23.8±1.9 | 22.1±0.8a | 22.6±0.6a | 23.9±0.5 | 25.8±0.5b |
| Na (mg) | 958.1±187.6a | 1164.1±256.2 | 1648.4±156.7 | 2056.4±144.6b | 2162.6±93.2b | 2402.6±93.2b |
| RDI Na% | 58.3 | 45.5 | 33.3 | 29.7 | 29.1 | 18.0 |
CH%: percentage energy as carbohydrates; RDI%: percentage of recommended daily intake for sodium (Na) in children aged 4–8 years (≤1200mg/day) and 9–13 years (≤1500mg/day); Lip%: percentage energy as lipids; Prot%: percentage energy as proteins.
Intervals were stratified according to urinary iodine concentration. Different letters on the same line indicate p<0.05 according to two-way ANOVA with post test Bonferroni correction or Dunnett's T3 test. Continuous values represent the mean±standard deviation.
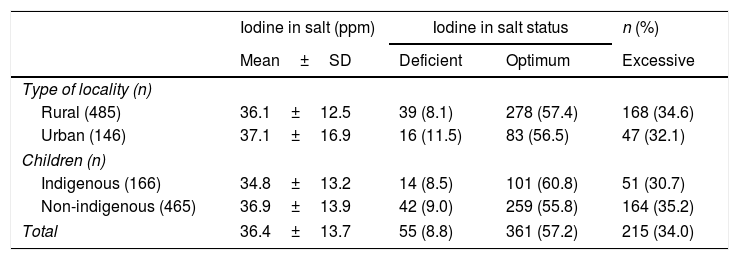
Poor iodination of table salt was recorded in 8.8% of the samples (<20ppm). Most samples revealed adequate iodination values, and over 30% of these had values of >40ppm, thus defining them as containing excess iodine. In turn, 10.6% of the salt samples had iodine levels >50ppm. There were no differences according to the type of locality and indigenous status (Table 5).
Iodine levels and iodization status in salt samples in Mexican children according to type of locality and indigenous status.
| Iodine in salt (ppm) | Iodine in salt status | n (%) | ||
|---|---|---|---|---|
| Mean±SD | Deficient | Optimum | Excessive | |
| Type of locality (n) | ||||
| Rural (485) | 36.1±12.5 | 39 (8.1) | 278 (57.4) | 168 (34.6) |
| Urban (146) | 37.1±16.9 | 16 (11.5) | 83 (56.5) | 47 (32.1) |
| Children (n) | ||||
| Indigenous (166) | 34.8±13.2 | 14 (8.5) | 101 (60.8) | 51 (30.7) |
| Non-indigenous (465) | 36.9±13.9 | 42 (9.0) | 259 (55.8) | 164 (35.2) |
| Total | 36.4±13.7 | 55 (8.8) | 361 (57.2) | 215 (34.0) |
Iodine intake according to urinary iodine concentration. Deficient = < 20ppm; optimum = 20–40ppm and excessive = > 40ppm of iodized salt.
The mean negative Z-scores of height for age (HAz) indicate that the study population was characterized by insufficient linear growth, a condition that has been associated with deficiencies of nutrients such as iodine, iron, zinc and vitamin A,21 as well as with a deficient intake of energy or proteins of high biological value.22 One third of the schoolchildren evaluated were indigenous language speakers. This condition has also been associated with delayed growth23 and multiple nutrient deficiencies.24
Although iodine deficiency was declared eradicated in Mexico, the indigenous schoolchildren and those living in rural settings showed the highest prevalence of ioduria <100μg/l (26.5% and 15.4%, respectively). In contrast to the median ioduria level (278.4μg/l), 13.2% of all the children evaluated presented values of <100μg/l, and mainly in the range of 50–99μg/l. In the population of the state of Hidalgo, this proportion historically has been high, reaching 32.8% of the population according to research conducted in 1991.25
Reports in Mexican children aged 5–12 years found the prevalence of ioduria <100μg/l to be 8.4% in 199926 and 4.3% in 2000.7 However, a study from 2014 in the adult population of Mexico City found 12.7% of the subjects to present urinary iodine levels of <100μg/l. The median urinary iodine level of this population was 221.0μg/l.27 The data reported herein indicate that the rural and indigenous population of schoolchildren in Hidalgo (Mexico) continues to be characterized by a proportion of >15% of the children with ioduria <100μg/l. This is higher than the national average, but similar to the figures reported in Mexico City, despite the fact that the median urinary iodine levels indicate sufficient iodine intake in the population of Hidalgo and Mexico City.
In the present study, over 40% of the schoolchildren presented ioduria ≥300μg/l, which is in clear contrast to the 4.3% reported in the year 2000.7 Excess iodine consumption may alter thyroid function, despite the fact that many individuals tolerate high iodine intakes.28 Excessive consumption causes hypothyroidism due to the Wolff-Chaikoff effect and hyperthyroidism – also known as the Jod-Basedow effect - in susceptible subjects.29
Non-indigenous schoolchildren and children from urban populations (49.0% and 59.6%, respectively) recorded the greatest number of individuals with high urinary iodine levels. This may be explained in part by the high consumption of processed foods combined with the intake of iodized salt in urban populations,30 in contrast to rural localities where the consumption of processed foods is lower. In some marginalized Mexican populations low iodine concentrations have been observed to be related to humidity and to the high temperatures to which the consumed salt is exposed.13 This circumstance was not seen in the population of our study, since the mean salt iodine levels were similar between urban and rural locations.
Thirty-four percent of the salt consumed by the children in their homes registered >40ppm, while 10.6% registered >50ppm. In 2003, in Mexico, 5.7% of the samples were in this latter concentration range,7 which indicates that the number of salt samples with high iodization values has increased by 5 percentage points in 15 years. This makes it necessary to monitor not only nutritional iodine status in the Mexican population, but also the evaluation of salt produced by the salt industry in Mexico.
In the present study, a mean sodium intake of 958.1g was recorded among the schoolchildren with lower ioduria values (<20μg/l), representing a salt intake of close to 2.4g/day, in contrast to the group with ≥300μg/l, where the figure was 6.1g/day. These differences in salt intake can be explained by the processed foods eaten by the children. The median iodine content in salt was 35.9ppm. It can therefore be assumed that schoolchildren with a salt intake of 2.4g/day obtain 86.2μg of iodine, while a salt intake of 6.1g/day provides 219μg of iodine. The former situation is insufficient to cover the recommended daily iodine intake (120μg/day).20 Moreover, from these estimates we must subtract the iodine losses that occur during the cooking of food (20%), a circumstance that further affects the availability of iodine consumed by these children.
ConclusionsIt can be concluded that iodine nutritional status is polarized in Mexican schoolchildren, with indigenous children having a two-fold higher risk of ioduria <100μg/l. By contrast, schoolchildren from urban areas and non-indigenous populations have up to a three-fold higher risk of ioduria ≥300μg/l. This situation requires a prompt response through compliance with salt iodization standards, the education of the population regarding the consumption of processed foods, the monitoring of nutritional iodine status in vulnerable populations, and the implementation of public health policies in Mexico.
Financial supportThis project was funded by the Secretary of Health of the State of Hidalgo (Mexico).
Authorship/collaboratorsAll four authors contributed to the design of the present study. M. Galván performed the data analysis and development of the methodology. T. Suárez-Diéguez designed and conducted the experimental analysis of the urine and salt samples, and also performed the analysis of the results. L. Fernández-Cortes designed the instruments and analyzed the diet results. G. López-Rodríguez coordinated all the project work, drafted the introduction and interpreted the results. All the authors collaborated in drafting the discussion and in the review and final approval of the manuscript.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Galván M, Fernández Cortés TL, Suárez-Diéguez T, López-Rodríguez G. Estado nutricional de yodo en niños escolares mexicanos de zonas urbanas y rurales. Endocrinol Diabetes Nutr. 2020;67:228–234.