The important prevalence and morbidity of obesity has generated an increase in bariatric surgery. It has a positive effect in obesity-related comorbidities. However, it's detrimental to bone health. The underline pathophysiological mechanisms are complex and heterogeneous. The knowledge of these factors may lead us to develop an adequate therapeutic intervention.
La importante prevalencia y morbimortalidad de la obesidad ha ocasionado un aumento de pacientes sometidos a cirugía bariátrica. Son numerosos los beneficios reportados de la cirugía de la obesidad en distintas esferas de la salud. Sin embargo, no ocurre lo mismo sobre el hueso, donde tiene un impacto negativo. Los mecanismos fisiopatológicos que subyacen en el deterioro del tejido óseo de estos pacientes son complejos y requieren de un estudio en profundidad. El adecuado conocimiento de estos factores permitirá adoptar las herramientas más oportunas para un adecuado abordaje terapéutico.
Obesity is a leading health problem due to its high prevalence and consequences in terms of morbidity and mortality. According to the World Health Organization (WHO), obesity and overweight have reached epidemic proportions worldwide. In 2014, over 1900 million adults were overweight, and of these, more than 500 million were obese. Furthermore, if this trend is not reversed, the figures can only be expected to worsen.1 In Spain, data from the ENPE trial (a cross-sectional study designed to represent the Spanish population of adults between 25 and 64 years of age) estimated the prevalence of overweight and general obesity to be 39.3% and 21.6%, respectively.2
This alarming rise has led to an increase in patients undergoing surgical treatment for obesity. In this regard, bariatric surgery (BS) is an effective treatment option for such problems, not only to secure weight control, but also to deal with the associated comorbidities (type 2 diabetes mellitus, arterial hypertension, respiratory disease, dyslipidemia, etc.).3,4 By contrast, with regard to bone tissue, such surgery appears to have a negative effect that must be taken into account, with changes in bone mineral density (BMD) and bone microarchitecture.5 The unfortunate consequence of this is the occurrence of fragility fractures. A number of retrospective studies have shown a significant increase in fracture risk among patients subjected to bariatric surgery as compared to controls.6–8 This increased risk of fracture is greater in the case of surgeries characterized by a greater malabsorptive component.9 However, considering the limitations inherent in retrospective studies, further prospective investigations are needed.
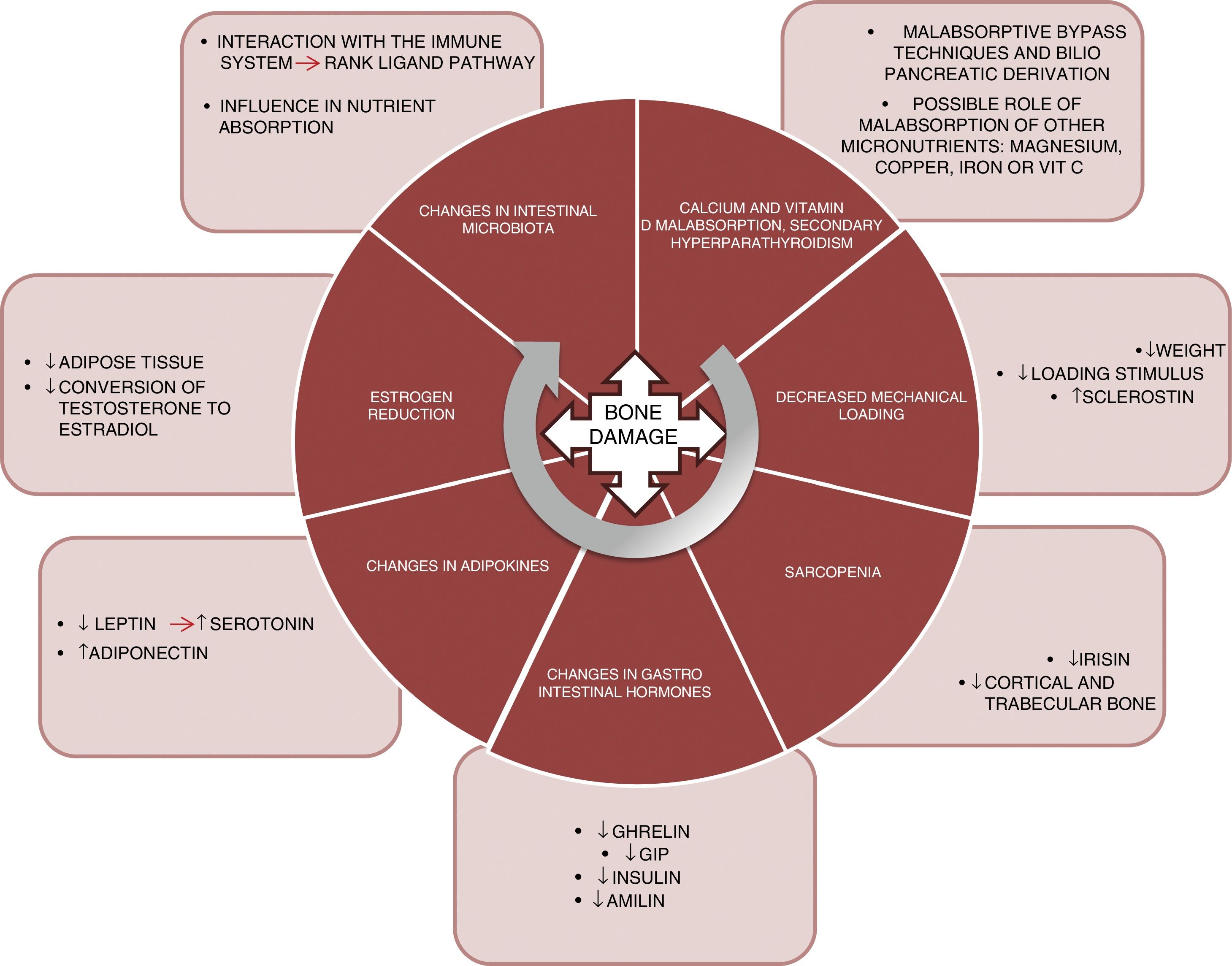
PhysiopathologyThe mechanisms underlying this deleterious effect upon bone are varied and are not yet fully understood. Fig. 1 summarizes the main factors known to date and which are detailed below:
Malabsorption. The first implicated factor is calcium and vitamin D malabsorption. Techniques such as bypass surgery or biliopancreatic diversion alter the absorption of these nutrients, which may lead to secondary hyperparathyroidism.10 Calcium and phosphorus are essential nutrients in bone mineralization, and thus condition bone resistance to fragility fractures. In addition, vitamin D plays a key role in bone metabolism. Vitamin D absorption takes place in the jejunum and ileum, and is therefore affected by surgical procedures that exclude part of that intestinal segment. Vitamin D deficiency after bariatric surgery has been widely documented,11 with prevalences of 44% after gastric bypass surgery and 50% after biliopancreatic diversion.12
Another aspect that should be taken into consideration is whether the deficiency of other micronutrients such as magnesium also plays a relevant role in worsened bone health among patients.13 Magnesium deficiency is relatively common after surgery. Dalcanale et al. reported a magnesium deficiency rate of 32.1% after gastric bypass surgery.14 Hypomagnesemia is associated with decreased bone mass, because it influences PTH secretion and bone response to this hormone, giving rise to hypocalcemia refractory to calcium supplementation. Other micronutrients such as vitamin C and certain trace elements (copper, manganese and zinc) act as cofactors in the synthesis of bone matrix proteins and matrix metalloproteinase collagenases, needed for normal bone metabolism, and a deficiency of these nutrients could constitute another cause of decreased bone mass in these patients. Problems with the absorption of these nutrients after gastric bypass surgery15 and biliopancreatic diversion have also been described.16
Lastly, there are doubts about the role of iron in fracture risk. A cross-sectional cohort trial, the Osteoporotic Fractures in Men Study (MrOs), showed that transferrin saturation and serum iron were independent predictors of FGF-23 levels.17 Accordingly, decreased iron levels could cause FGF-23 elevation, resulting in decreased phosphorus reabsorption and lowered 1,25(OH)2D3 synthesis, and thereby promote bone demineralization. However, further studies are needed to confirm the correlation between iron metabolism and FGF-23 in these patients, since some sources report the 10-year prevalence of iron deficiency after bypass surgery to be about 20%.18
Load reduction. Osteocytes are the bone tissue cells that act as mechanosensors, detecting changes in load through variations in canalicular flow that modulate the levels of both sclerostin and DKK1 (Wnt anabolic signaling pathway inhibitors). Thus, in patients subjected to bariatric surgery, significant weight loss causes a decrease in this mechanical stimulus, with a resulting increase in sclerostin levels. Muschitz et al. conducted a study in 52 premenopausal women subjected to bariatric surgery (bypass or sleeve technique) and followed-up on for 24 months. A significant increase was seen in sclerostin and in the CTX resorption marker, as well as a decrease in BMD after surgery.19 Another study conducted in 107 obese adults randomized to four groups (control, diet, exercise, and exercise with diet) documented a significant weight loss in two of the groups (diet and diet with exercise). However, in these two groups, sclerostin only increased in the diet group. It was therefore concluded that the mechanical stimulus produced by exercise is essential for regulating sclerostin levels.20
Sarcopenia. Weight loss caused by bariatric surgery does not occur only at the expense of fat mass. Just as in hypocaloric diet plans, such surgery is also accompanied by a loss of muscle mass.21 Muscle plays an important role in bone metabolism, and there is increasing documentation in this regard. In effect, there is a “dialogue” between bone and muscle, involving important myokines such as irisin. In a murine model of bone mass loss induced by suspension, the administration of recombinant irisin was shown to preserve trabecular and cortical bone density, as well as trabecular volume measured by micro-CT.22 In the MrOS cohort (5994 males), fracture risk was assessed in patients with sarcopenia, sarcopenic obesity, obesity without sarcopenia, or none of these conditions. A significant increase in fracture risk was observed in subjects with sarcopenia alone (hazard ratio [HR] 2.02; 1.06–3.84) and in patients with sarcopenic obesity (HR 3.11; 1.18–8.17).23
Changes in gastrointestinal hormones. The gastrointestinal tract is the largest endocrine organ of the body. The concentrations of a number of hormones are affected after surgery for obesity. Ghrelin is one of the most important. This hormone is mainly secreted by the gastric fundus and duodenum. It has a positive effect on bone, stimulating bone formation,24 as well as an indirect effect, since it stimulates growth hormone (GH) secretion. Changes in ghrelin levels after surgery depend on whether the gastric reservoir excludes the fundus or not. Thus, as expected, a significant decrease in ghrelin is seen in patients subjected to vertical gastrectomy or duodenal switch, where the reservoir is vertical.25–27
Gastric inhibitory polypeptide (GIP) is secreted in the duodenum and proximal jejunum, and exerts a positive effect upon bone, preventing bone resorption and promoting bone formation, as has been demonstrated in animal models.28 A significant decrease in GIP has been observed in surgeries that exclude the duodenum, such as bypass surgery and biliopancreatic diversion.29,30
Secretion of GLP-1 and peptide YY occurs in the terminal ileum. Although animal models have shown a positive effect upon bone,31 the role of these peptides in human bone metabolism is not well understood, as heterogeneous results have been obtained.32 After surgery for obesity, a very significant increase in postprandial GLP-1 secretion has been reported.33,34
Lastly, the pancreatic hormones insulin35 and amylin36 are similarly affected after bariatric surgery, and are significantly decreased in the context of diminished insulin resistance. As is well known, both of these hormones exert an anabolic effect upon bone.37
Adipokine changes. Leptin is mainly secreted by white adipose tissue and experiences a significant decrease after bariatric surgery, related to fat mass loss.38 By contrast, adiponectin increases after bariatric surgery.39 The role of leptin in bone has been subject to controversy. In the ob/ob mouse model (animals with genetic leptin deficiency), treatment with leptin induces an increase in bone formation,40 and in the experimental setting appears to have a beneficial effect, favoring osteoblast differentiation.41 However, this effect has not been demonstrated in clinical studies. Recently, a study has proposed a new pathway for leptin action in bone remodeling through serotonin. The study was carried out in genetically modified mice lacking cerebral serotonin (due to the suppression of the gene encoding for tryptophan hydroxylase, Tph2 −/−).42 Serotonin has been shown to have a potent effect in the inhibition of bone formation, and one would expect serotonin levels to be elevated after surgery for obesity, this being consistent with the loss of bone mass seen after surgery.
Estrogen reduction. Bariatric surgery results in a rapid loss of adipose tissue that can affect the aromatase-mediated conversion of testosterone into estradiol.43,44 Estrogens play an important and well documented role in regulating bone metabolism, particularly in the inhibition of bone resorption, which promotes osteoclast apoptosis through increased TGF-β. In addition, they inhibit the production of IL-6 (a major resorption stimulus), and participate in the regulation of the main resorption pathway (the RANK ligand pathway).45,46
Lastly, a further field for research is the manner in which changes in intestinal microbiota affect the bone metabolism of these surgical patients. Modifications in the intestinal microbiota have been reported after different bariatric surgical procedures.47 The microbiota may influence bone metabolism through various mechanisms such as nutrient absorption, or through interaction with the immune system, regulating osteoclastogenesis through the RANK ligand pathway.48
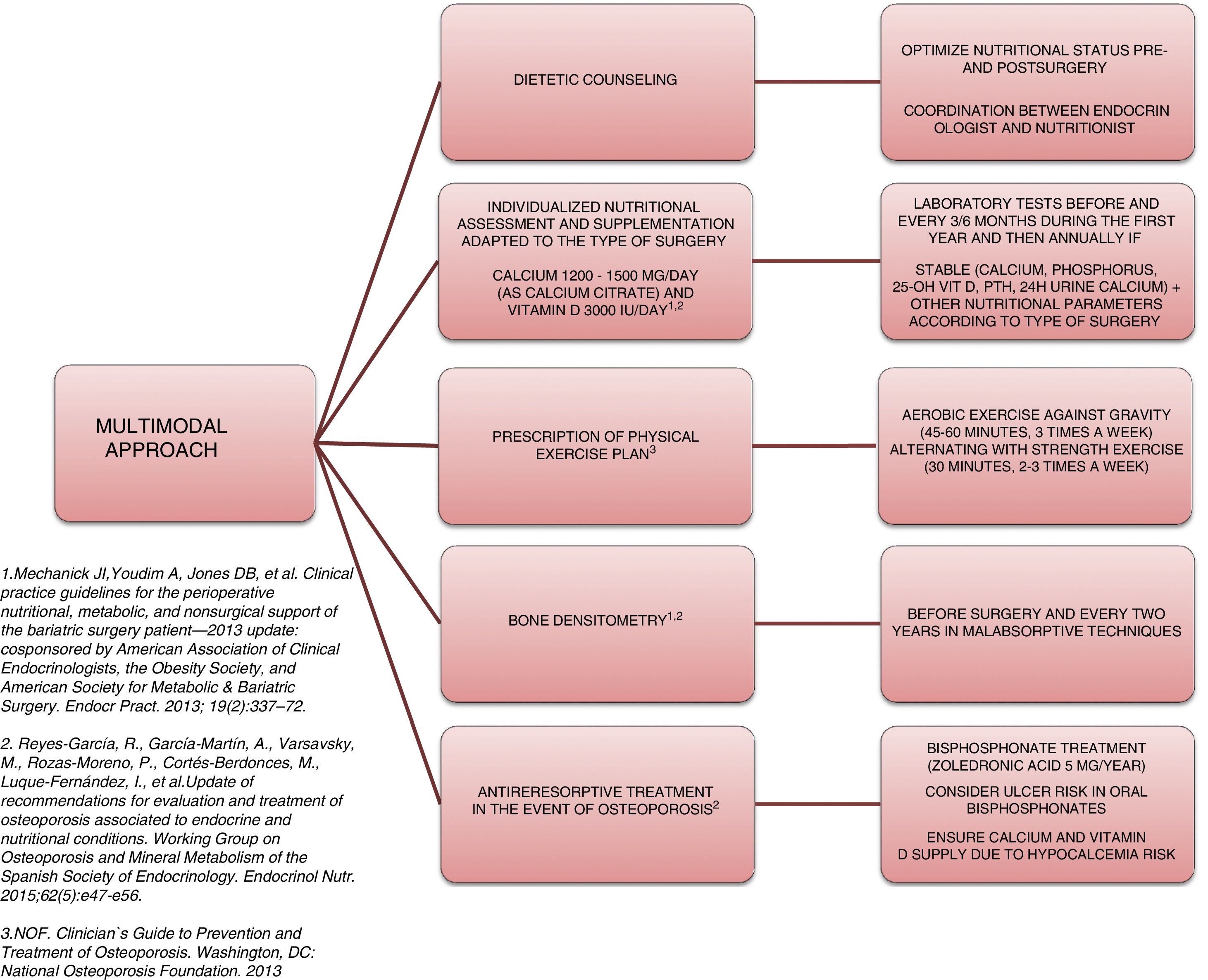
Approach and treatment planThe complexity and heterogeneity of the physiopathology underlying the bone alterations in these patients requires a multimodal approach (Fig. 2).
The first step is to ensure adequate nutritional status. This requires patient evaluation and follow-up by a specialist in endocrinology and nutrition, providing nutritional counseling before and after surgery, and the prescription of supplements to avoid nutritional deficiencies. The recommendations of most scientific bodies, including the American Society for Metabolic and Bariatric Surgery49 and the osteoporosis and mineral metabolism working group of the SEEN,50 recommend supplementation with 1200–1500mg/day of calcium (as calcium citrate) and 3000IU/day of vitamin D. White et al., in a cohort of 283 patients supplemented after surgery with 1200mg of calcium citrate and 1000IU of vitamin D3, recorded a secondary hyperparathyroidism incidence of 46.6%.51 In this regard, when supplementing, it is advisable to titrate the vitamin D levels periodically until concentrations of 30ng/ml 50have been reached.
Another important aspect in the multimodal approach to these patients is the need to prescribe physical exercise in order to prevent or treat sarcopenia and accelerated muscle mass loss, thereby favoring bone metabolism. An interventional study involving 220 patients who had been subjected to sleeve or bypass surgery and randomized to the intervention or control group reported lesser BMD loss and a significantly lower increase in bone remodeling markers.52 In that study, patients in the intervention group were supplemented with 28,000IU of cholecalciferol weekly during the 8 weeks prior to surgery, followed by supplementation with 16,000IU/week, 1000mg of calcium citrate, and a daily protein supplement adjusted to the BMI (range 35–60g/day). All the patients in this group started an exercise program, monitored by experts, two weeks after surgery: Nordic walking adapted to heart rate (minimum 45min, 3 times a week) and strength exercises (minimum 30min, 2 times a week). These results are consistent with those reported by Campanha-Versiani et al. in a group of 37 obese patients subjected to bypass surgery and enrolled in a supervised aerobic physical exercise program (against gravity and strength).53 Taking into account the published evidence, the National Osteoporosis Foundation 54recommends two types of effective exercises for preserving bone density: 1) body weight-bearing exercises, i.e., activities performed against gravity (such as walking, jumping, Nordic walking, etc.); and 2) strength exercises, where muscle contraction is performed against external resistance. Moreover, regular exercise improves agility, strength and balance, and may help in preventing falls. Such measures therefore should be present in the management plan of patients subjected to bariatric surgery.
Bone assessment using densitometry (DXA) is useful for evaluating these patients. Many prospective studies have reported BMD loss after bariatric surgery.55 Although it has been speculated that this decrease in density could represent a normal adaptive mechanism, the fact is that there is a significant increase in fractures in this population. Thus, the osteoporosis and mineral metabolism working group of the SEEN recommends DXA in order to monitor BMD at baseline before surgery and every two years thereafter in patients subjected to surgery with a malabsorptive component (Roux-en-Y gastric bypass, biliopancreatic diversion). The working group also recommends the determination of parathyroid hormone, calcium, phosphorus, 25-OH-vitamin D, and 24-hour urine calcium at baseline and then every 6–12 months.50 The main limitation of DXA is that it only offers quantitative and non-qualitative information regarding bone; as a result, other bone assessment methods are required. Few studies have analyzed the usefulness of new bone assessment techniques such as the Trabecular Bone Score. The results in this regard do not appear to be consistent. A prospective study involving 38 patients subjected to bypass surgery revealed no significant decrease in Trabecular Bone Score after three years of follow-up.56 By contrast, Muschitz et al.,52 in the abovementioned study, observed reductions after surgery in both the control group and in the patients subjected to surgery with calcium, vitamin D and protein supplements, plus exercise, though the decrease was significantly greater in the control group (10.5% vs. 3.4%; p=0.03). As with DMX, the main limitation of this technique is that excess fat in the lumbar region attenuates and limits the evaluation. It is therefore not advisable to use this technique in patients with a BMI>37kg/m2.
Bone remodeling markers are a simple tool for assessing bone metabolism. A number of studies have documented significant increases in such markers after surgery, and these changes are moreover maintained over the long term. A prospective study of patients who had been subjected to gastric bypass surgery recorded a significant increase (at 1 and 2 years) in both the bone formation marker P1NP and in the bone resorption marker CTX.57 Similar results were reported by Biagioni et al. likewise after gastric bypass surgery, with significant increments three months following the intervention in the markers CTX (0.2 [0.1; 2.2] vs. 0.6 [0.4; 6.0]ng/ml), RANK ligand (0.1 [0.0; 0.5] vs. 0.3 [0.0; 2.0]pmol/l) and sclerostin (21.7 [3.2; 75.1] vs. 34.8 [6.4; 80.5]pmol/l) (p<0.005).39 The CTX resorption marker has shown a strong correlation to the resorption process. Its measurement in serum affords more reproducible results and reduces the variability of measurement in urine (which should be corrected for creatinine excretion). Furthermore, the latest techniques developed for the measurement of CTX in serum have been shown to markedly decrease both within- and between-test variations. As a result, the consensus document published by the IOF and the IFCC58 recommends its measurement as a reference bone resorption marker in the design of observational and interventional studies. However, the usefulness of this tool in monitoring these patients remains to be established.
Lastly, when osteoporosis is diagnosed, treatment should be started with an antiresorptive agent. Bisphosphonates should be considered in such cases, though the fact that oral formulations can cause ulceration of the anastomotic mouth, as well as absorption problems should be taken into account. Accordingly, the osteoporosis and mineral metabolism working group of the SEEN50 recommends zoledronic acid 5mg once a year or ibandronate 3mg every three months as the treatment of choice. In the presence of a high risk of fracture, denosumab or an anabolic agent such as teriparatide may be considered. In any case, an adequate calcium and vitamin D supply should be ensured when treatment for osteoporosis is started, since the introduction of antiresorptive therapy can cause severe hypocalcemia.
ConclusionsIn conclusion, the physiopathology underlying bone tissue impairment in patients subjected to bariatric surgery is complex. A multimodal approach to such patients is required in the pre- and postsurgery period. Specialists in endocrinology and nutrition play a key role in the adequate management of these patients. Imaging tools for bone assessment (such as DXA and the Trabecular Bone Score) are limited by excess adipose tissue at the lumbar level. Regarding the clinical usefulness of other tools such as bone remodeling markers, there is insufficient evidence as to their clinical usefulness, a fact that makes the management of these patients more difficult. Further studies are needed to optimize follow-up and prevent the occurrence of fragility fractures.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Botella Martínez S, Petrina Jauregui E, Escalada San Martín J. Impacto de la cirugía bariátrica en el tejido óseo. Endocrinol Nutr. 2019;66:62–68.