Type 2 diabetes mellitus (T2DM) constitutes a global pandemic with increasing prevalence, with cardiovascular disease being a common comorbidity, accounting for half of deaths among the affected subject.1 Sodium-glucose co-transporter 2 (SGLT-2) inhibitors have gained significant ground in the management of T2DM over recent years, due to their multiple pleiotropic effects, with emphasis on cardio- and reno-protection.2
Acute hyperglycemic crises, namely diabetic ketoacidosis (DKA) and hyperglycemic hyperosmolar state (HHS), are diabetic emergencies that require prompt recognition and therapeutic intervention, since they are associated with significant morbidity and mortality, even in the developed world, while they add a substantial economic burden to national health systems.3 In addition, a major adverse cardiovascular event may be identified as the trigger for the development of such a complication, leading to increased in-hospital morbidity and mortality and greater length of hospitalization.4,5 Treatment of acute hyperglycemic crises may be accompanied with significant complications, such as electrolyte disorders and iatrogenic pulmonary edema, which can be fatal for patients with established atherosclerotic cardiovascular disease.5 A former meta-analysis of the first three hallmark cardiovascular outcome trials with SGLT-2 inhibitors demonstrated that this novel class increases by almost two times the risk for DKA,6 which is mainly explained by the fact that it enhances ketogenesis, an action that may be implicated in the observed cardioprotection.7 However, another, previous meta-analysis of randomized controlled trials in patients with T2DM failed to prove a significant association between the use of SGLT-2 inhibitors and DKA.8
Initial concerns expressed by the Food and Drug Administration (FDA) authorities regarding the risk for DKA with SGLT-2 inhibitors were confirmed in a former FDA Adverse Event Reporting System (FAERS) retrospective analysis.9 Recent real-world data are rather confirming regarding the true risk for DKA.10–13 In their multicenter cohort study, Douros and colleagues demonstrated that patients initiated on SGLT-2 inhibitors experience a 1.85-fold increase in the risk for DKA compared to those initiated on dipeptidyl-peptidase-4 (DPP-4) inhibitors.10 In another propensity score matched cohort study from the Scandinavian Registry it was shown that patients on SGLT-2 inhibitors feature an increase in the risk for DKA by 114% compared to those administered glucagon-like peptide-1 receptor agonists (GLP-1RAs).11 Fralick et al. have also shown in their retrospective analysis that patients with T2DM administered SGLT-2 inhibitors feature a 1.5-fold increase in the risk for DKA compared to those prescribed DPP-4 inhibitors.12 Of note, a most recent nationwide retrospective analysis from Denmark by Laursen and colleagues documented that patients on SGLT-2 inhibitors do not feature increased risk for DKA compared to those initiated on GLP-1RAs based regimens, however, patients on SGLT-2 inhibitor and insulin combination have a significantly higher risk for DKA (almost 4.5-fold increase) compared to those on GLP-1RA and insulin combination.13
Therefore, we sought to update the results of former meta-analyses, based on the recently published cardiovascular and renal outcome trials, and to assess the impact of SGLT-2 inhibitors on HHS, as well. The rationale behind assessing their impact on the risk for HHS is based on the osmotic diuresis and volume depletion that they can induce, which can lead to dehydration and hyperosmosis, especially in the elderly population. The motive for the assessment of HHS as an efficacy endpoint is that there is only one case report available in the literature describing an atypical presentation of mixed DKA and HHS in an elderly patient.14
We searched PubMed from its inception to 27 November 2020, to identify relevant cardiovascular and renal outcome trials with SGLT-2 inhibitors. Two independent reviewers (D.P. and C.P.) extracted the data from the eligible reports, by using a pilot tested, data extraction form. We set as primary safety outcome the incidence of DKA and as secondary safety outcome the incidence of HHS.
As we assessed only dichotomous variables, differences were calculated with the use of risk ratio (RR), with 95% confidence interval (CI), after implementation of the Mantel–Haenszel (M–H) random effects formula. Statistical heterogeneity among studies was assessed by using I2 statistics. Heterogeneity was considered to be low if I2 was between 0% and 25%, moderate if I2 was between 25% and 50%, or high if I2 was greater than 75%.15 All analyses were performed at the 0.05 significance level, while they were undertaken with RevMan 5.3 software.
The reviewers assessed the quality of the included RCTs, by using the Revised Cochrane risk of bias tool for randomized trials (RoB 2.0) for the primary efficacy outcome. Discrepancies between them were solved by discussion, consensus or arbitration by a third senior reviewer (M.D.).
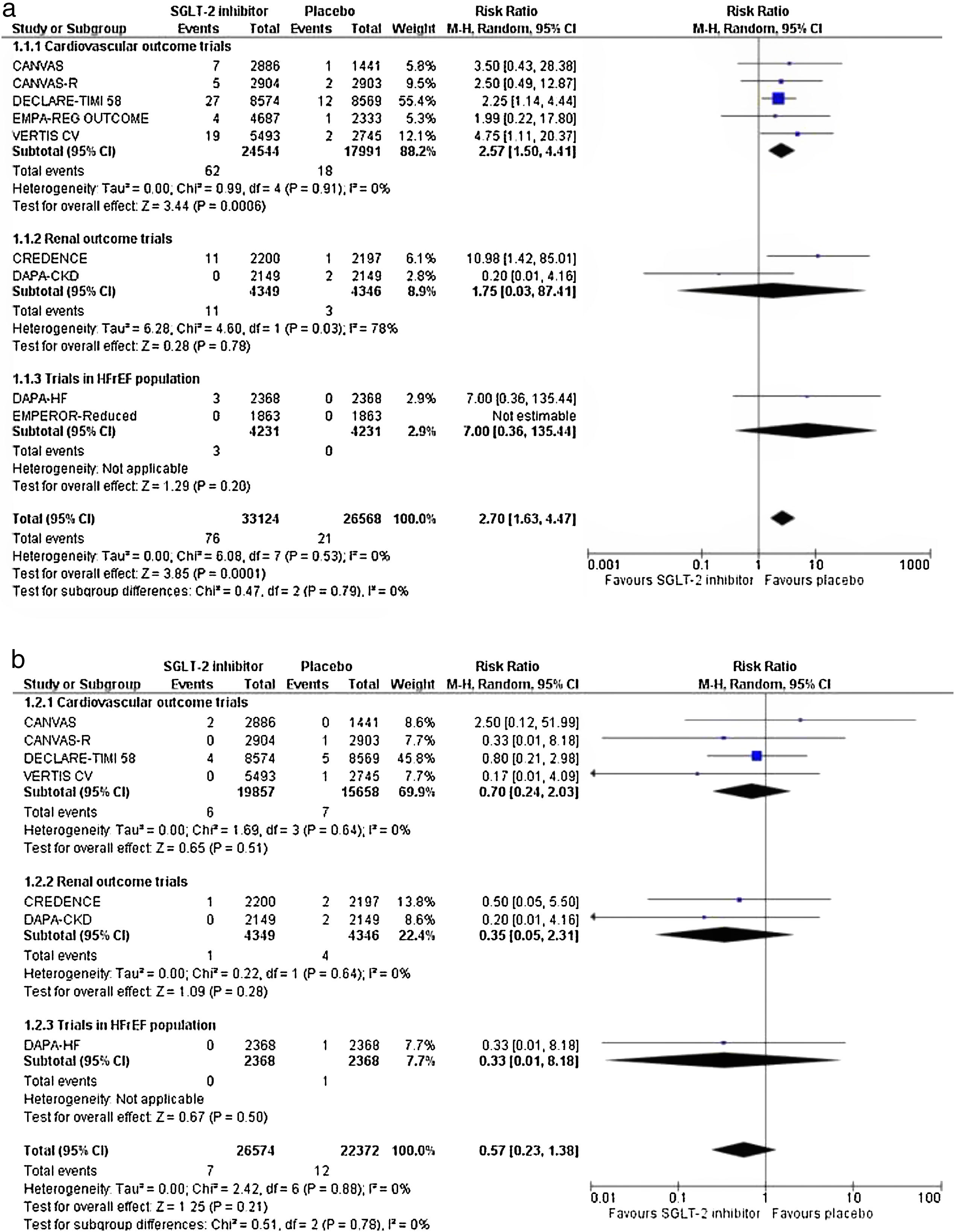
We pooled data from 9 trials in a total of 70,269 enrolled participants assigned either to SGLT-2 inhibitor treatment or placebo. Risk of bias is considered as low across all selected trials.
SGLT-2 inhibitors increase the risk for DKA by 1.47 times compared to placebo (RR=2.47, 95% CI; 1.66–3.66, I2=0%), as shown in Fig. 1a. Results were primarily driven by cardiovascular outcome trials, while no significant risk was documented in the renal outcome trials and in the trials enrolling patients with heart failure with reduced ejection fraction. The latter finding might be partially explained by the fact that DAPA-CKD (67.5% with T2DM at baseline vs. 22.5% non-diabetic subjects), DAPA-HF (42% with T2DM at baseline vs. 58% non-diabetic participants) and EMPEROR Reduced (50% with T2DM at baseline vs. 50% non-diabetic participants) trials enrolled a high proportion of non-diabetic subjects, in whom ketogenesis is rather beneficial, as speculated previously.7
However, their use is not associated with a significant increase in the risk for HHS (RR=0.57, 95% CI; 0.24–2.03, I2=0%), as shown in Fig. 1b. Unfortunately, trialists of the Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk (SCORED) and the EMPEROR Reduced trials did not report data regarding the incidence of HHS across the two study groups.
The present meta-analysis confirms that SGLT-2 inhibitors precipitate to the development of DKA, which might boost morbidity and mortality in the very high-risk patients enrolled in the cardiovascular and renal outcome trials. Despite the fact that mild, persistent hyperketonemia can confer significant cardiovascular benefit in patients with T2DM, their use should be made with extra caution, especially in the context of an acute illness, in these high-risk patients.
Authors’ contributionsDimitrios Patoulias and Christodoulos Papadopoulos conceived the study and drafted the first version. All authors critically revised the manuscript and approved its final version.