Autoimmune thyroid diseases are reported in no treated hepatitis C virus (HCV) infection. The standard interferon alpha (IFNα) treatment is associated with an increase of thyroid damage and dysfunction. The present cohort prospective study compared thyroid function and autoimmunity in HCV patients’ monoinfected and coinfected HCV–HIV at baseline, during and after IFNα therapy.
MethodsWe studied 790 HCV infected patients: G1 (monoinfected HCV: N=580) and G2 (HCV–HIV coinfected: N=210). They were evaluated for thyroid function and thyroid tiroperoxidase antibodies (TPOAb) at baseline and 235 patients (G1: 183; G2: 52) post IFNα therapy.
If thyroid dysfunction (TD) was diagnosed, they were reevaluated at 12 month after discontinuation to determine whether the TD was transitory or definitive.
ResultsNo difference was found in the prevalence of TD at baseline in G1 (7.6%) and G2 (9%). However, monoinfected patients showed a higher prevalence of TPOAb positivity with a women preponderance in this group. There was no difference in TD between both groups during IFNα therapy (G1 23.5% vs G2 19.2%). In G1 the autoimmune TD was higher than in G2 (67.4% vs 30%, p=0.02). Autoimmune TD during IFNα tended to evolve to definitive hypothyroidism and non-autoimmune TD recovered euthyroidism after IFNα discontinuation. The presence of positive TPOAb (RR 3.55) and female gender (RR 2.4) were associated with the development of TD with IFNα therapy.
ConclusionOur hypothesis is the importance of HCV in G1 and G2, combined with IFNα in triggering TD and TPOAb positivity, not described in other diseases’ applications.
El presente estudio prospectivo de cohorte evaluó y comparó la función y la autoinmunidad tiroidea en pacientes con virus de la hepatitis C (VHC) monoinfectados y coinfectados con VHC-virus de la inmunodeficiencia humana (VIH) al inicio, durante y después de la terapia estándar con interferón alfa (IFNα).
MétodosSe estudiaron 790 pacientes infectados por VHC: G1 (VHC monoinfectados: N=580) y G2 (coinfección por VHC-VIH: N=210). Se evaluó la función tiroidea y los anticuerpos anti-tiroperoxidasa (ATPO) al inicio del estudio, y a 235 pacientes tratados con IFNα (G1: 183; G2: 52). Si se diagnosticó disfunción tiroidea (DT), estos fueron reevaluados 12 meses posteriores al tratamiento para determinar si esta era transitoria o definitiva.
ResultadosNo se encontraron diferencias en la prevalencia de DT en forma basal G1 (7,6%) vs. G2 (9%). Los pacientes monoinfectados mostraron una mayor prevalencia de positividad de ATPO, siendo preponderante el sexo femenino en el G1. No hubo diferencias en la DT entre ambos grupos con IFNα (G1 23,5 vs. G2 19,2%). En G1, la DT autoinmune fue mayor que en G2 (67,4 vs. 30%; p=0,02). La DT autoinmune con IFNα tendió a evolucionar hacia un hipotiroidismo definitivo, mientras que la DT no autoinmune fue transitoria. Tuvieron mayor riesgo de DT durante el tratamiento los pacientes que presentaban ATPO positivos previos (RR: 3,55) y el sexo femenino (RR: 2,4).
ConclusionesPlanteamos como hipótesis la importancia del VHC en G1 y G2, combinado con IFNα en el desarrollo de la DT y positividad de los ATPO, no descripta en su uso en otras enfermedades.
Chronic hepatitis C (HCV) is a global health problem; 170 million people are chronically infected worldwide. In Latin America its prevalence is estimated to be between 1.0 and 2.3%, while in Argentina this percentage oscillates between 1.0 and 1.5%.1 Specifically, in the city of Buenos Aires, Argentina,2 there is an estimated 21% of HCV–HIV coinfection.
In addition to hepatic complications, chronic HCV has been suggested to cause extrahepatic disorders related to autoimmunity and malignancy. These include hematological disease, lymph proliferative disorders, renal disease and endocrine disease.
Autoimmune thyroid disease is reported in HCV infected patients and the standard IFNα in combination with ribavirin based treatment, is associated with an increase of the immune mediated thyroid damage. The mechanism of thyroid damage had been analyzed, by focusing on the balance between the environment (virus infection with potential cross-reaction) and the host (susceptibility genes with consistent immune response).3
Since 19924 there have been many articles published about the prevalence of thyroid autoantibodies in HCV infected patients, which mainly differed on autoantibodies methodology and ethnicity. The prevalence of TPOAb varied between 3.5 and 30.5%.5,6
It is important to remark, that there is a debate surrounded thyroid autoimmunity in HCV and coinfected HCV–HIV patients. By analyzing the literature, it can be observed that it depends on many factors: gender, HAART therapy, and disease’ outcome.7–10
Thyroid autoimmune or non-autoimmune dysfunction is associated with HCV infection due to IFNα plus ribavirin based treatment. The incidence has been reported to occur in 4.7% to 27.8% of cases.11 There are few reports who compared TD between HCV infected and coinfected HCV–HIV population.12
The aim of the present cohort clinical study was to evaluate prospectively thyroid function and autoimmunity in 790 patients HCV infected and coinfected with HIV between 1995 until 2014. There were 235 patients treated with IFNα analyzing them during the therapy. If TD was diagnosed they were tested 12 months after the therapy was discontinued. Eventually, both groups were compared and it was possible to determine the risk factors for thyroid function and autoimmunity in patients recruited from two Hepatology Sections (JM Ramos Mejia Hospital and Infectious Disease F Muñiz Hospital). They were clinically and laboratory evaluated by the same professionals in one Endocrinology Division (JM Ramos Mejia Hospital).
Materials and methodsSeven hundred ninety HCV infected subjects were enrolled consecutively from 1995 until 2014 at the Endocrinology Division, Hospital JM Ramos Mejia. They were sent from two Hepatology Units: Infectious Disease Hospital F Muñiz and Hospital JM Ramos Mejia, Buenos Aires, Argentina.
All patients underwent a clinical and laboratory evaluation including measurement of serum levels of TSH, free thyroxine (FT4) and thyroid tiroperoxidase antibodies (TPOAb). They were divided into two groups:
Group 1 (G1): 580 monoinfected HCV patients.
Group 2 (G2): 210 HCV–HIV coinfected patients.
If a thyroid dysfunction (TD) was diagnosed at baseline, the patient was treated to reach euthyroidism and to authorize the hepatologist the HCV treatment.
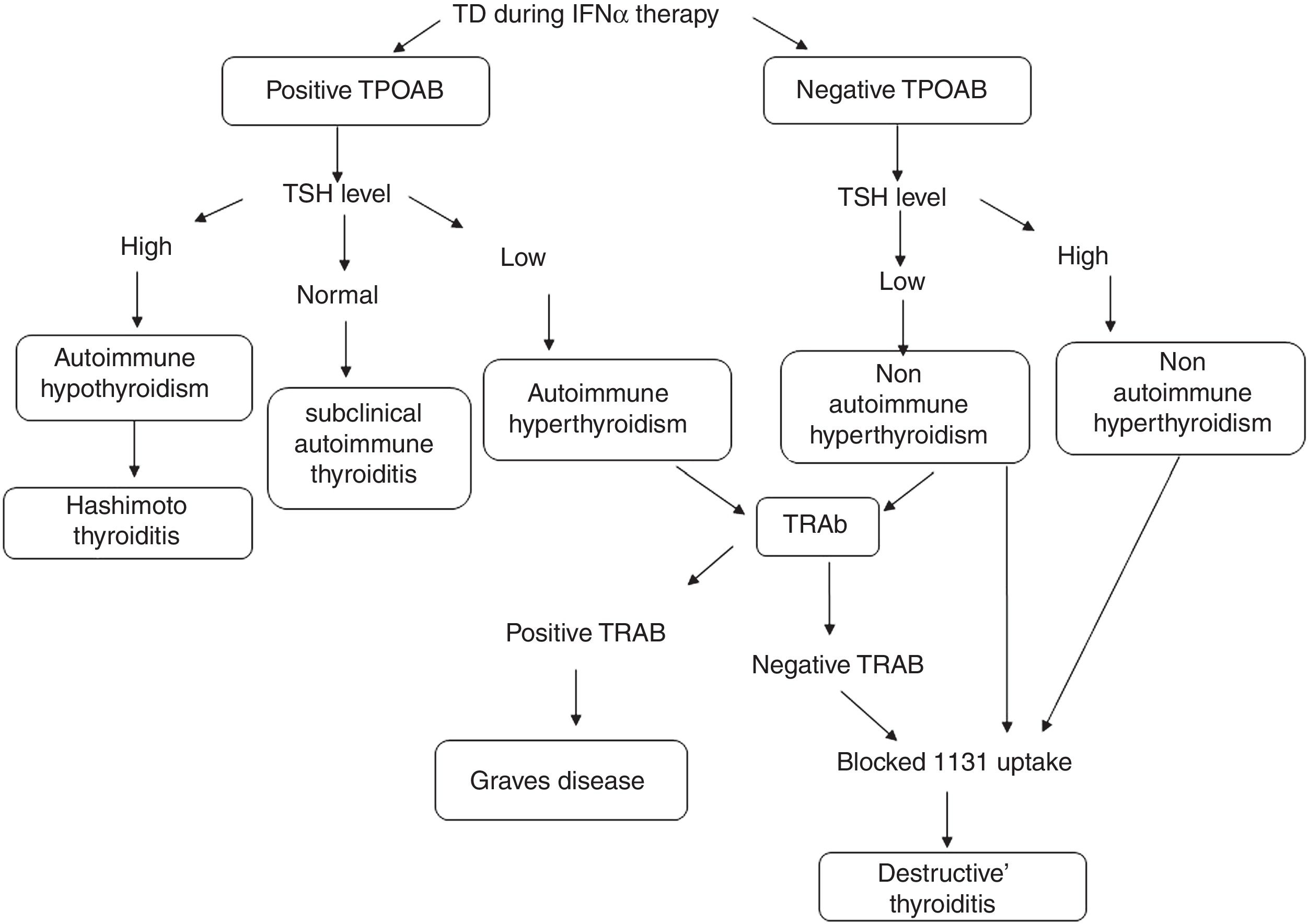
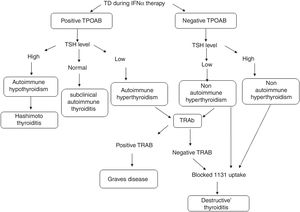
TD was defined as a TSH level >4μIU/ml (hypothyroid) or <0.4μIU/ml (hyperthyroid). Patients who developed biochemical TD were classified into five types: (1) subclinical hypothyroidism, TSH>4miu/ml with normal FT4; (2) hypothyroidism, TSH>4μIU/ml with decreased FT4; (3) subclinical hyperthyroidism, TSH<0.4μIU/ml with normal FT4; (4) hyperthyroidism, TSH<0.4μIU/ml with increased FT4; and (5) thyroiditis, hyperthyroidism diagnosed initially, followed by gradual resolution (sometimes with hypothyroid phase) and blocked 131I uptake.
Patients with low TSH levels (<0.4μUI/ml) also had anti-TSH receptor (TRAb) antibody levels measured.
183/580 patients from G1 and 52/210 from G2 were treated with IFNα plus ribavirin.
Inclusion criteria for therapy with IFNα plus ribavirin in G1: All HCV patients with compensated chronic liver disease, who are willing to be treated and who have no contraindications to treatment. It should be scheduled, rather than deferred, in patients with advanced fibrosis, and in those patients with clinically significant extrahepatic manifestations (symptomatic cryoglobulinemia or HCV immune complexes nephropathy). For patients with minimal or no fibrosis, the timing of therapy is debatable, considering the patient's preference and priorities, the natural history and risk of progression, the patient's age and the development and availability of new therapies. Exclusion criteria for therapy in G1: Uncontrolled depression, psychosis or epilepsy, pregnancy, cardiovascular disease, renal insufficiency and hepatic cirrhosis.
Progression of liver disease in patients is accelerated with HIV–HCV co-infection. Inclusion criteria for therapy with IFN plus ribavirin in G2: Advanced hepatic fibrosis, stable antiretroviral treatment during the preceding 3 months (or no antiretroviral treatment); and a CD4 cell count higher than 200×106/L. Exclusion criteria for therapy in G2: Patients were not eligible if they had neutropenia, thrombocytopenia or anemia.
A detailed history, physical examination and thyroid function tests were performed every three months and twelve months after the therapy was completed if TD was diagnosed.
Elisa 3 test was used for the detection of Antibodies against the virus C. The viral load of virus C was detected by the real time PCR method.
Serum TSH concentration was determined by a Solid-phase immuno-chemiluminescent immunoassay (IMM I, IMM 2000, Siemmens). Reference range: 0.4–4.0μUI/ml.
Serum free thyroxine (FT4) was measured from 1995 to 2010 by a sequential competitive enzyme-chemiluminescent assay with solid phase analogs (IMM I, IMM 2000, Siemmens). Reference range: 0.8–1.91ng/dl and from 2010 to 2014 by a competitive solid phase enzymatic chemiluminescent assay (IMM 2000, Siemmens). Reference range: 0.79–1.40ng/dl.
At the beginning, TPOAb was measured in serum samples between 1995 until 2003, by using a direct radioimmunoassay (RSR) Cut-off>0.5U/ml. In a second period, from 2004 until 2014, it was analyzed through a solid phase sequential immunomimetic immunologicalimmunition assay (IMM I, IMM 2000, Siemmens). Reference range: up to 35IU/ml. 1U/ml (RIA RSR)=5U/ml (NIBSC 66/387).13
Thyroglobulin antibodies (TGAb) were not measured routinely in all patients.
TSH Receptor Autoantibody (TRAb) was measured using a direct radioimmunoassay, RSR TRAb RIA Kit. Cut-off <15% binding.
TD during IFNα therapy was defined as was defined as the diagnostic classification described by Mandac et al.14: Fig. 1.
(1) Autoimmune: With TPOAb or TRAb positive clinically manifested as Hashimoto thyroiditis (HT), Graves’ disease (GD) or presence of thyroid autoantibodies without clinical dysfunction.
Patients with Hashimoto thyroiditis (HT) or Graves’ disease (GD) were diagnosed based on previously established clinical and biochemical criteria.15
Those patients that developed thyroid autoantibodies, without evidence of clinical disease resulted of IFNα therapy, were classified as subclinical autoimmune thyroiditis.
(2) Non-autoimmune: with TPOAb negative. Clinically manifested as Destructive Thyroiditis or non-autoimmune hypothyroidism.
Patients who developed clinical TD, defined by initial hyperthyroid phase followed by gradual resolution (sometimes with hypothyroid phase) without the presence of thyroid antibodies were diagnosed as Destructive Thyroiditis. They were confirmed by a blocked or low radioiodine uptake.
Individuals with clinical symptoms and biochemical evidence of hypothyroidism without the presence of thyroid antibodies, were classified as non-autoimmune hypothyroidism.
The duration of treatment for HCV depended on the virus genotype, viral response and adverse effects analyzed by the hepatologist.
If the TD was diagnosed during the therapy with IFNα, the patient remained in observation or with a thyroid treatment. Cessation of IFNα therapy was not advised.
After three months of finishing IFNα treatment, the patients were clinically evaluated through thyroid function tests. If the patient had had a TD during the therapy, they were tested twelve months after discontinuation, to determine if the TD was transitory or definitive.
Statistical analysisThe data were analyzed using SPSS version 21 (SPSS Inc., Chicago, IL, USA). Continuous variables are expressed as mean±standard deviation.
Comparisons between groups were performed using the chi-square and Fisher tests for qualitative data and the unpaired t test for continuous variables.
Relative Risk was calculated to estimate the strength of the association between the following factors and patient's outcome: patient's age, gender, TSH values, TPOAb, and HAART therapy in coinfected patients.
The p values <0.05 were considered to indicate significance.
The Ethics Committees of Ramos Mejia and Muñiz Hospitals approved this study. Informed consent was obtained from each patient before the study was initiated.
ResultsThe baseline features of the population are shown in Table 1.
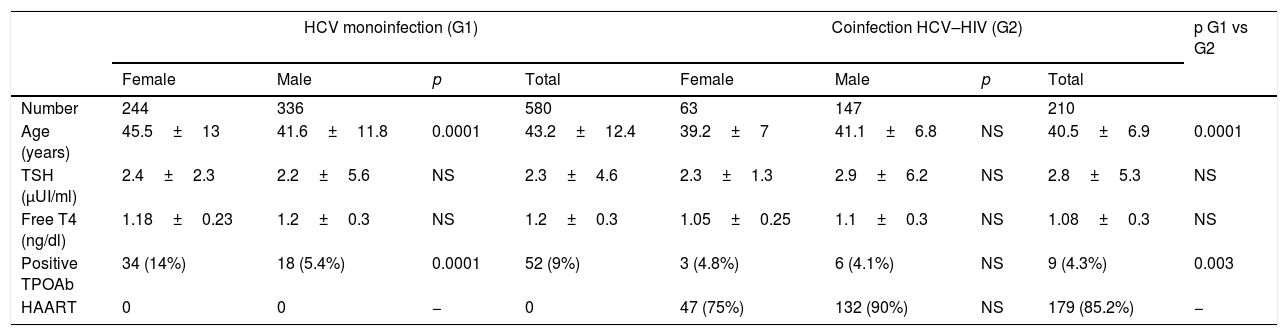
Baseline features of the population.
| HCV monoinfection (G1) | Coinfection HCV–HIV (G2) | p G1 vs G2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Female | Male | p | Total | Female | Male | p | Total | ||
| Number | 244 | 336 | 580 | 63 | 147 | 210 | |||
| Age (years) | 45.5±13 | 41.6±11.8 | 0.0001 | 43.2±12.4 | 39.2±7 | 41.1±6.8 | NS | 40.5±6.9 | 0.0001 |
| TSH (μUI/ml) | 2.4±2.3 | 2.2±5.6 | NS | 2.3±4.6 | 2.3±1.3 | 2.9±6.2 | NS | 2.8±5.3 | NS |
| Free T4 (ng/dl) | 1.18±0.23 | 1.2±0.3 | NS | 1.2±0.3 | 1.05±0.25 | 1.1±0.3 | NS | 1.08±0.3 | NS |
| Positive TPOAb | 34 (14%) | 18 (5.4%) | 0.0001 | 52 (9%) | 3 (4.8%) | 6 (4.1%) | NS | 9 (4.3%) | 0.003 |
| HAART | 0 | 0 | − | 0 | 47 (75%) | 132 (90%) | NS | 179 (85.2%) | − |
The results are expressed as mean±standard deviation and percentage of the total.
TPOAb: Anti-thyroid peroxidase antibodies/HAART: highly active antiretroviral therapy.
790 patients diagnosed with HCV infection were enrolled, 580 (73.4%) monoinfected (G1) and 210 (26.6%) coinfected HIV (G2). The mean age was 42.54±11.2 years, and 483 (61.1%) of the patients were men. When evaluating the distribution by gender, in G2 there was a higher percentage of men than in G1 (70% vs 59%, p=0.002).
With regard to baseline autoimmunity, patients in G1 had a higher percentage of positive TPOAb than those in G2 (G1 9% vs G2 4.3%, p=0.003) and a higher prevalence of autoimmunity in G1 versus G2 women (14% vs 4.8% respectively, p=0.0001). The diagnosis of HCV was a predisposing factor for the presence of positive TPOAb after correcting the data by age and sex (RR 2.11, 95% CI 1.06–4.2, p: 0.02). Age was not related to the prevalence of TPOAb.
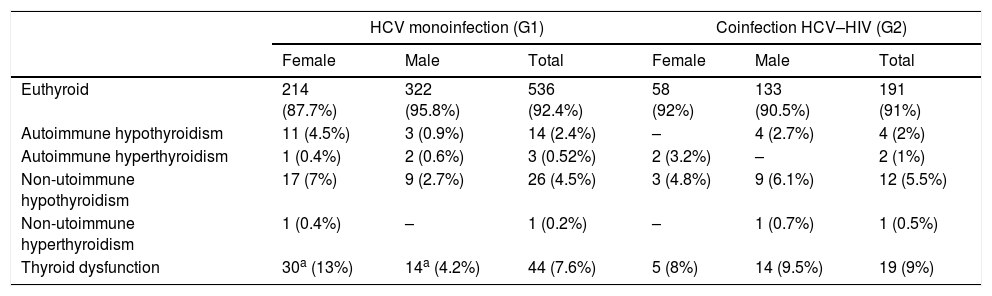
Thyroid function at baselineTable 2 shows thyroid function at baseline.
Thyroid function at baseline.
| HCV monoinfection (G1) | Coinfection HCV–HIV (G2) | |||||
|---|---|---|---|---|---|---|
| Female | Male | Total | Female | Male | Total | |
| Euthyroid | 214 (87.7%) | 322 (95.8%) | 536 (92.4%) | 58 (92%) | 133 (90.5%) | 191 (91%) |
| Autoimmune hypothyroidism | 11 (4.5%) | 3 (0.9%) | 14 (2.4%) | – | 4 (2.7%) | 4 (2%) |
| Autoimmune hyperthyroidism | 1 (0.4%) | 2 (0.6%) | 3 (0.52%) | 2 (3.2%) | – | 2 (1%) |
| Non-utoimmune hypothyroidism | 17 (7%) | 9 (2.7%) | 26 (4.5%) | 3 (4.8%) | 9 (6.1%) | 12 (5.5%) |
| Non-utoimmune hyperthyroidism | 1 (0.4%) | – | 1 (0.2%) | – | 1 (0.7%) | 1 (0.5%) |
| Thyroid dysfunction | 30a (13%) | 14a (4.2%) | 44 (7.6%) | 5 (8%) | 14 (9.5%) | 19 (9%) |
The results are expressed as number and percentage of the total.
Autoimmune hypothyroidism was detected at baseline laboratory test in 14 of G1 patients: Three overt and eleven subclinical. All of them were treated with LT4. Four of them received IFN α therapy and among this group, two of them needed an increase in LT4 daily dose.
In G2, 2/4 overt hypothyroidism were diagnosed at baseline. Two male patients with subclinical hypothyroidism were treated with IFNα, one of them required more LT4 doses, and the other one had a Destructive Thyroiditis with transient hyperthyroidism.
Autoimmune hyperthyroidism was diagnosed in three patients at baseline in G1: two Graves’ disease and one Hashimoto thyroiditis. In G2 in two patients: one Graves’ disease and one Hashimoto thyroiditis. Graves’ disease was treated with radioiodine and Hashimoto thyroiditis evolved to hypothyroidism.
Non-autoimmune hypothyroidism was diagnosed at baseline in 26 in G1 patients: 16 overt and 10 subclinical hypothyroidism. In G2: 12 patients, 2 presented overt hypothyroidism and 10 subclinical.
Non-autoimmune hyperthyroidism was established as a Plummer disease in a G1 woman. A Destructive silent Thyroiditis in a man was diagnosed in G2. The first one was treated with radioiodine, while the second remained in observation. IFNα therapy was not indicate in this case because of their liver status.
Baseline TD was similar in both groups: G1:7.6% versus G2: 9% of the patients (p=0.77). In G1, women had greater TD at baseline than men (p=0.0001).
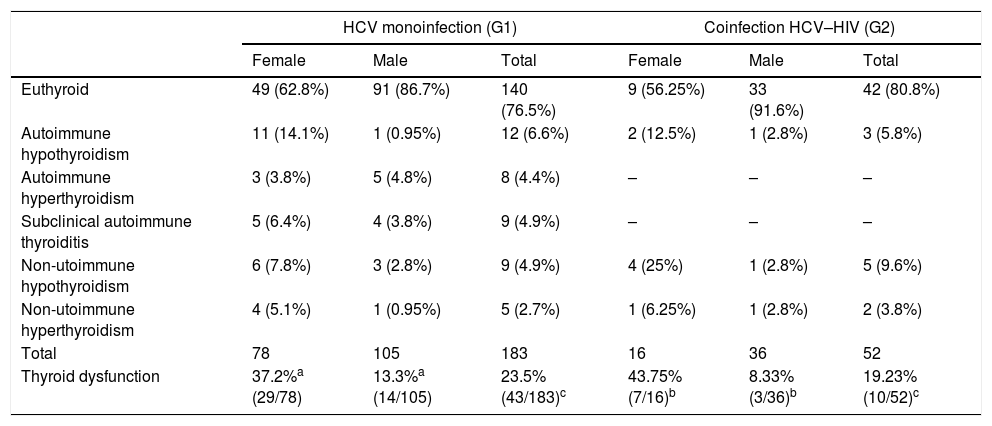
Thyroid function with IFNα therapyTable 3 shows thyroid function during IFNα therapy.
Thyroid function during IFNα therapy.
| HCV monoinfection (G1) | Coinfection HCV–HIV (G2) | |||||
|---|---|---|---|---|---|---|
| Female | Male | Total | Female | Male | Total | |
| Euthyroid | 49 (62.8%) | 91 (86.7%) | 140 (76.5%) | 9 (56.25%) | 33 (91.6%) | 42 (80.8%) |
| Autoimmune hypothyroidism | 11 (14.1%) | 1 (0.95%) | 12 (6.6%) | 2 (12.5%) | 1 (2.8%) | 3 (5.8%) |
| Autoimmune hyperthyroidism | 3 (3.8%) | 5 (4.8%) | 8 (4.4%) | – | – | – |
| Subclinical autoimmune thyroiditis | 5 (6.4%) | 4 (3.8%) | 9 (4.9%) | – | – | – |
| Non-utoimmune hypothyroidism | 6 (7.8%) | 3 (2.8%) | 9 (4.9%) | 4 (25%) | 1 (2.8%) | 5 (9.6%) |
| Non-utoimmune hyperthyroidism | 4 (5.1%) | 1 (0.95%) | 5 (2.7%) | 1 (6.25%) | 1 (2.8%) | 2 (3.8%) |
| Total | 78 | 105 | 183 | 16 | 36 | 52 |
| Thyroid dysfunction | 37.2%a (29/78) | 13.3%a (14/105) | 23.5% (43/183)c | 43.75% (7/16)b | 8.33% (3/36)b | 19.23% (10/52)c |
The results are expressed as number and percentage of the total.
183 patients of G1 (78 females and 105 males) and 52 patients of G2 (16 women and 36 men) received IFNα therapy plus ribavirin.
Analyzing both groups, patients in G2 were younger than those in G1 (G1: 44.1±12.3 and G2: 40.7±6.4 years, p=0.008), and men in G1 were younger than women (men: 41.14±12 and women: 48.3±12 years, p=0.0001). The distribution by gender was similar in both groups. TD and TPOAb positivity at baseline before IFNα therapy were not different from the total cohort.
We describe the following TD during IFNα therapy:
Autoimmune thyroid dysfunctionAutoimmune hypothyroidism: Twelve G1 patients, evolved with autoimmune hypothyroidism during IFNα therapy. Seven had positive TPOAb at baseline. They required LT4, during HCV treatment.
Three G2 patients presented autoimmune hypothyroidism. They were treated with LT4; two had positive TPOAb at baseline.
Autoimmune hyperthyroidism: In G1, four patients were diagnosed with Graves’ disease. They were treated with radioactive iodine and quickly developed definitive hypothyroidism at three months after starting IFNα therapy. One woman had had in a first IFNα treatment a non-autoimmune Destructive Thyroiditis described in the literature reviewed as the “swinging thyroid”.16
The other four patients presented an autoimmune thyroiditis, with a thyrotoxic and hypothyroid phase, clinically compatible with a Destructive Thyroiditis with low radioiodine uptake and negative TSH receptor antibodies (TRAb).
No G2 patients in our cohort, presented autoimmune hyperthyroidism.
Subclinical autoimmune thyroiditis: According to the TD definition, nine G1 patients with negative TPOAb at baseline became positive and remained euthyroid during and after IFNα treatment. None G2 became TPOAb positive during IFNα therapy.
Non-autoimmune thyroid dysfunctionNon-autoimmune hypothyroidism: In G1, nine patients presented non-autoimmune hypothyroidism during IFNα therapy, three were treated with LT4. In G2, five patients presented non-autoimmune hypothyroidism, only one woman required LT4 treatment. G1 and G2 were clinically destructive thyroiditis.
Non-autoimmune hyperthyroidism: In G1, five patients were presented clinically as Destructive Thyroiditis with a hyperthyroid and hypothyroid phase. In G2, two patients presented Destructive Thyroiditis.
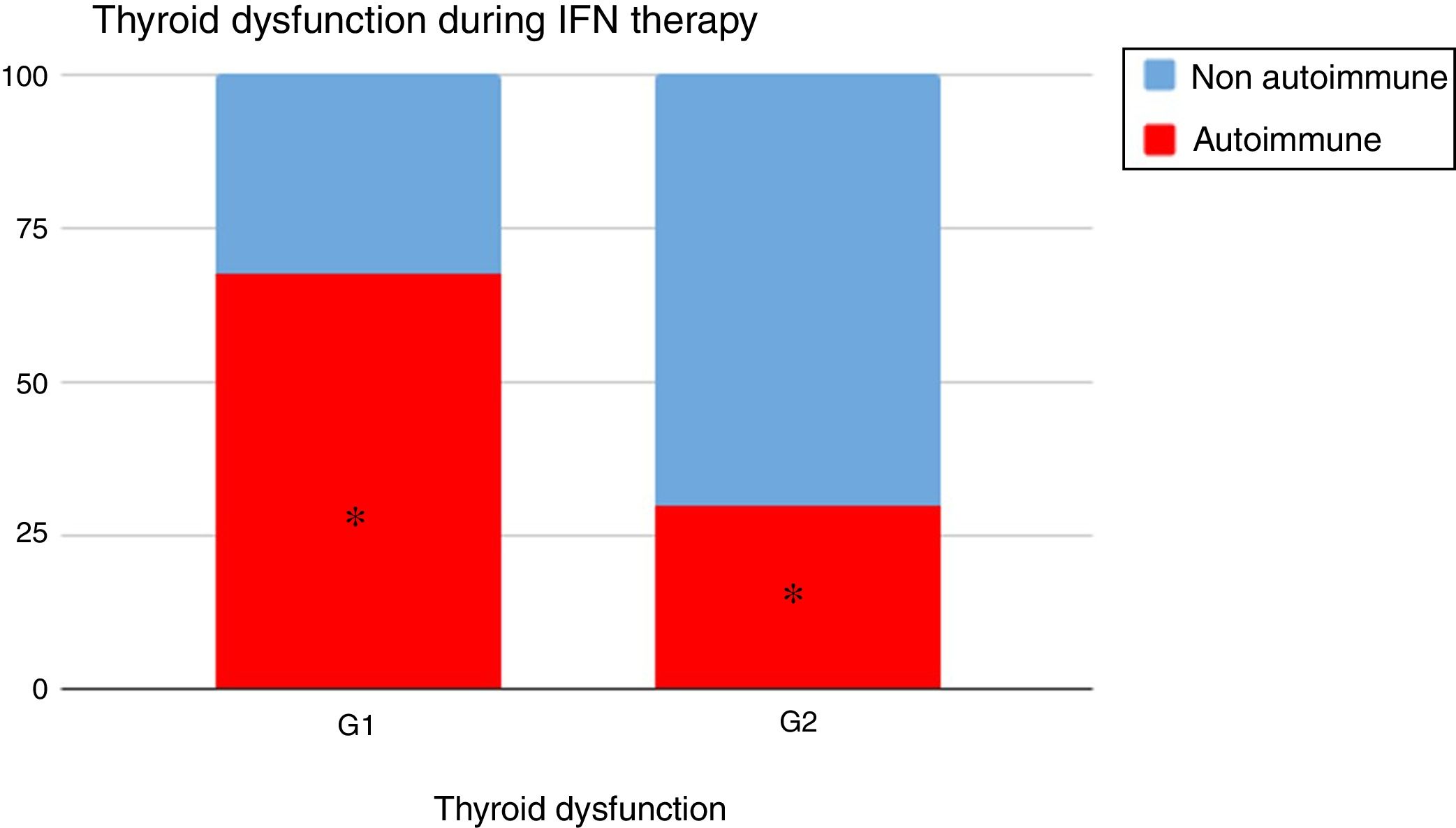
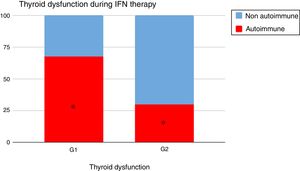
When analyzing the type of TD in G1 and G2, it could be seen that autoimmune dysfunction was more frequent in G1 than in G2 (67.4% vs 30%, p=0.02) (Fig. 2).
TD with IFNα occurred at 5.05±3 months in G1 and at 6.2±4.2 months in G2. Overall, there was no difference in the mean time of the TD development, neither in the dysfunction evolution nor in the requirement of a specific medical treatment.
There were no differences between G1 and G2 in the percentage of TD.
Concerning the gender, in both groups female had a higher percentage of TD post IFNα treatment than men did. (G1 p=0.002 and G2 p=0.01).
There were no differences in TD in G2 patients treated with HAART and those without it during IFNα therapy (13.8% vs 30.8%) p=0.09.
TD outcome after 12-month IFNα’s discontinuationA total of 53 TD in 235 HCV patients monoinfected and coinfected were reevaluated after twelve months of finishing IFNα therapy.
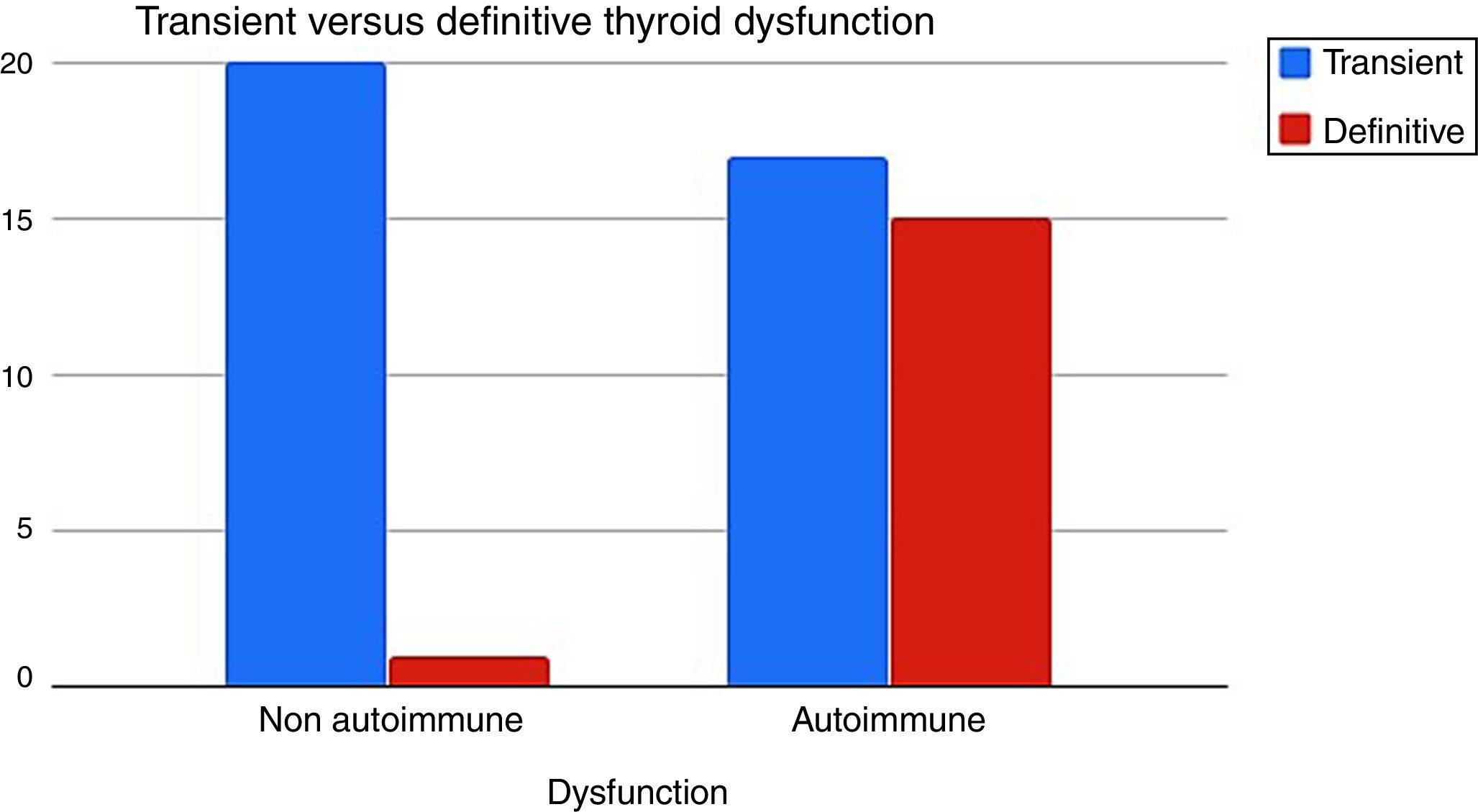
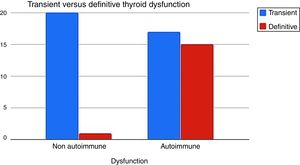
It was determined that when the TD had an autoimmune etiology, definitive hypothyroidism was more frequent than the non-autoimmune dysfunction 15/32 (46%) vs 1/21 (5%) respectively (p=0.001) (Fig. 3).
Predictors of TD in HCV patients treated with IFNαPositive TPOAb (RR 3.5, 95% CI 2.4–5.1, p: 0.0001), and female gender (RR 2.4, 95% CI 1.5–3.8, p: 0.0001) were associated with the development of TD with IFNα therapy. Patient's age was not a risk factor for the development of thyroid dysfunction.
DiscussionThis cohort clinical study is based on thyroid function and autoimmunity in 790 HCV infected patients at baseline: 580 monoinfected (G1), 210 coinfected HCV–HIV (G2).
235 patients were treated with IFNα plus ribavirin (G1: 183 patients, G2: 52 patients). Thyroid function and autoimmunity were evaluated every three months and three months after ending IFNα therapy.
If TD was diagnosed, the patients were treated according etiology and reevaluated in the next twelve months after HCV therapy was finished.
We compared thyroid autoimmunity and type of TD in both groups, at baseline, during and after IFNα treatment and determined the risk factors and therapeutic behavior.
Hepatitis C virus (HCV) infection is a liver disease that may be associated with extra hepatic manifestations: autoimmune disorders or malignant tumors, defining the HCV syndrome as a result of multifactorial process with significant genetic predisposition and/or environmental triggering factors.3,17
In large cohort studies, two thirds of patients with HCV infection experienced extrahepatic manifestations, which included thyroid disorders and autoimmunity.
The prevalence of thyroid antibodies in HCV, varied from 2% to 48% of individuals in several studies.17,18 Such a remarkable variation may be attributed to the different methodology used, and/or to the different ethnicity, age, and sex of the populations studied in these reports.
Shen et al.,19 in a meta-analysis identified observational studies which compared the prevalence of antithyroid antibodies in IFNα naive chronic HCV infected subjects with non-HCV infected controls. Twelve studies were included; involving 1735 HCV infected and 1868 non-HCV infected subjects. TPOAb were 1.96 fold higher in HCV-infected subjects than in controls. They did not discriminate if they were only monoinfected or if they were also coinfected HIV in the cohorts studied. Regarding TD, in the same review it could be found prevalence of hypothyroidism in HCV infected, with a pooled risk of 3.19. They concluded that chronic HCV infection might be an independent risk factor for thyroid disturbance.
It is important to remark that HIV and HCV can interact in several ways. HIV itself, may be a cause for dysregulation of the autoimmune system. Alternatively, immunodeficiency associated with HIV may either down regulate or stimulate autoimmune features, which are normally associated with HCV. Woitas et al.20 studied prospectively extrahepatic manifestations and the presence of autoantibodies in HCV monoinfected (n=78), HIV monoinfected (n=45) and HIV–HCV coinfected (n=98) patients. In TPOAb, determined by ELISA (>18GPL-U/ml), they were present in 6.6%, 11.8% and 13.3% of the patients with HIV-mono, HCV-mono and HIV–HCV coinfection respectively. There was no difference among the three groups with preponderance of male gender in the HIV groups.
The association between TD and HIV infection, specially hypothyroidism, in combination with the impact of highly active anti-retroviral therapy (HAART) has been demonstrated in several studies, with the historical use of stavudine.21 Recently, Harsløf et al.,22 evaluated well treated people living with HIV, and did not find any evidence of an increased risk of TD, compared to uninfected controls.
By examining monoinfected HCV and coinfected HCV–HIV at baseline features in our cohort, it was possible to observe that TPOAb positivity was more frequent in G1 than G2 (9% vs 3.8%, p=0.003), this might be probably because of female preponderance in G1, in an iodine sufficient area.23 G2 could have had beside gender, the immunodeficiency of the coinfection. The prevalence in both groups was not different than the one described for general population in the NHANES III study.24
There was no difference in TD in both groups G1: 7.6% versus G2: 9%, even though there was more autoimmunity in G1, which was not reflected in TD.
Treatments for hepatitis C virus infection have rapidly improved over the last decade. IFNα alone, in combination with ribavirin allowed sustained virological response (SVR) rate in 50% of the patients. In 2011, the first direct-acting antivirals (DAA), the first-generation protease inhibitors: boceprevir and telaprevir, was added to this treatment. Consequently, the SVR rate increased to 75%. The new DAA currently available does not require the combination with IFN and eventually, SVR greater than 90–95% can be achieved. In Argentina, DDA for HCV therapy was approved in 2012.
The relationship between the cytokines and TD was first described in 1985 in patients treated for breast cancer.25 IFN is also used to treat kidney cancer, malignant melanoma, multiple myeloma, certain types of lymphoma, leukemia and carcinoid tumors. Most of the TD described after its use is related with HCV infection. This interaction, allows the discussion of virus C role in thyroid function.
The immunostimulatory effects of IFN have been well described, and it can be identified that the thyroid is the most commonly affected organ. Studies had reported various incidence in infected HCV patients treated with IFN across countries (4.6–33.3%).26 Nowadays, the reversibility of TD remains controversial after the IFN therapy.
For instance, the Montes-Ramírez et al.’s12 work showed that 12 patients HCV, who were monoinfected and 22 coinfected had an incidence of TD from 25% and 0% (p<0.01) respectively. Likewise, in this report, the CD4 count was more than 400, which could be inferred as a condition to be treated with IFNα.
Tran et al.27 assessed the prevalence of TD in HCV patients treated with triple combination therapy: IFN-α, ribavirin and protease inhibitors (boceprevir and telaprevir), by comparing them with the dual therapy IFNα and ribavirin. It could be noticed a significant absence of TD in the protease inhibitor group. It is important to mention that there are still few reports with small number of patients about thyroid function in HCV treated with DAA.
The treatment with IFNα and ribavirin in our study induced different autoimmune and non-autoimmune TD in G1 and G2. In this study, it was evaluated the TD induced by IFNα, based on the classification described by Mandac et al.14 The patients were tested during their treatment; if TD was diagnosed, they were followed for twelve months after discontinuing therapy.
There was no difference on the mean time when the TD occurred: 5.05±3 months in G1 and at 6.2±4.2 months in G2.
Both groups, HCV monoinfected and coinfected with HIV, presented similar TD prevalence: 23.5% in G1 and 19.2% in G2.
Thyroid dysfunction's etiology was different: autoimmunity was more preponderant in G1 than in G2: 67.4% vs 30% (p=0.02) (Fig. 2).
When we reevaluated the TD of G1 and G2 (n=53) after twelve months of discontinuation IFNα therapy, autoimmune TD evolved more into definitive hypothyroidism than the non-autoimmune one (46% vs 5%) (Fig. 3).
The predictors of TD with IFNα treatment in HCV patients were the presence of positive TPOAb and female sex (RR: 3.5 and 2.4 respectively). Patient's age was not a risk factor for the development of thyroid dysfunction.
Many hypotheses have tried to explain why HCV patients have developed autoimmune response at baseline and TD when they were treated with IFNα.
A potential mechanism exists whereby HCV itself infects thyroid cells, or its envelope proteins bind to thyroid cells, triggering cytokine secretion that can activate resident T-cells. In genetically susceptible individuals, this results in thyroid autoimmunity through a bystander mechanism.28 In this sense, Blackard et al.,29 who belongs to the same investigation group, demonstrated that HCV could infect human thyroid cells in vitro and suggested that this infection might play a role in thyroid autoimmunity.
The presence of TPOAb or antithyroglobulin antibodies is strongly associated with IFN-α induced TD. HCV itself is believed to induce production of thyroid autoantibodies in addition to its direct effects on thyrocytes; IFN activated lymphocytes, which lead to increased cytokine production and induction of thyroid antibodies. These results suggested that assessing pretreatment thyroid autoantibodies in patients with HCV would facilitate prediction of the occurrence of IFN-induced TD.30
The high levels of IFN-α had numerous immunomodulatory functions, including activating both innate and adaptive immune responses. Some reports had also stated the direct toxic effect of IFN-α on thyroid cells, without the participation of immunological factors.6
In our study in monoinfected and coinfected patients, TD prevalence at baseline was low. TD with IFN-α was important and without difference (23.5% in G1 and 19.2% in G2), probably detected by periodical screening. Autoimmune TD was more frequent in G1 and definitive in the follow up.
The main hypothesis of this study is that HCV infection would have a stronger autoimmune response than the one presented in coinfected patients. Moreover, it is shown that coinfection would not act as a protection factor for TD before or after IFNα.
Symptoms of TD can easily be mistaken for adverse effects of the HCV therapy, and eventually remain undiagnosed if patients do not undergo routine periodic screening of TSH and FT4 levels. Regardless of symptoms, all patients should be screened for thyroid antibodies (TPOAb) and function (serum TSH, FT4) prior to starting IFNα therapy and every three months, particularly women. Coinfection with HIV had no difference in incidence of TD induced by IFNα than monoinfected. Autoimmune TD developed often to definitive hypothyroidism. Otherwise, non-autoimmune TD was generally transitory and reversible. These findings have to be taken in account, when patients were treated with IFNα in the past.
The limitation of this study is that there is not a control group, and the autoimmunity was diagnosed only with TPOAb, as it is the most frequent antibody measured in this entity.
The fortress of this clinical study is that is a large prospective cohort (N=790), evaluated at baseline, every three month during IFNα therapy and after discontinuation. The HCV population was analyzed comparing monoinfected and coinfected patients. If a TD was diagnosed during IFN therapy, the patient was followed 12 months after discontinuation, to evaluate if the TD was definitive or transitory. The patients were evaluated and followed up by the same Endocrinology laboratory and endocrinologist, with the same diagnostic and therapeutic criteria.
Conflicts of interestThe authors declare that there is no conflict of interest.
We wish to thank Laura Delfino for her collaboration in the preparation of the statistical analysis.