The accurate typification of the different Pituitary Neuroendocrine Tumours (PitNETs) is essential to their proper management and follow-up. The classification of PitNETs has evolved over time depending on advances in pathological techniques, ranging from the cellular staining properties to the immunohistochemical (IHC) and ultrastructural criteria of the tumour cells. The 2004 World Health Organization (WHO) classification of Tumours of the Pituitary Gland1 was based on IHC techniques. However, while there is good concordance between clinical and IHC diagnosis in Functioning PitNETs, the information provided by IHC is not as accurate in Non-functioning PitNETs, mainly attributed to the absence of protein secretion by tumour cells (Null Cell Tumours) or to the secretion of proteins coming from different cell lineage types (Plurihormonal Tumours). That is why the recently published 2017 WHO classification of Tumours of the Pituitary Gland2,3 considers the study of the adenohypophyseal cell lineages as the pivotal aspect to classify the PitNETs. This concept is based on the IHC identification of the main transcription factors involved in the cellular differentiation of the adenohypophysis, mainly Pit-1, T-Pit and ESR-1.
However, the immunohistochemistry is a semi-quantitative technique that depends largely on the antibodies chosen and the observer. Indeed, we have recently observed an important variability in the IHC results between four different centres analysing two large series of PitNETs.4,5 In addition, in the Lyon's pathological series, using more sensitive IHC techniques, the percentage of Null Cell adenomas were reduced from 10% in 1992 to 1% in 2012.6 Moreover, the well-differentiated pituitary tumours mimic the normal anterior pituitary cells.7 Therefore, it is essential to be sure that the analyzed tissue corresponds to the tumour specimen and not to the normal pituitary gland. As pituitary tumours are small, the amount of tissue given by the surgeon to the pathologist is usually scarce, and this can make the pathological studies difficult.
Unlike the 2017 WHO classification of Tumours of the Pituitary Gland, the 2016 Classification of Tumours of the Central Nervous System3 incorporated molecular and genetic patterns in the diagnosis of gliomas, medulloblastomas and other embryonal tumours. Accordingly, we published the complementary role of the molecular typing of pituitary-specific hormone genes to the IHC identification of PitNETs.4,5 However, its clinical applicability has not been established.
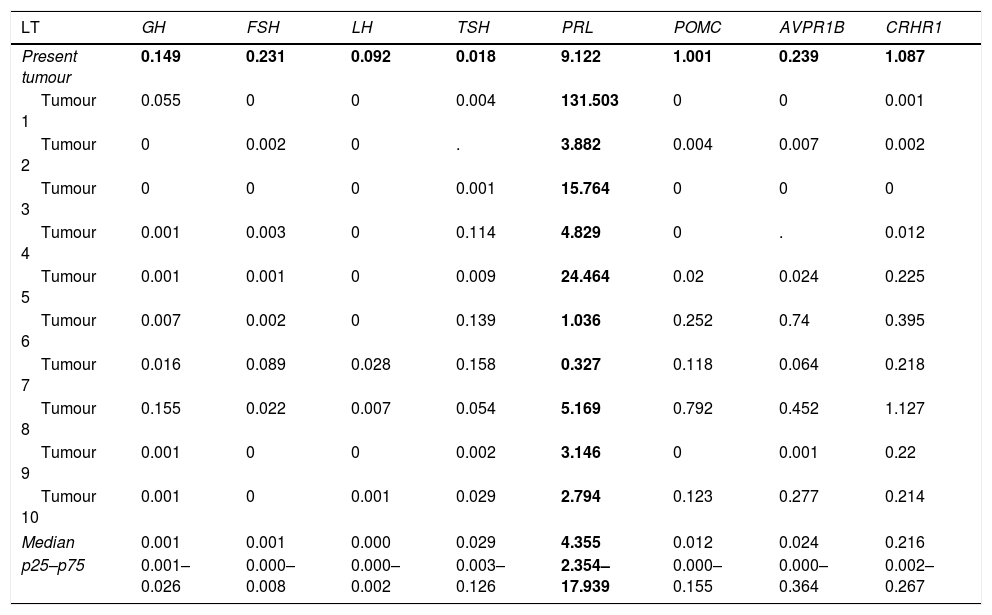
We have recently had the opportunity to study a 37-year-old man who came in our surgery because of sexual dysfunction and moderately high levels of Prolactin (PRL) (serial determinations of PRL of 58, 59 and 62ng/mL; nv 4–21) with low levels of Total Testosterone (TT) (2ng/mL; nv 3.12–10) and Gonadotropins (LH 2.1 UL (nv 2–11.2) and FSH 3.6U/L (nv 1–8). The rest of pituitary function was preserved. The Magnetic Resonance Image revealed a 12×9mm nodule on the left side of the gland, without extraselar extension or displacement of the pituitary stalk. The nodule was hypointense in T1 and hyperintense in T2, suggesting a cystic component. Because of the uncertainty of the clinical diagnosis, our Neurosurgical team performed an Endoscopic Endonasal Surgery with complete resection of the tumour, disappearance of the sexual dysfunction and normalization of the hormone levels (PRL 2.7ng/mL; TT 4.6ng/mL, LH 3.7U/L and FSH 5.7U/L). The tissue sent for anatomopathological study consisted of adenohypophyseal fragments. The study with techniques for reticulin fibres as well as the IHC evaluation confirmed normal adenohypophyseal tissue without tumour component. Therefore, it was not possible to identify the subtype of PitNET, conditioning the follow-up of the patient in case of relapse of the tumour. The molecular study was carried out by Real-Time Quantitative PCR (RT-qPCR) with TaqMan® Assays and the relative expression (Fold Change, FC) was quantified using a pool of cDNA of normal pituitary glands as calibrator and TBP, PGK1 and MRPL19 as reference genes. This molecular study found a dominant expression of PRL (FC of PRL: 9.122) compared to the rest of anterior pituitary hormone genes (FC of GH1: 0.149; FSHβ: 0.231; LHβ: 0.092; TSHβ: 0.018; POMC: 1.001; AVPR1B: 0.239, and CRHR1: 1.087). According to the median and 25th and 75th percentiles of PRL mRNA expression (4.344 (2.354–17.939)) of our series of prolactinomas (Table 1),5 the tumour was compatible with this subtype, whose clinical management is completely different from a non-functioning PitNET.
Expression of the dominant specific genes of anterior pituitary hormones in a series of lactotroph PitNETs.
| LT | GH | FSH | LH | TSH | PRL | POMC | AVPR1B | CRHR1 |
|---|---|---|---|---|---|---|---|---|
| Present tumour | 0.149 | 0.231 | 0.092 | 0.018 | 9.122 | 1.001 | 0.239 | 1.087 |
| Tumour 1 | 0.055 | 0 | 0 | 0.004 | 131.503 | 0 | 0 | 0.001 |
| Tumour 2 | 0 | 0.002 | 0 | . | 3.882 | 0.004 | 0.007 | 0.002 |
| Tumour 3 | 0 | 0 | 0 | 0.001 | 15.764 | 0 | 0 | 0 |
| Tumour 4 | 0.001 | 0.003 | 0 | 0.114 | 4.829 | 0 | . | 0.012 |
| Tumour 5 | 0.001 | 0.001 | 0 | 0.009 | 24.464 | 0.02 | 0.024 | 0.225 |
| Tumour 6 | 0.007 | 0.002 | 0 | 0.139 | 1.036 | 0.252 | 0.74 | 0.395 |
| Tumour 7 | 0.016 | 0.089 | 0.028 | 0.158 | 0.327 | 0.118 | 0.064 | 0.218 |
| Tumour 8 | 0.155 | 0.022 | 0.007 | 0.054 | 5.169 | 0.792 | 0.452 | 1.127 |
| Tumour 9 | 0.001 | 0 | 0 | 0.002 | 3.146 | 0 | 0.001 | 0.22 |
| Tumour 10 | 0.001 | 0 | 0.001 | 0.029 | 2.794 | 0.123 | 0.277 | 0.214 |
| Median | 0.001 | 0.001 | 0.000 | 0.029 | 4.355 | 0.012 | 0.024 | 0.216 |
| p25–p75 | 0.001–0.026 | 0.000–0.008 | 0.000–0.002 | 0.003–0.126 | 2.354–17.939 | 0.000–0.155 | 0.000–0.364 | 0.002–0.267 |
LT, lactotroph tumours.
This clinical case highlights the complementary role of molecular studies to immunohistochemistry in the typifying of PitNET subtypes. Obviously, there is a bias in the interpretation of the results, because the pathological and molecular studies were performed in different tissues, and it is possible that the pathologist only received normal pituitary gland. This has been the main criticism of the molecular studies: the difficulty to differentiate tumour from normal pituitary tissues. However, in our large experience in 256 tumours, we have had no difficulty differentiating normal from tumour tissue. Moreover, the cystic degeneration of the tumour could reduce the protein expression of the PRL gene hindering its IHC identification but not its molecular one.
In conclusion, we think that the molecular study of PitNETs can be very useful in the identification of the different subtypes when the IHC diagnosis is not definitive providing the clinician with an excellent tool for the personalized management of their patients. In addition, it is of utmost importance that an expert neuropathologist properly identifies the tissue where the studies should be performed.
Ethical approvalAll procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consentInformed consent was obtained from all individual participants included in the study.
We thank Dr. Javier Abarca Olivas (Neurosurgery Department, Hospital General Universitario de Alicante, Alicante, Spain) and Dr. Irene Monjas (ORL Department, Hospital General Universitario de Alicante, Alicante, Spain) for their contribution in surgeries. We also thank the Biobank of the Hospital General Universitario de Alicante. Finally, we thank M. Eugenia Torregrosa (Clinical Analysis Department, Hospital General Universitario de Alicante, Alicante, Spain) for her contribution in the biochemical and hormonal analysis.