Thyroid nodule detection has increased with widespread use of ultrasound, which is currently the main tool for detection, monitoring, diagnosis and, in some instances, treatment of thyroid nodules. Knowledge of ultrasound and adequate instruction on its use require a position statement by the scientific societies concerned.
The working groups on thyroid cancer and ultrasound techniques of the Spanish Society of Endocrinology and Nutrition have promoted this document, based on a thorough analysis of the current literature, the results of multicenter studies and expert consensus, in order to set the requirements for the best use of ultrasound in clinical practice. The objectives include the adequate framework for use of thyroid ultrasound, the technical and legal requirements, the clinical situations in which it is recommended, the levels of knowledge and learning processes, the associated responsibility, and the establishment of a standardized reporting of results and integration into hospital information systems and endocrinology units.
La ecografía se ha convertido en un instrumento imprescindible en la asistencia a los pacientes con enfermedades tiroideas. La detección de los nódulos tiroideos se ha incrementado con el uso generalizado de la misma, siendo la herramienta principal para su detección, orientación diagnóstica, seguimiento y, en ocasiones, también terapéutica.
Los Grupos de Trabajo de Cáncer de Tiroides y de Técnicas ecográficas de la Sociedad Española de Endocrinología y Nutrición han promovido este documento en el que se resumen los requisitos necesarios para la mejor práctica clínica posible con esta técnica.
Los objetivos del trabajo incluyen encuadrar su utilización dentro de la especialidad, los requisitos técnicos y legales necesarios, las situaciones clínicas de su aplicación, los niveles de conocimiento y aprendizaje, la responsabilidad asociada, la comunicación estandarizada de resultados e integración en los sistemas de información hospitalarios, posicionando la técnica ecográfica dentro de la cartera de servicios en las actuales unidades de Endocrinología y Nutrición.
Ultrasonography (US) allows for understanding the characteristic patterns of the thyroid gland, collecting relevant cytological samples of adequate quality, assessment of vascularization and thyroid cancer, and development of minimally invasive treatments, and has therefore revolutionized diagnosis, monitoring, and treatment of benign and malignant thyroid diseases, becoming one of the most significant advances in our specialty in recent decades.
US is currently the best imaging test for selecting the puncture pathway and optimizing access to the lesion to be studied or treated with a minimum risk of complications. However, actions derived from a deficient interpretation of US and inadequate performance of invasive procedures may involve an unnecessary risk for the patient and legal consequences for the physician. Thus, US should only be performed at clinical units integrated in the functional plan by experienced staff.
In order to help clinical teams integrate all of these factors into the design and implementation of the diagnostic, therapeutic, and monitoring plan for patients with thyroid disease, and at the proposal of the working groups on thyroid cancer and US techniques of the Spanish Society of Endocrinology and Nutrition (SEEN), a consensus document (http://www.seen.es/docs/apartados/439/Documento.Consenso.Ecografia.pdf) and this summary have been prepared to establish the knowledge and skills required of endocrinologists in this field, as well as all other conditions to be met by the units. The document is intended to be used as guidance for the medical community and the healthcare administration.
Rational use and indications of thyroid USThe prevalence of thyroid nodules detected by palpation ranges from 4% and 8%1,2, and is up to 66% when US is performeda.3–6 However, only a small proportion of nodules (2%–15%) are found to be malignant.7–10 Moreover, widespread use of imaging tests to study thyroid and other diseases has led to an almost exponential increase in detection of nodular thyroid disease.11–16. However, only a few of these incidentalomas are malignant: 5% to 13% of those found by US, computed tomography, or magnetic resonance imaging,15,17 and 27%–42% of those detected by positron emission tomography.16,18
Today, thyroid US is indicated for incidentalomas found by another imaging test, patients at high risk of thyroid cancer, with palpable nodules or suspicious adenopathies,19 but is not recommended as a screening test in the general population or in patients with normal palpation and low risk for thyroid cancer.19,20 On the other hand, ultrasonographic characteristics do not allow for accurate differentiation of benign and malignant nodules, but may identify malignant characteristics,8,10,11,21 which allows for selecting those that require fine needle aspiration (FNA),20 thus optimizing diagnosis22 and providing information on the functional status of the gland.23
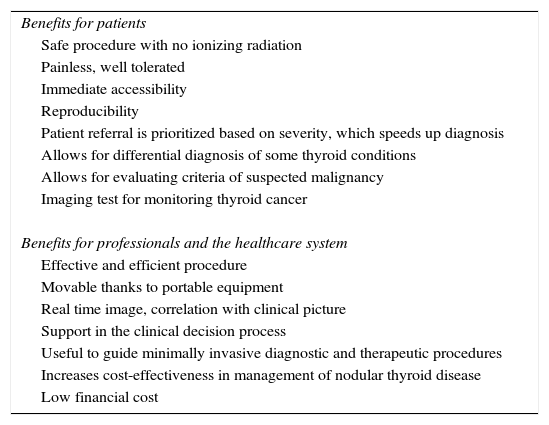
Table 1 shows the advantages of US. It should be noted, however, that US results depend on the operator, and reliability of the test is related to the training, experience, and skill of the operator.23–26 The main current indications of thyroid US are listed below.27–36
- 1.
Evaluation of the presence, size, and situation of the thyroid gland and cervical masses.
- 2.
Assessment of the condition of vocal cords and adjacent structures.
- 3.
Assessment of functional thyroid abnormalities (hypothyroidism and hyperthyroidism), thyroiditis, and benign nodular disease before or after treatment with radioactive iodine.
- 4.
Thyroid nodule management: identification of lesions amenable to cytological study and guidance for FNA. Support for minimally invasive procedures.
- 5.
Differentiated thyroid carcinoma: (a) preoperative assessment: extrathyroid invasion, contralateral and nodal involvement; (b) intraoperative location: in repeat surgery or complicated locations, locating and signaling specific lesions; (c) follow-up: location of recurrence, persistent tumor, or nodal metastases; and (d) treatment: as guide for percutaneous ablation both in the thyroid bed and metastatic adenopathies.
- 6.
Hyperparathyroidism: identification and location of parathyroid glands. Percutaneous treatment of parathyroid adenomas and location and monitoring of autologous parathyroid implants.
Benefits of ultrasonography for patients, professionals, and the healthcare system.
| Benefits for patients |
| Safe procedure with no ionizing radiation |
| Painless, well tolerated |
| Immediate accessibility |
| Reproducibility |
| Patient referral is prioritized based on severity, which speeds up diagnosis |
| Allows for differential diagnosis of some thyroid conditions |
| Allows for evaluating criteria of suspected malignancy |
| Imaging test for monitoring thyroid cancer |
| Benefits for professionals and the healthcare system |
| Effective and efficient procedure |
| Movable thanks to portable equipment |
| Real time image, correlation with clinical picture |
| Support in the clinical decision process |
| Useful to guide minimally invasive diagnostic and therapeutic procedures |
| Increases cost-effectiveness in management of nodular thyroid disease |
| Low financial cost |
Current standard treatment for benign symptomatic nodules is surgery. However, surgery is expensive, will often require replacement therapy, is sometimes associated to a unsightly scar, and may cause potentially severe permanent complications.37 On the other hand, impact of surgery on quality of life and patient reluctance to undergo a surgical procedure are increasingly taken into account.38 Because of this, US-guided, minimally invasive non-surgical procedures have been developed in the past decades to treat thyroid nodules when surgery is contraindicated or refused by the patient. Such procedures include percutaneous ethanol injection and ablation by radio frequency, microwaves, laser, and high-frequency ultrasound.39–41
Infrastructure and administrative requirements for performing thyroid USFacilities and basic equipment24,25,42–44Although radiological protection measures are not required, installation of US equipment close to magnetic fields should be avoided. The number of rooms should be adapted to the demand and the characteristics of procedures to be performed. Two or three diagnostic examinations per hour may be performed on average at the specialist's office, provided the minimum requirements are met and an additional area for interventional therapeutic procedures is available. Integration of the latter into the facilities of the day hospital, room for function tests, or outpatient major surgery room is sufficient to meet the requirements for facilities, equipment, and staff.
Legal and administrative framework46–48From 2005, the SEEN has recommended that our departments have US equipment available to mainly perform thyroid US examination, and performance of FNA by endocrinologists. Order SCO/3122/2006, detailing the training program of the specialty, stated the need for “knowledge” and “skills” in US examination and FNA biopsy of the thyroid gland,49 and in 2011, the care committee of the Society of Endocrinology and Nutrition recommended inclusion of US equipment as “necessary equipment” at hospital and outpatient clinics,50 and urged the different endocrinology units to “organize multidisciplinary units” at the hospitals, particularly “high resolution thyroid nodule units”.
Implementation of these units requires a specific care context that allows for defining not only their field of action and competences, but also the requirements and standards they must meet.44,51–53 In addition, their care activities must be oriented to patients, and their final goal must be “to improve system efficiency, decreasing the number of patient visits, avoiding delays in tests and appointments for subsequent visits”, as stated in Royal Decree 63/1995, of January 20, on the regulation of the healthcare benefits of the National Health System and on the guide for management of outpatient clinics in specialized care.54 Because of the possibility of an oncological thyroid disease, the recommendations of the Ministry of Health, Social Services and Equality entitled “Standards and recommendations for quality and safety at care units in the area of cancer”53 and clinical consensus guidelines and current legal regulations should also be taken into account.52,55
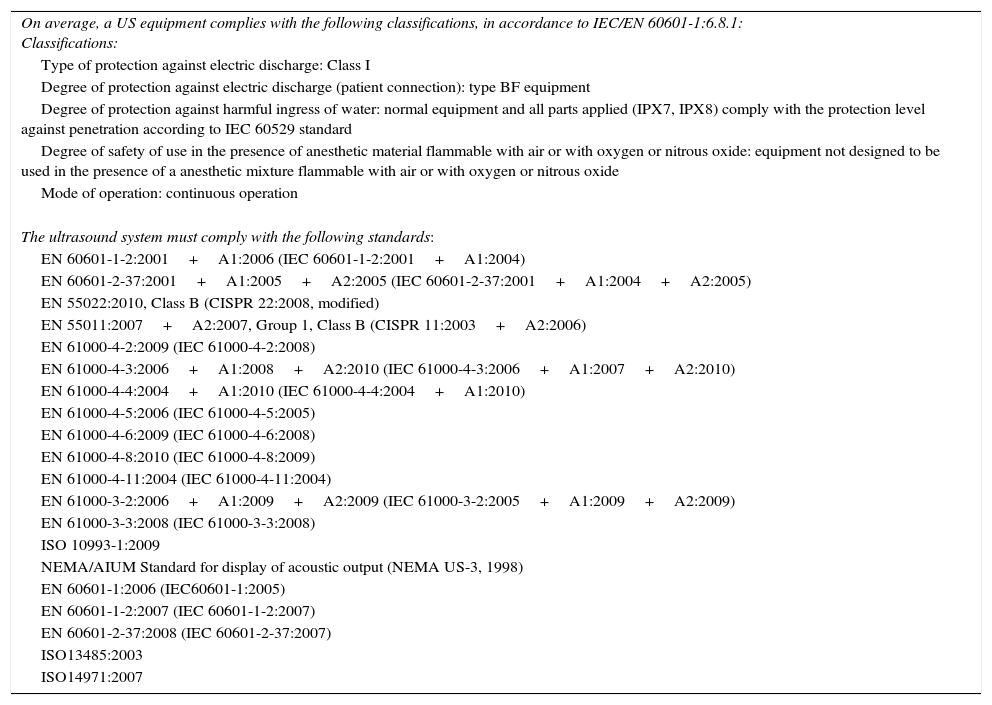
Minimum technical specifications of US equipment for diagnosis of thyroid diseaseAdequate performance of thyroid US examination requires an equipment with a high-frequency linear transducer (7.5–15MHz or greater), with a penetration of 5cm and a resolution of 2–3mm.56 A low frequency convex (curved array) transducer may be required for deep or very big lesions. The equipment should also be able to operate in real time in the B and doppler (pulsed, color and power) modes, and be fitted with an analog-digital converter for manipulation, graphic recording, and exporting of images for local or central storage. Any equipment has to comply with the quality regulations related to technical and safety conditions (Table 2).47,57
Quality rules related to the technical and safety conditions of the equipment.
| On average, a US equipment complies with the following classifications, in accordance to IEC/EN 60601-1:6.8.1: Classifications: |
| Type of protection against electric discharge: Class I |
| Degree of protection against electric discharge (patient connection): type BF equipment |
| Degree of protection against harmful ingress of water: normal equipment and all parts applied (IPX7, IPX8) comply with the protection level against penetration according to IEC 60529 standard |
| Degree of safety of use in the presence of anesthetic material flammable with air or with oxygen or nitrous oxide: equipment not designed to be used in the presence of a anesthetic mixture flammable with air or with oxygen or nitrous oxide |
| Mode of operation: continuous operation |
| The ultrasound system must comply with the following standards: |
| EN 60601-1-2:2001+A1:2006 (IEC 60601-1-2:2001+A1:2004) |
| EN 60601-2-37:2001+A1:2005+A2:2005 (IEC 60601-2-37:2001+A1:2004+A2:2005) |
| EN 55022:2010, Class B (CISPR 22:2008, modified) |
| EN 55011:2007+A2:2007, Group 1, Class B (CISPR 11:2003+A2:2006) |
| EN 61000-4-2:2009 (IEC 61000-4-2:2008) |
| EN 61000-4-3:2006+A1:2008+A2:2010 (IEC 61000-4-3:2006+A1:2007+A2:2010) |
| EN 61000-4-4:2004+A1:2010 (IEC 61000-4-4:2004+A1:2010) |
| EN 61000-4-5:2006 (IEC 61000-4-5:2005) |
| EN 61000-4-6:2009 (IEC 61000-4-6:2008) |
| EN 61000-4-8:2010 (IEC 61000-4-8:2009) |
| EN 61000-4-11:2004 (IEC 61000-4-11:2004) |
| EN 61000-3-2:2006+A1:2009+A2:2009 (IEC 61000-3-2:2005+A1:2009+A2:2009) |
| EN 61000-3-3:2008 (IEC 61000-3-3:2008) |
| ISO 10993-1:2009 |
| NEMA/AIUM Standard for display of acoustic output (NEMA US-3, 1998) |
| EN 60601-1:2006 (IEC60601-1:2005) |
| EN 60601-1-2:2007 (IEC 60601-1-2:2007) |
| EN 60601-2-37:2008 (IEC 60601-2-37:2007) |
| ISO13485:2003 |
| ISO14971:2007 |
Inclusion of US images into the clinical records of the patient allows for their review and management using specialized tools, for comparison of periodical examinations, for simultaneous access to them from different places, and for consultation with other professionals, including radiologists.
PACS (picture archiving and communication system) is the main tool for including images in the clinical records through a system for acquisition, storage, recovery and distribution of digital images that allows for subsequent visualization with adequate quality.58 Using DICOM (Digital Imaging and Communication in Medicine), medical images may be sent and received in a standardized manner regardless of equipment brand and model. US equipment should therefore incorporate the necessary license for DICOM services, and the staff in charge of information systems at each hospital should be asked to ensure inclusion of thyroid US images in the PACS of the hospital.
Accreditation and certification system of US procedures in endocrinologyThe recommendations for training in ultrasonography and related procedures of the American (ATA) and European (ETA) Thyroid Associations only suggest as experience at least 600 neck US examinations annually, including 30 cases of thyroid cancer, metastatic adenopathies, and local recurrence annually, and 150 FNAs annually with less than 10% of samples inadequate for diagnosis.19,20,23,31 Such recommendations recognize that no restrictions exist in most countries as to who may perform US examinations, and assume the difficulty of establishing a given number of hours, US examinations, and FNAs to consider that the training period has been overcome.
In this regards, the SEEN has started to implement an accreditation and training system in neck US, diagnostic and treatment procedures, based on the experience of national and international endocrinology associations, in order to ensure a clinical practice of quality in Spain.59–63
This system has three competence acquisition levels for training of specialists:
- I.
Basic level: US examination and neck and vascular anatomy. Ultrasonographic diagnosis of the main endocrine changes.
- II.
Intermediate level: ultrasonographic diagnosis of common neck changes and US-guided puncture. Introduction to thyroid cancer monitoring.
- III.
Advanced level: complex ultrasonographic diagnosis. US monitoring of high-risk thyroid cancer. Minimally invasive therapeutic procedures.
Accreditation of these skills requires that a number of different evaluable conditions, specific for each level, are detailed in the consensus document and evaluated by a SEEN committee.
US examination protocol and interpretation of resultsA standardized ultrasonographic report must include clinical, morphological, monitoring, and therapeutic concepts that allow for taking decisions about a particular patient.10,64 It is therefore indispensable to have a reproducible examination protocol that is comparable between various operators, a report using standardized terminology and contents, and information classification systems that allow for taking clinical and treatment decisions.
As regards study characteristics, the report must include the following concepts: location, size, shape and symmetry, echogenicity, homogeneity, vascularization, glandular margins, nerve structures (vagus, recurrent, sympathetic, etc.), esophagus, parathyroid glands, lymphadenopathies and, of course, any intraparenchymal lesions that may be detected (including their number and position), as well as the following characteristics of each of them: size, contour, contents, echogenicity, margins, vascular calcifications, and elasticity (if elastography is available).8,20,21,30
Systems grouping or classifying different combinations of ultrasonographic characteristics have been developed in recent years to improve the sensitivity and specificity of US examinations and to stratify the risk of malignancy of each particular nodule. Such systems help make decisions on the diagnostic means and therapeutic indications most adequate for patients.
The Thyroid Imaging Reporting and Data System, developed by Horvath et al.65 following the one devised for the breast (Breast Imaging Reporting and Data System of the American College of Radiology), and systems evolved from them are used in the guidelines for thyroid cancer of the British Thyroid Association (BTA),33 ATA,32 and American Association of Clinical Endocrinologists.20
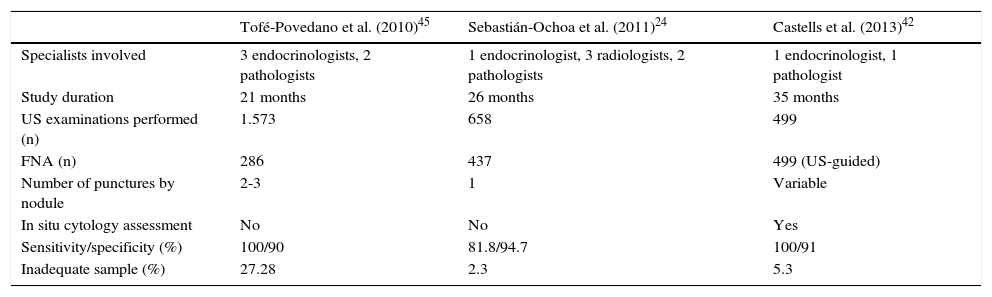
High resolution units and specific thyroid nodule clinicsThere are three reports of clinical experience in Spain in a clinic specific for nodular thyroid disease.24–26 Organization of these three work groups was different, and their results cannot therefore be compared (Table 3). The main differences between them are whether US is performed by a radiologist or an endocrinologist, whether one or several samples are taken of a same nodule, and whether or not sample suitability is verified in situ.24,42,45 These three models reported indicate the main barriers for implementation: the lack of experience and training in this field, and insufficiency of resources and qualified staff at the different units.
Comparison of results in different high-resolution thyroid nodule clinics.
| Tofé-Povedano et al. (2010)45 | Sebastián-Ochoa et al. (2011)24 | Castells et al. (2013)42 | |
|---|---|---|---|
| Specialists involved | 3 endocrinologists, 2 pathologists | 1 endocrinologist, 3 radiologists, 2 pathologists | 1 endocrinologist, 1 pathologist |
| Study duration | 21 months | 26 months | 35 months |
| US examinations performed (n) | 1.573 | 658 | 499 |
| FNA (n) | 286 | 437 | 499 (US-guided) |
| Number of punctures by nodule | 2-3 | 1 | Variable |
| In situ cytology assessment | No | No | Yes |
| Sensitivity/specificity (%) | 100/90 | 81.8/94.7 | 100/91 |
| Inadequate sample (%) | 27.28 | 2.3 | 5.3 |
FNA: fine needle aspiration.
The most basic model, which is adequate when no US equipment or endocrinologists experienced in ultrasonography are available, is based on coordination with other departments (usually radiodiagnosis and pathology), with the resultant burden for work agendas and organization, assuming that the managing role of endocrinologists is previously accepted or agreed. In the intermediate model, endocrinologists perform US examination and FNA, and pathologists ensure suitability of the sample in situ. This model has great acceptance at many units, provides few inadequate samples, and does not require patients to be moved, but implies a significant collaboration between professionals and coordination of their work schedules.66
Finally, performance of the whole process of ultrasonographic diagnosis and FNA by endocrinologists is clearly efficient in terms of cost, management and patient satisfaction, but requires adequate resources and continuous assessment of sample quality. If inadequate sample rates less than 5%–10% are not achieved, the process is no longer efficient. Operator skill and learning curve are therefore the limiting factor in the model.67–69
At any rate, units should be implemented ensuring their administrative and functional inclusion in the particular care context of each hospital, and that their actions, in addition to being effective, are based on the current clinical consensus guidelines and are approved and included in the care portfolio of each hospital.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Martín-Hernández T, Díez Gómez JJ, Díaz-Soto G, Torres Cuadro A, Navarro González E, Oleaga Alday A, et al. Criterios sobre la utilización y requerimientos técnicos de la ecografía tiroidea en los servicios de endocrinología y nutrición. Endocrinol Diabetes Nutr. 2017;64:23–30.