Gender affirming hormone therapy (HT) in transgender men both improves and impairs several surrogate cardiovascular risk markers. However, few prospective works with long follow-up and control group are available. In this context, this work aimed to assess the changes in the metabolic and cardiovascular risk pattern after 12 months of HT in transgender men. Furthermore, we aimed to investigate early effects on target tissues that may reflect an initial vascular damage.
MethodsProspective observational study, including 20 transgender men, attended in the Gender Identity Unit (UIG) of the Hospital Clinic from July 2013 to November 2015. Anthropometric and body composition by dual-energy X-ray absorptiometry (DXA), hormonal, metabolic and coagulation parameters, endothelial dysfunction by flow-mediated dilation (FMD) and intima–media thickness (IMT) by carotid ultrasound, were assessed at baseline, at 6 and 12 months of HT.
ResultsWe observed an impairment of lipid profile, and increase of homocysteine and leucocytes count, as well as changes in body composition with increased total lean mass together with decreased total fat mass. In addition, higher mean-maximum common IMT was observed after 12 months of HT.
ConclusionOur work shows changes in metabolic and inflammatory parameters after HT after short-medium follow-up, which could increase cardiovascular risk in this setting, together with initial evidence of vascular changes.
Objetivo En los transexuales masculinos (FtM) el tratamiento hormonal (TH) cruzado produce cambios tanto positivos como negativos en diversos marcadores subrogados de riesgo cardiovascular. Por otro lado, existen pocos estudios prospectivos con un grupo control y con un seguimiento prolongado que valoren los cambios en el perfil del riesgo cardiovascular. En este contexto, nuestro trabajo tiene como objetivo evaluar los cambios en el patrón de riesgo metabólico y cardiovascular tras 12 meses de TH en transexuales masculinos. Además, estudiamos los cambios tempranos en tejidos diana que puedan reflejar un daño vascular inicial.
MetodologíaEstudio observacional prospectivo en 20 transexuales masculinos atendidos en la unidad de identidad de género (UIG) del Hospital Clínic desde julio de 2013 a noviembre de 2015. Se valoraron los cambios antropométricos y de composición corporal mediante una absorciometría de rayos X de doble energía (DXA), así como las variaciones en los parámetros metabólicos y trombóticos. La disfunción endotelial fue evaluada mediante la dilatación mediada por flujo (FMD), y el grosor de íntima-media carotídea (IMT) a través de una ecografía carotídea, a los 6 y 12 meses del TH.
ResultadosObservamos un deterioro en el perfil lipídico, y un aumento de los niveles de homocisteína y del recuento de leucocitos, así como cambios en la composición corporal con aumento de la masa magra y disminución de la masa grasa. Además, se observó un incremento en el grosor de la IMT tras 12 meses del TH.
ConclusiónEn un seguimiento a mediano-corto plazo tras TH, nuestro trabajo muestra cambios en los parámetros metabólicos inflamatorios que podrían incrementar el riesgo cardiovascular en los transexuales masculinos, sumado a la evidencia de cambios vasculares incipientes.
Gender affirming hormone therapy (HT) is necessary for sex reassignment in most transgender people. Among them, some seek maximum feminization/masculinization, while others experience relief with an androgynous look resulting from hormonal attenuation of existing secondary sex characteristics.1
In post-puberal subjects HT leads to sex reassignment by both the reduction of the birth assigned sex characters and the development of those of the identified gender, usually to the greatest extent possible. Transgender women are treated with estrogens together with antiandrogens; transgender men are generally treated with testosterone.2
However, the use of sex steroids is associated to several risks. Potential adverse effects include arteriosclerosis, hypertension and dyslipidemia.3–5 Hormone treatment in transgender men produces positive and negative changes in several surrogate cardiovascular risk markers. Weight gain and increased visceral fat, triglycerides, insulin resistance and decreased HDL-cholesterol have been observed in transgender men, together with an increase in acute phase reactants such as C-reactive protein (CRP), homocysteine and prothrombotic factors.6–8 According to available data, some surrogate cardiovascular risk markers are impaired in transgender men while others are improved; however, it is not yet clear whether these changes affect cardiovascular morbidity and mortality. Several studies have addressed morbidity and mortality associated to HT in transgender people.9–13
The current evidence largely suggests that cardiovascular risk associated to HT of transgender women is higher than in transgender men.14 According to available information, testosterone therapy seems to be quite safe in relation to short and medium-term CV health. However, risk may become more apparent as subjects get older and the duration of hormone exposure increases.12
In this context, this work aims to assess the changes in the metabolic and cardiovascular risk pattern after 12 months of HT in transgender men. Furthermore, we aimed to investigate early effects on target tissues that may reflect an initial vascular damage.
Subjects and methodsWe carried out a prospective observational study in transgender men that attended the Gender Identity Unit (UIG) of the Hospital Clinic from July 2013 to November 2015. All subjects with comorbidities either contraindicating HT, requiring non-standard therapeutic schedules or with possible confounding effect on CV risk (unstable psychiatric comorbidity, severe liver dysfunction, drugs abuse or tobacco) or who had previously received hormonal treatment were excluded, as well as those with personal or family history of cardiovascular disease or HIV infection. Twenty transgender men were included, with mean age at the beginning of the HT of 27.1±8.0 years (range: 17–43 years). We studied body composition by dual-energy X-ray absorptiometry (DXA), endothelial dysfunction by flow-mediated dilation (FMD), and arterial intima–media thickness by carotid ultrasound and metabolic parameters.
Eighteen transgender men were treated with intramuscular testosterone undecanoate (1000mg every 2–3 months) while other two received transdermal testosterone (50mg/day). Subjects were assessed at baseline, 6 and 12 months of HT.
Ethics statementThis study was approved by the ethics committees of the Hospital Clinic (register number 2012/7430) in accordance with all laws and international ethics guidelines outlined in the Declaration of Helsinki. All subjects provided written informed consent.
Metabolic assessmentAnthropometric parameters and body compositionBody weight was measured wearing light clothing and without shoes to the nearest 0.1kg. Height was measured to the nearest half centimetre. BMI was calculated as weight in kilograms divided by height in metres squared (kg/m2). Waist circumference was measured at minimal inspiration to the nearest 0.1cm, midway between the last rib and the iliac crest. Hip circumference was measured at the widest of the buttocks, and then the waist hip ratio (WHR) was calculated.
Body composition was assessed by DXA with the GE Lunar iDXA device (GE Healthcare, Madison, WI) according to the manufacturer's specifications. Encore Software global GE Healthcare TM 2008 version 12.30.008 was used for measuring fat-free mass (FFM), fat mass (FM), each segment (trunk, upper and lower extremities) and gynoid or android distribution.
Biomarkers of metabolic and cardiovascular riskBlood samples were collected at 8 a.m., after overnight fast. After 6 and 12 months of HT in most subjects blood samples were adjusted to the timing of testosterone undecanoate injections (one month before next injection).
Main biochemical parameters were measured in serum with standard methods of the Core Laboratory of our hospital. Plasma glucose, total and HDL cholesterol and triglycerides were measured using ADVIA 2400 (Siemens Healthcare Diagnostics, Tarrytown, NY, USA), and glycated haemoglobin (HbA1C) was measured using high-performance liquid chromatography (Menarini Diagnostics, Firenze, Italy). Basic haemogram parameters were analyzed on an ADVIA 120 haematology analyzer (Siemens Healthcare Diagnostic, Tarrytown, NY, USA). High sensitivity C-reactive protein (hs-CRP) was determined using an immunonephelometric method (Boehring Nephelometer analyzer; Dade Boehring, Marburg, Germany). Insulin was determined in duplicate by an Elisa kit (Mercodia AB, Uppsala, Sweden), following the manufacturer's instructions. The intra and inter-assay CVs were lower than 4% and the assay sensitivity was 1mU/L. Insulin resistance was calculated according to homeostasis model assessment (HOMA-IR): insulin resistance=fasting plasma insulin (μU/ml)×fasting plasma glucose (mmol/l)/22.5.
Intercellular adhesion molecules (ICAM-s) was determined by an Elisa Kit (Human ICAM-1/CD54 DuoSet ELISA Development Kit, R&D Systems, Cat. No. DY720), following the manufacturer's instructions. The lower limit of detection was 0.35ng/ml. Measurement range between 15.6 and 1000pg/ml. Normal value between 98.9 and 320ng/ml.
Homocysteine was measured by electro-chemiluminiscence immunoassay in an Advia Centaur analyzer (Siemens Healthcare, Barcelona, Spain) using the same manufacturer's reagents. Bio-Rad quality controls were routinely used and the inter-assay variation coefficient was less than 6.3%.
Tissue plasminogen activator (t-PA) was measured using a commercially available kit: t-PA Combi Actibind (Technoclone GmbH, Vienna, Austria). This assay uses a catching antibody, which does not interfere with t-PA functional activity, coated in an ELISA plate. After the binding of the t-PA contained in the sample, t-PA functional activity is determined using plasminogen, cyanogen bromide fragments of fibrinogen, and a plasmin substrate.
Plasminogen activator inhibitor type 1 (PAI-1) was determined by a commercially available kit: Asserachrom PAI-1 (Diagnostica Stago SAS, 92665 Asnières sur Seine, France). This assay is a sandwich ELISA employing two monoclonal antibodies.
We selected these parameters as surrogate markers of cardiovascular and thrombotic risk because previous studies in both general and transgender population have provided evidence of their association with cardiovascular and thrombotic events.4–8,15
Hormone measurementThe hormonal analysis were performed in the hormonal laboratory of our centre, applying the standard procedures as follows: sex hormone binding globulin (SHBG), estradiol, luteinizing hormone (LH), follicle stimulating hormone (FSH), dehydroepiandrosterone sulfate (DHEAs), thyroid stimulating hormone (TSH), free thyroxine (FT4) and prolactin were measured using chemiluminometric immunoassays run on the ADVIA Centaur Immunochemistry analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY). Total testosterone was measured by a chemiluminometric immunoassay (Testosterone II, Elecsys; Roche, Mannheim, Germany). Androstenedione was measured by using an immunoradiometric assay (ZenTech, Angleur, Belgium).
Study of endothelial dysfunctionFlow mediated dilation (FMD)The study was conducted using a 10MHz linear probe Ultrasonix SonixTouch (brachial ultrasound) and Brachial Analyzer v.6 MIA software. For the evaluation of FMD in the brachial artery, patients were assessed after 10–12h of fasting and caffeinated beverages abstinence, at rest, in a dark room with a stable temperature of 22°C.
Brachial ultrasound was performed with the patient at supine position, after resting 10min. The brachial artery was assessed longitudinally, 5–10cm above the antecubital fossa. A sphygmomanometer was placed on the forearm to create flow stimulation in the brachial artery. Sphygmomanometer was inflated until the systolic pressure was above 50mmHg for 5min, thus stopping the antegrade flow and creating ischaemia. When the sphygmomanometer was deflated a reactive hyperemia occurs in the brachial artery. The % difference between the diameter measured after reactive hyperemia and the basal diameter was taken as FMD. Reference images were recorded continuously for 4min. Values were expressed as percentages.
Carotid ultrasound imaging studyB-mode ultrasound imaging was performed using a Siemens Acuson X300. A standardized imaging protocol was performed in all the subjects to evaluate carotid intima–media thickness (CIMT), defined as the distance between the lumen–intima and the media–adventitia ultrasound interfaces (intima–media complex), and plaque presence in the carotid arteries. The patients were placed in the supine position with the head turned 45° contralateral to the side of scanning. Images were obtained in longitudinal sections, the B-mode images of the left and right vessels were recorded and electronically stored. The data (mean IMT and mean-maximum IMT) from each segment were provided, and the right and left-side values were averaged to obtain the mean and mean-maximum common carotid (CC), carotid bulb (BULB), and internal carotid (ICA) measurements. Plaques were identified using B-mode and colour Doppler examinations in both longitudinal and transverse planes to considerer circumferential asymmetry and were defined as a “focal structure that encroaches into the arterial lumen of at least 0.5mm or 50% of the surrounding IMT value or demonstrates a thickness of 1.5mm, as a measured from the media adventitia interference to the intima–lumen surface” according to the Mannheim consensus.16
We chose FMD and IMT measurement as markers of pre-clinic atherosclerosis following previous findings in the general population.
Statistical analysisDue to our small sample size and the non-normal distribution of most of the variables observed, we underwent non-parametric tests to analyze the contrast between paired samples. Descriptive data are presented as the median and interquartile range (IQR) for continuous variables, or number and percentage (%) for categorical variables. Non-parametric test for paired samples (Wilcoxon) was used to observe differences between baseline data and at 6 and 12 months of treatment. Spearman correlation coefficients were obtained to assess the association between quantitative data (metabolic parameters, FMD and IMT). All analyses were performed using SPSS version 22 (SPSS, Chicago, IL, USA) and the level of significance was established at the two-sided 5% level.
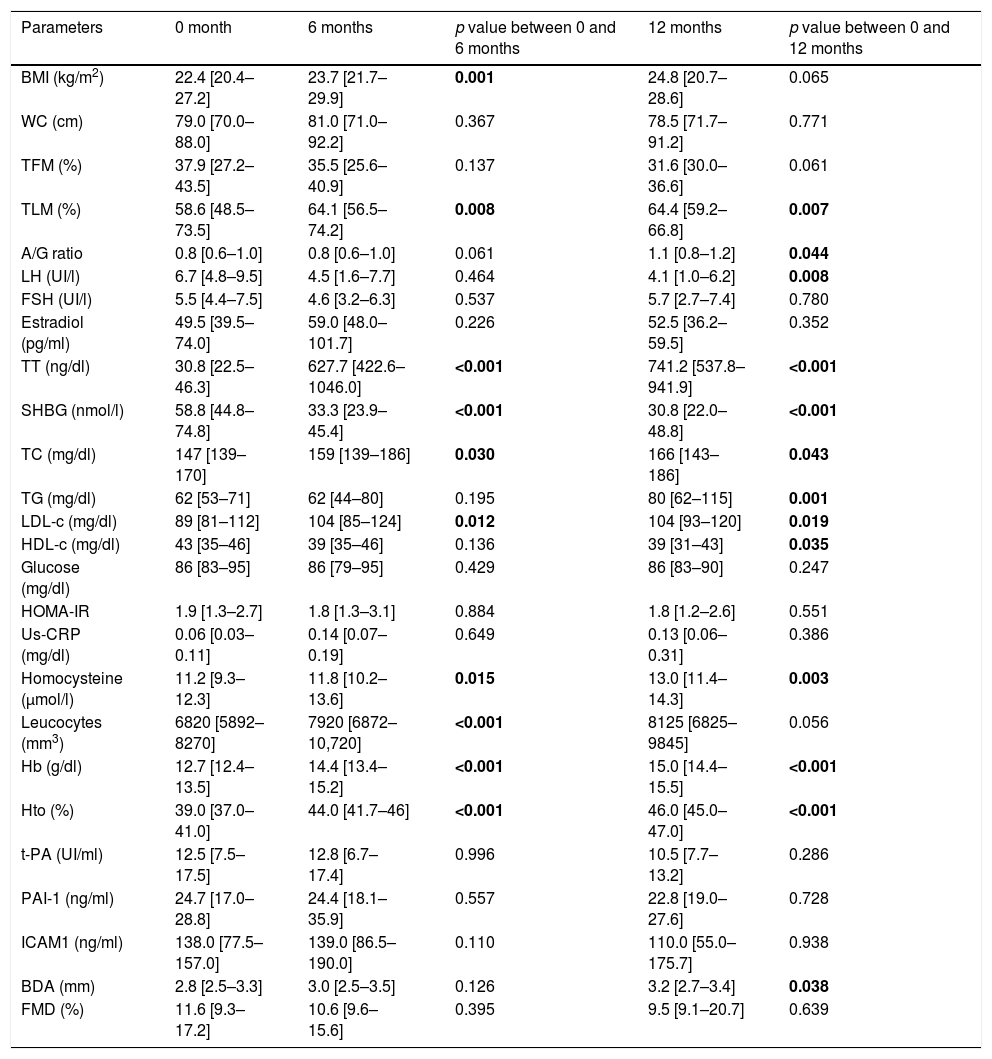
ResultsAnthropometric, hormonal and metabolic changes after 6 and 12 months of HTAn increase in BMI at 6 months in comparison to basal point (22.4 [20.4–27.2] vs 23.7 [21.7–29.9]kg/m2) (p=0.001) was observed, which remains stable at 12 months. Also, an increase in total testosterone were observed at 6 months (30.8 [22.5–46.3] vs. 627.7 [422.6–1046.0]ng/dl) and 12 months (741.2 [537.8–941.9]ng/dl) (p<0.001) together with a decrease of LH (6.7 [4.8–9.5] vs. 4.1 [1.0–6.2]UI/l) (p=0.001) at 12 months, SHBG (58.8 [44.8–74.8] vs 33.3 [23.9–45.4]nmol/l) (p<0.001) at 6 months (30.8 [22.0–48.8]nmol/l) (p<0.001) at 12 months.
Lipid profile was modified, with increased total cholesterol at 6 months (147 [139–170] vs. 159 [139–186]mg/dl) (p=0.030) and 12 months (166 [143–186]mg/dl) (p=0.043), LDL-c at 6 months (89 [81–112] vs 104 [85–124]mg/dl) (p=0.012) and 12 months (104 [93–120]mg/dl) (p=0.019) and triglycerides at 12 months (62 [53–71] vs 80 [62–115]mg/dl) (p=0.001), and decreased HDL-c at 12 months (43 [35–46] vs. 39 [31–43]mg/dl) (p=0.035), while no changes in glucose metabolism were observed. In addition, increases in homocysteine at 6 months (11.2 [9.3–12.3] vs. 11.8 [10.2–13.6]μmol/l) (p=0.015), at 12 months (13.0 [11.4–14.3]μmol/l) (p=0.003) and leucocytes at 6 months (6820 [5892–8270] vs 7920 [6872–10,720]mm3) (p<0.001), with no changes in hs-CRP. An increase in haemoglobin at 6 months (12.7 [12.4–13.5] vs 14.4 [13.4–15.2]g/dl) (p<0.001) at 12 months (15.0 [14.4–15.5]g/dl) (p<0.001) and haematocrit at 6 months (39.0 [37.0–41.0] vs. 44.0 [41.7–46]%) (p<0.001), and 12 months (46 [45–47]%) were observed, while there were no changes in fibrinogen, t-PA, PAI-1 and ICAMs (Table 1).
Changes after 6 and 12 months of HT in transgender men.
| Parameters | 0 month | 6 months | p value between 0 and 6 months | 12 months | p value between 0 and 12 months |
|---|---|---|---|---|---|
| BMI (kg/m2) | 22.4 [20.4–27.2] | 23.7 [21.7–29.9] | 0.001 | 24.8 [20.7–28.6] | 0.065 |
| WC (cm) | 79.0 [70.0–88.0] | 81.0 [71.0–92.2] | 0.367 | 78.5 [71.7–91.2] | 0.771 |
| TFM (%) | 37.9 [27.2–43.5] | 35.5 [25.6–40.9] | 0.137 | 31.6 [30.0–36.6] | 0.061 |
| TLM (%) | 58.6 [48.5–73.5] | 64.1 [56.5–74.2] | 0.008 | 64.4 [59.2–66.8] | 0.007 |
| A/G ratio | 0.8 [0.6–1.0] | 0.8 [0.6–1.0] | 0.061 | 1.1 [0.8–1.2] | 0.044 |
| LH (UI/l) | 6.7 [4.8–9.5] | 4.5 [1.6–7.7] | 0.464 | 4.1 [1.0–6.2] | 0.008 |
| FSH (UI/l) | 5.5 [4.4–7.5] | 4.6 [3.2–6.3] | 0.537 | 5.7 [2.7–7.4] | 0.780 |
| Estradiol (pg/ml) | 49.5 [39.5–74.0] | 59.0 [48.0–101.7] | 0.226 | 52.5 [36.2–59.5] | 0.352 |
| TT (ng/dl) | 30.8 [22.5–46.3] | 627.7 [422.6–1046.0] | <0.001 | 741.2 [537.8–941.9] | <0.001 |
| SHBG (nmol/l) | 58.8 [44.8–74.8] | 33.3 [23.9–45.4] | <0.001 | 30.8 [22.0–48.8] | <0.001 |
| TC (mg/dl) | 147 [139–170] | 159 [139–186] | 0.030 | 166 [143–186] | 0.043 |
| TG (mg/dl) | 62 [53–71] | 62 [44–80] | 0.195 | 80 [62–115] | 0.001 |
| LDL-c (mg/dl) | 89 [81–112] | 104 [85–124] | 0.012 | 104 [93–120] | 0.019 |
| HDL-c (mg/dl) | 43 [35–46] | 39 [35–46] | 0.136 | 39 [31–43] | 0.035 |
| Glucose (mg/dl) | 86 [83–95] | 86 [79–95] | 0.429 | 86 [83–90] | 0.247 |
| HOMA-IR | 1.9 [1.3–2.7] | 1.8 [1.3–3.1] | 0.884 | 1.8 [1.2–2.6] | 0.551 |
| Us-CRP (mg/dl) | 0.06 [0.03–0.11] | 0.14 [0.07–0.19] | 0.649 | 0.13 [0.06–0.31] | 0.386 |
| Homocysteine (μmol/l) | 11.2 [9.3–12.3] | 11.8 [10.2–13.6] | 0.015 | 13.0 [11.4–14.3] | 0.003 |
| Leucocytes (mm3) | 6820 [5892–8270] | 7920 [6872–10,720] | <0.001 | 8125 [6825–9845] | 0.056 |
| Hb (g/dl) | 12.7 [12.4–13.5] | 14.4 [13.4–15.2] | <0.001 | 15.0 [14.4–15.5] | <0.001 |
| Hto (%) | 39.0 [37.0–41.0] | 44.0 [41.7–46] | <0.001 | 46.0 [45.0–47.0] | <0.001 |
| t-PA (UI/ml) | 12.5 [7.5–17.5] | 12.8 [6.7–17.4] | 0.996 | 10.5 [7.7–13.2] | 0.286 |
| PAI-1 (ng/ml) | 24.7 [17.0–28.8] | 24.4 [18.1–35.9] | 0.557 | 22.8 [19.0–27.6] | 0.728 |
| ICAM1 (ng/ml) | 138.0 [77.5–157.0] | 139.0 [86.5–190.0] | 0.110 | 110.0 [55.0–175.7] | 0.938 |
| BDA (mm) | 2.8 [2.5–3.3] | 3.0 [2.5–3.5] | 0.126 | 3.2 [2.7–3.4] | 0.038 |
| FMD (%) | 11.6 [9.3–17.2] | 10.6 [9.6–15.6] | 0.395 | 9.5 [9.1–20.7] | 0.639 |
BMI: body mass index; WC: waist circumference; SBP: systolic blood pressure; DBP: diastolic blood pressure; TFM: total fat mass; TLM: total lean mass; A/G R: android/gynoid ratio; LH: luteinizing hormone; FSH: follicle stimulating hormone; TT: total testosterone; SHBG: sex hormone binding globulin; TC: total cholesterol; TG: triglycerides; LDL-c: low density lipoprotein-cholesterol; HDL-c: high density lipoprotein-cholesterol; HOMA-IR: homeostasis model assessment-insulin resistance, Hb: haemoglobin; Hto: haematocrit.; ICAMs: intercellular adhesion molecules; t-PA: tissue plasminogen activator; PAI-1: t-PA inhibitor; BDA: baseline diameter of artery; FMD: flow mediated dilation.
In the study of body composition with DXA we observed a decrease in total fat mass (TFM) at 12 months (37.9 [27.2–43.5] vs 31.6 [30.0–36.6]%) (p=0.061), increase in total lean mass (TLM) at 6 months (58.6 [48.5–73.5] vs. 64.1 [56.5–74.2]%) (p=0.008) and 12 months (64.4 [59.2 – 66.8] %) (p=0.008), and increased android/gynecoid ratio at 12 months (0.8 [0.6 – 1.0] vs. 1.1 [0.8 – 1.2]) (p=0.044) (Table 1).
Endothelial dysfunctionNo changes in vascular response studied by FMD were observed in this group at 6 and 12 months of HT (Table 1).
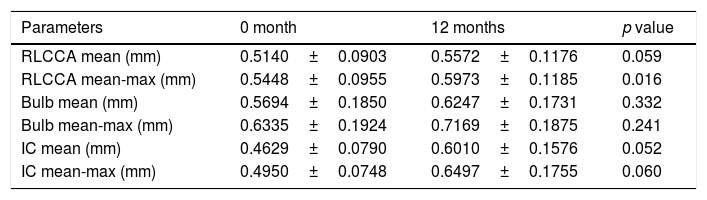
Carotid ultrasound – CIMTThe mean and mean-maximum IMT at different territories (common, bulb, and internal carotid) did not differ between baseline and at 12 months after HT, except that higher mean-maximum common IMT was observed at 12 months (0.5448±0.0955 vs. 0.5973±0.1185) (p=0.016). Only in one patient a carotid plaque was observed before beginning HT (Table 2).
Carotid ultrasound results in transgender men.
| Parameters | 0 month | 12 months | p value |
|---|---|---|---|
| RLCCA mean (mm) | 0.5140±0.0903 | 0.5572±0.1176 | 0.059 |
| RLCCA mean-max (mm) | 0.5448±0.0955 | 0.5973±0.1185 | 0.016 |
| Bulb mean (mm) | 0.5694±0.1850 | 0.6247±0.1731 | 0.332 |
| Bulb mean-max (mm) | 0.6335±0.1924 | 0.7169±0.1875 | 0.241 |
| IC mean (mm) | 0.4629±0.0790 | 0.6010±0.1576 | 0.052 |
| IC mean-max (mm) | 0.4950±0.0748 | 0.6497±0.1755 | 0.060 |
RLCCA: right-left common carotid artery; IC: internal carotid artery.
Our study describes the effects of testosterone on the metabolic pattern and cardiovascular risk profile, together with its early vascular effects, in a cohort of previously untreated transgender men under standard HT along 12 months. We observed an impairment of lipid profile, increase of homocysteine and leucocytes count, while changes in body composition with increased total lean mass together with decreased total fat mass were shown by the DXA study. Furthermore, higher mean-maximum common IMT was observed after 12 months of HT.
Both favourable and deleterious effects of HT on cardiovascular risk factors in transgender men have been described in literature, with little evidence on their actual clinical significance. The point of interest is to assess the cardiovascular situation in comparison with both their assigned and their desired sex. The clue to fill the gap between contradictory changes in surrogate risk factors and epidemiological evidence on morbidity and mortality may lay in the study of early vascular changes.
Hyperandrogenism in women, usually resulting from the polycystic ovarian syndrome, is associated with an unfavourable cardiovascular risk profile. However, hyperandrogenism in this context is usually clustered with features of the metabolic syndrome (hyperinsulinemia, visceral obesity, hypertension and dyslipidemia).5 In our group, we have seen only some features of hyperandrogenism related with cardiovascular risk, like worsening of lipid profile, android fat distribution, and increased total homocysteine levels; in this setting, CIMT has been found to be increased.
Regarding to lipid profile, changes were observed after 6 and 12 months of HT, with a significant increase in total cholesterol, triglycerides and LDL-c, and decrease in HDL-c; in a retrospective study of our population by Quirós et al., we have seen the same findings.17 Even so, clinical relevance is still uncertain as all of the parameters except for HDL-c remained within normal range. A longer follow-up would be interesting to assess the changes in lipid profile in older transgender men and to see its repercussion at the level of clinical cardiovascular disease.
Many studies in both men and women have shown that the administration of androgens reduces HDL-c levels.6,17 An extreme reduction of androgen levels has been shown to increase HDL-c levels in young men, with subsequent decrease to normal male levels when serum androgens returned to normal.18 Gooren and Giltay concluded that testosterone is the major determinant of the sex difference in HDL-c levels.6
Although HDL-c exerts several potentially antiatherogenic actions, it is still unclear whether the HDL-lowering effect of testosterone leads to increased cardiovascular risk, as atherogenicity seems to be more related to its metabolism rather than to circulating levels.19
We have found an increase of total homocysteine levels after HT. Homocysteine is an independent risk factor for atherosclerotic and thrombotic disease.20 Higher total plasma homocysteine in healthy men in comparison to premenopausal women may be explained by their differences in sex steroids.21 Besides direct effects, anabolic effects of androgens on muscle mass and creatine–creatinine metabolism must be considered. Changes in serum creatinine levels correlated positively with changes in homocysteine levels in transgender men.7,22
Regarding to body composition under HT, we have seen an increase in total lean mass, android fat distribution and decrease in total fat mass; despite this last change, the data suggest that visceral fat may be increased in relation with android fat distribution. These findings are consistent with previous studies that showed an android pattern of fat distribution in transgender men after HT.23
Gooren et al., have shown that androgens lead to a quick depletion of subcutaneous fat, while it takes between 1 and 3 years for visceral fat accumulation to become manifest.24 Testosterone decreases total body fat mass through the activation of hormone sensitive lipase in adipocytes. Visceral fat accumulation has been found to be a risk factor for cardiovascular disease and for type 2 diabetes mellitus (T2DM).24
CIMT is an early marker of systemic atherosclerosis and provides a noninvasive method for the risk assessment of cardiovascular disease.25–28 CIMT is a well-established index of atherosclerosis that correlates with prevalence and incidence of coronary artery disease and stroke.29,30
A number of risk factors have been associated with the development of atherosclerosis in the carotid arteries. Risk factors that predict CIMT are the same that also predict coronary artery disease, in accordance with the evidence that atherosclerosis is a diffuse disease. These risk factors include increasing age, male sex, smoking, blood pressure, measures of adiposity such as body mass index waist to hip ratio, sedentary lifestyles, family history, ethnicity and the presence of diabetes or glucose intolerance. CIMT has also been reported to be associated with serum cholesterol, triglycerides levels, HDL-c, LDL-c, high sensitivity C-reactive protein, and asymmetric dimethylarginine.26–30
In our study, we have seen an increase of mean-maximum common IMT after 12 months of HT and compared to Catalan population with the same age and birth assigned sex (75th percentile) and identified gender (50th percentile), indicating initial evidence of vascular changes.31 To our knowledge, there are no previous studies on CIMT in transgender; on the other hand, arterial stiffness has been investigated in transgender and hypogonadal patients, showing that testosterone did not affect arterial stiffness.32,33 In accordance with these works, we did not find changes in FMD after HT.
LimitationsOur study has several limitations. Although ours is a transgender referral centre, the number of included patients was low due to our stringent inclusion criteria intended to avoid confounding factors (like previous hormonal treatment, tobacco use, cardiovascular risk factors or HIV infection). Follow-up time may be too short to detect vascular changes or certain effects on body composition. The strength of our study is that our subjects had received no previous hormonal treatment and had no other risk factors that could interfere in our results; in addition, practically all of them followed the same HT (testosterone undecanoate). This may partially explain the differences between our results and the current evidence.
ConclusionOur work has shown changes in metabolic and inflammatory parameters after HT in a short-medium follow-up. These results could suggest an increase in cardiovascular risk in transgender people with hormonal treatment, together with initial evidence on vascular changes. Long-term follow-up is essential to determine whether these changes may lead to an increased cardiovascular morbidity and mortality.
Authors’ contributionsThe experiments were conceived and designed by Irene Halperin, Mireia Mora. The experiments were performed by Gloria Aranda, Emilio Ortega and Josep Vera. Data was analyzed by Gloria Aranda, Mireia Mora and Irene Halperin. Gloria Aranda managed the literature searches, performed statistical analysis and prepared the main draft of the manuscript. Irene Halperin, Felicia Hanzu contributed with reagents/material/analysis tools. Irene Halperin contributed to the writing of the manuscript. Irene Halperin, Mireia Mora, Felicia Hanzu, Emilio Ortega revised the manuscript and contributed significantly to the intellectual value of the paper.
Conflict of interestsThe authors declare that there is no conflict of interests.
We are grateful to transgender people for their willingness to participate in the study. We thank Yaiza Esteban from CIBERDEM/IDIBAPS and Mónica Romo from the Group of Endocrine Disorders – IDIBAPS for her help with the study protocol.
Special thanks to Sean Calvo for his English version of the manuscript.