The incidence of papillary thyroid microcarcinoma (PTMC) has increased in recent years, especially in patients operated on for presumably benign thyroid disease. The aim of this study was to analyze the differences between PTMC incidentally diagnosed and PTMC clinically diagnosed, as well as its long-term prognosis.
Material and methodsThe study population consisted of patients with a histological diagnosis of PTMC. Patients with previous thyroid surgery, other synchronous thyroid or extrathyroid malignancies and an ectopic location of PTMC were excluded. Two groups were compared: patients with PTMC incidentally diagnosed (group 1) and patients with PTMC clinically diagnosed (group 2). A multivariate analysis of differentiating factors was performed.
ResultsPTMC clinically diagnosed had a high frequency of hypothyroidism (4,6% vs. 18,9%; p = 0,004), extrathyroidal extension (5,7% vs. 17,6%; p = 0,018), metastatic lymph nodes (1,1% vs. 18,9%; p < 0,001) and lymphocytic thyroiditis (5,7% vs. 27%; p < 0,001). In the multivariate analysis, metastatic lymph nodes (OR 22,011, IC95% 2,621-184,829; p = 0,004) and lymphocytic thyroiditis (OR 4,949, IC95% 1,602-15,288; p = 0,005) were associated with the clinical diagnosis of PTMC. During a mean follow-up of 119,8 ± 65,1 months, one recurrence was detected in group 2 (0 vs. 1,4%; p = 0,460). No patient died due to the disease.
ConclusionsPTMC clinically diagnosed, although it has more aggressive histopathological characteristics (extrathyroidal extension and metastatic lymph nodes), presents a long-term prognosis similar to the PTMC incidentally diagnosed. The presence of metastatic lymph nodes and lymphocytic thyroiditis were independent factors associated with PTMC clinically diagnosed.
La incidencia del microcarcinoma papilar de tiroides (MCPT) está aumentando en los últimos años, especialmente en pacientes intervenidos por enfermedad tiroidea presumiblemente benigna. El objetivo de este estudio es analizar las diferencias entre el MCPT de diagnóstico incidental y de diagnóstico clínico preoperatorio, así como su pronóstico a largo plazo.
Material y métodosLa población a estudio la constituyen los pacientes con diagnóstico histológico de MCPT. Se excluyeron los pacientes con cirugía tiroidea previa, con otras patologías tiroideas o extratiroideas malignas sincrónicas y localización ectópica del MCPT. Se compararon dos grupos: pacientes con diagnóstico incidental (grupo 1) y con diagnóstico clínico (grupo 2). Se realizó un análisis multivariante de los factores diferenciadores.
ResultadosEl MCPT de diagnóstico clínico presentó mayor frecuencia de hipotiroidismo (4,6% vs. 18,9%; p = 0,004), invasión extracapsular (5,7% vs. 17,6%; p = 0,018), adenopatías metastásicas (1,1% vs. 18,9%; p < 0,001) y tiroiditis linfocitaria (5,7% vs. 27%; p < 0,001). En el análisis multivariante, la presencia de adenopatías metastásicas (OR 22,011, IC95% 2,621-184,829; p = 0,004) y la tiroiditis linfocitaria (OR 4,949, IC95% 1,602-15,288; p = 0,005) se asociaron con el diagnóstico clínico del MCPT. Durante un seguimiento medio de 119,8 ± 65,1 meses, se detectó una recidiva en el grupo 2 (0 vs. 1,4%; p = 0,460). Ningún paciente falleció debido a la enfermedad.
ConclusionesEl MCPT de diagnóstico clínico, aunque presenta características histopatológicas más agresivas (invasión extracapsular y de adenopatías metastásicas), presenta un pronóstico a largo plazo similar al MCPT incidental. La presencia de adenopatías metastásicas y tiroiditis linfocitaria fueron factores independientes asociados al MCPT de diagnóstico clínico.
Papillary thyroid microcarcinoma (PTMC) is papillary carcinoma measuring 1 cm or less in size.1 The incidence of PTMC has increased considerably in recent years,2 particularly in patients undergoing surgery for presumably benign thyroid disease, and may range from 1.3-21.6%.3–7
In addition to the increase in incidental diagnoses of PTMC, clinical diagnoses of the disease are also increasing due to the general use of high-resolution neck ultrasound. This technique allows for the identification of increasingly small nodules; assessment of the morphological and structural characteristics associated with possible malignancy; and more precise guidance of fine needle aspiration biopsy (FNAB) for subsequent cytological analysis.8
On comparing incidental and non-incidental differentiated thyroid cancer, without differentiation in terms of size (less than or greater than 1 cm) or histological type (papillary or follicular), an increased incidence of persistence, relapse and mortality associated with the disease has been observed in the non-incidental cases.9
Because of the increased incidence of PTMC, awareness of the differentiating factors and prognosis related to the type of diagnosis is important, since the findings in this regard may justify more or less aggressive treatments, making it possible for us to reduce surgical morbidity and mortality, disease persistence, recurrence and disease-related mortality.
The present study was carried out to analyze and compare the differentiating factors and the long term prognosis of PTMC diagnosed incidentally and diagnosed according to clinical criteria.
Material and methodsThe present study was carried out in compliance with the principles of the Declaration of Helsinki, and was approved by the Ethics Committee of our hospital. Informed consent was obtained from the patients.
Study populationThe study population consisted of patients with a histopathological diagnosis of PTMC (papillary carcinoma ≤ 1 cm1 in size) operated upon during the period 1995-2015.
Patients in whom the histopathological sample was available and with a minimum follow-up period of two years were included in the study.
Patients with a history of thyroid surgery before surgery due to the diagnosis of PTMC were excluded, as were subjects with other synchronous malignant thyroid or extrathyroid disease processes and ectopic locations of PTMC (sublingual, thyroglossal cyst, struma ovarii, etc.).
Study groupsThe following groups were analyzed and compared:
- •
Group 1: PTMC as an incidental diagnosis. An incidental diagnosis is defined as a casual diagnosis made by the pathologist in thyroidectomy specimens from patients undergoing surgery for presumably benign thyroid disease, or as an intraoperative biopsy-based diagnosis in patients undergoing surgery for benign thyroid or parathyroid disease.
- •
Group 2: PTMC as a preoperative clinical diagnosis. A clinical diagnosis is defined as a diagnosis made in the study of a thyroid nodule measuring ≤ 1 cm in size, with cytology findings suggestive of or positive for malignancy (categories iii, iv, v or vi of the Bethesda classification), or as a diagnosis established from the study of adenopathies proving positive for malignancy in the FNAB-based cytological analysis.
The following were analyzed in both groups:
- •
Persistence. Persistence is defined as the presence of clinical, biochemical or radiological evidence of disease (ultrasound, CT, whole body scintigraphy with 131I or PET-CT) in the first 6 months after surgery. Patients initially undergoing lobectomy, and in whom thyroidectomy is completed in the first 6 months with the detection of PTMC, are classified as representing total thyroidectomy and not persistence.
- •
Relapse. Relapse is defined as the presence of clinical, biochemical or radiological evidence of disease (ultrasound, CT, whole body scintigraphy with 131I or PET-CT) from 6 months after surgery.
- •
Disease-related mortality.
The following variables were used to compare the two study groups:
- 1)
Sociodemographic: age and gender. In relation to age, we established a cut-off point of 55 years in accordance with the 8th edition of the American Joint Committee on Cancer Staging Manual.10
- 2)
Clinical: a family history of papillary thyroid carcinoma, the body mass index (BMI) measured in kg/m2, thyroid function (euthyroidism, hypothyroidism and hyperthyroidism), mean plasma concentration of thyroid-stimulating hormone (TSH) (normal range: 0.27-4.2 mIU/ml) and free thyroxine (normal range: 0.93-1.7 ng/ml), and Graves’ disease. Hypothyroidism is defined as thyroid function with TSH > 4.2 mIU/ml or when the patient is receiving replacement therapy with levothyroxine. Hyperthyroidism is defined as thyroid function with TSH < 0.1 mIU/ml or when the patient is treated with antithyroid drugs.
- 3)
Treatment and stage: this refers to the surgical technique (patients undergoing lobectomy and where thyroidectomy is completed within the first 6 months are classified as representing total thyroidectomy), metabolic therapy with 131I and disease staging (according to the 8th edition of the American Joint Committee on Cancer10 manual).
- 4)
Histopathological parameters: size (defined by maximum lesion diameter in millimeters; for multifocal tumors, size is defined by the lesion with the greatest diameter). In relation to focality, a tumor is considered to be unifocal if there is a single site or as multifocal if there is more than one site. The maximum diameter of each of the tumor foci must be ≤ 10 mm (even if the sum of all the maximum diameters of the foci exceeds 10 mm). In terms of laterality, a tumor is considered to be unilateral if it is only located in one thyroid lobe, and bilateral if it is multifocal and is located in both thyroid lobes. A tumor located in a thyroid lobe and in the isthmus is considered to be unilateral. Other parameters are histological variants, capsular invasion, extracapsular invasion, the infiltration of resection margins, metastatic adenopathies, and the presence of chronic lymphocytic thyroiditis.
The study data were entered in a database. Categorical variables were reported as frequencies and percentages, and comparisons were made using the Pearson chi-squared test or the Fisher exact test, as required. Continuous quantitative variables were reported as the mean ± standard deviation (SD). Normal data distribution was verified using the Kolmogorov-Smirnov test. Quantitative variables of both groups were compared using the Student t-test for independent data in the presence of a normal distribution. The nonparametric Mann-Whitney U-test was used for the comparison of quantitative variables with a non-normal distribution.
Multivariate analysis was performed using binary logistic regression, with the initial introduction of all variables subject to analysis, in order to assess the independent associations of those factors found to be statistically significant in the bivariate analysis. Results were presented with the odds ratio (OR) and 95% confidence interval (CI), and p-value.
Statistical significance was considered for p < 0.05.
ResultsStudy groupsGroup 1. The diagnosis of PTMC was incidental in 54% of the patients (n = 87).
In patients with an incidental diagnosis of PTMC, the surgical indications were: thyroid nodule in 9.2% (n = 8), multinodular goiter in 56.3% (n = 49), toxic goiter in 14.9% (n = 13), Graves’ disease in 14.9% (n = 13), prophylactic thyroidectomy in MEN 2A syndrome in 2.3% (n = 2), and primary hyperparathyroidism in 2.3% (n = 2).
Group 2. The diagnosis of PTMC was clinical in 46% of the patients (n = 74), based on the cytology findings of a thyroid nodule FNAB in 89.2% (n = 66) and on the cytology findings of a metastatic adenopathy FNAB in 10.8% (n = 8).
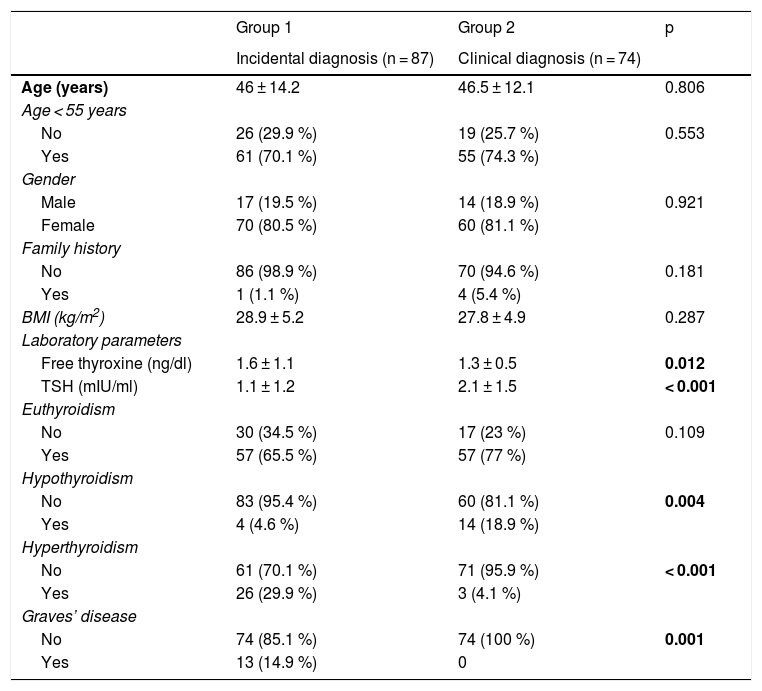
Differentiating factors between the two groupsSociodemographic and clinical factorsThe patients with clinically diagnosed PTMC had a higher frequency of hypothyroidism (18.9% vs. 4.6%; p = 0.004), less frequent hyperthyroidism (4.5% vs. 29.9%; p < 0.001), higher mean plasma TSH (2.1 ± 1.5 vs. 1.1 ± 1.2 mIU/ml; p < 0.001), lower mean plasma free thyroxine concentration (1.3 ± 0.5 vs. 1.6 ± 1.1 ng/ml; p = 0.012), and a lower prevalence of Graves’ disease (0% vs. 14.9%; p = 0.001) (Table 1).
Comparison of the patient sociodemographic and clinical variables according to the type of diagnosis of PTMC.
| Group 1 | Group 2 | p | |
|---|---|---|---|
| Incidental diagnosis (n = 87) | Clinical diagnosis (n = 74) | ||
| Age (years) | 46 ± 14.2 | 46.5 ± 12.1 | 0.806 |
| Age < 55 years | |||
| No | 26 (29.9 %) | 19 (25.7 %) | 0.553 |
| Yes | 61 (70.1 %) | 55 (74.3 %) | |
| Gender | |||
| Male | 17 (19.5 %) | 14 (18.9 %) | 0.921 |
| Female | 70 (80.5 %) | 60 (81.1 %) | |
| Family history | |||
| No | 86 (98.9 %) | 70 (94.6 %) | 0.181 |
| Yes | 1 (1.1 %) | 4 (5.4 %) | |
| BMI (kg/m2) | 28.9 ± 5.2 | 27.8 ± 4.9 | 0.287 |
| Laboratory parameters | |||
| Free thyroxine (ng/dl) | 1.6 ± 1.1 | 1.3 ± 0.5 | 0.012 |
| TSH (mIU/ml) | 1.1 ± 1.2 | 2.1 ± 1.5 | < 0.001 |
| Euthyroidism | |||
| No | 30 (34.5 %) | 17 (23 %) | 0.109 |
| Yes | 57 (65.5 %) | 57 (77 %) | |
| Hypothyroidism | |||
| No | 83 (95.4 %) | 60 (81.1 %) | 0.004 |
| Yes | 4 (4.6 %) | 14 (18.9 %) | |
| Hyperthyroidism | |||
| No | 61 (70.1 %) | 71 (95.9 %) | < 0.001 |
| Yes | 26 (29.9 %) | 3 (4.1 %) | |
| Graves’ disease | |||
| No | 74 (85.1 %) | 74 (100 %) | 0.001 |
| Yes | 13 (14.9 %) | 0 | |
Statistically significant differences in bold.
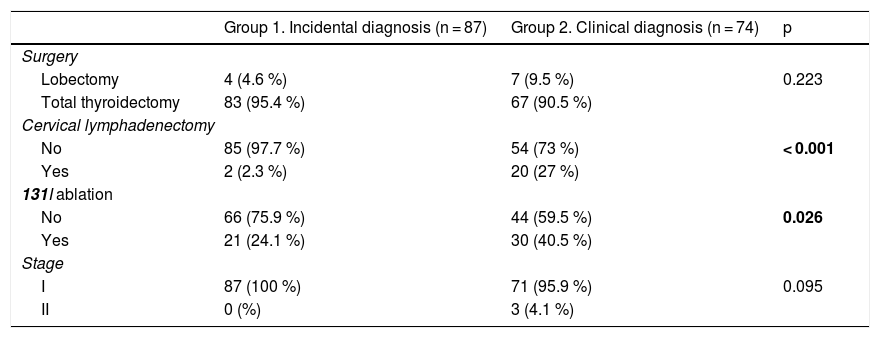
Cervical lymphadenectomy was more often performed in patients with clinically diagnosed PTMC (27% vs. 2.3%; p = 0.001), as was the ablation of thyroid remnants with 131I (40.5% vs. 24.1%; p = 0.026). There were no differences in terms of tumor stage (Table 2).
Comparison of treatment and staging variables according to the type of diagnosis of PTMC.
| Group 1. Incidental diagnosis (n = 87) | Group 2. Clinical diagnosis (n = 74) | p | |
|---|---|---|---|
| Surgery | |||
| Lobectomy | 4 (4.6 %) | 7 (9.5 %) | 0.223 |
| Total thyroidectomy | 83 (95.4 %) | 67 (90.5 %) | |
| Cervical lymphadenectomy | |||
| No | 85 (97.7 %) | 54 (73 %) | < 0.001 |
| Yes | 2 (2.3 %) | 20 (27 %) | |
| 131I ablation | |||
| No | 66 (75.9 %) | 44 (59.5 %) | 0.026 |
| Yes | 21 (24.1 %) | 30 (40.5 %) | |
| Stage | |||
| I | 87 (100 %) | 71 (95.9 %) | 0.095 |
| II | 0 (%) | 3 (4.1 %) | |
Statistically significant differences in bold.
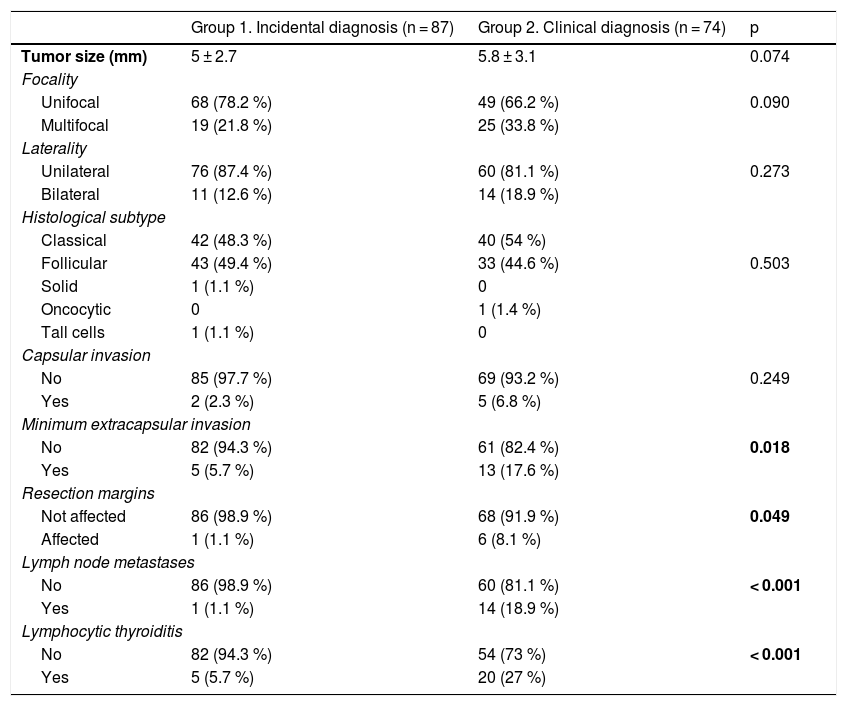
As seen in Table 3, patients with clinically diagnosed PTMC had a greater presence of minimal or microscopic extracapsular invasion (17.6% vs. 5.7%; p = 0.018), a higher frequency of infiltrated resection margins (8.1% vs. 1.1%; p = 0.049), a greater presence of metastatic adenopathies (18.9% vs. 1.1%; p < 0.001), and a higher frequency of lymphocytic thyroiditis (27% vs. 5.7%; p < 0.001).
Comparison of histopathological variables according to the type of diagnosis of PTMC.
| Group 1. Incidental diagnosis (n = 87) | Group 2. Clinical diagnosis (n = 74) | p | |
|---|---|---|---|
| Tumor size (mm) | 5 ± 2.7 | 5.8 ± 3.1 | 0.074 |
| Focality | |||
| Unifocal | 68 (78.2 %) | 49 (66.2 %) | 0.090 |
| Multifocal | 19 (21.8 %) | 25 (33.8 %) | |
| Laterality | |||
| Unilateral | 76 (87.4 %) | 60 (81.1 %) | 0.273 |
| Bilateral | 11 (12.6 %) | 14 (18.9 %) | |
| Histological subtype | |||
| Classical | 42 (48.3 %) | 40 (54 %) | |
| Follicular | 43 (49.4 %) | 33 (44.6 %) | 0.503 |
| Solid | 1 (1.1 %) | 0 | |
| Oncocytic | 0 | 1 (1.4 %) | |
| Tall cells | 1 (1.1 %) | 0 | |
| Capsular invasion | |||
| No | 85 (97.7 %) | 69 (93.2 %) | 0.249 |
| Yes | 2 (2.3 %) | 5 (6.8 %) | |
| Minimum extracapsular invasion | |||
| No | 82 (94.3 %) | 61 (82.4 %) | 0.018 |
| Yes | 5 (5.7 %) | 13 (17.6 %) | |
| Resection margins | |||
| Not affected | 86 (98.9 %) | 68 (91.9 %) | 0.049 |
| Affected | 1 (1.1 %) | 6 (8.1 %) | |
| Lymph node metastases | |||
| No | 86 (98.9 %) | 60 (81.1 %) | < 0.001 |
| Yes | 1 (1.1 %) | 14 (18.9 %) | |
| Lymphocytic thyroiditis | |||
| No | 82 (94.3 %) | 54 (73 %) | < 0.001 |
| Yes | 5 (5.7 %) | 20 (27 %) | |
Statistically significant differences in bold.
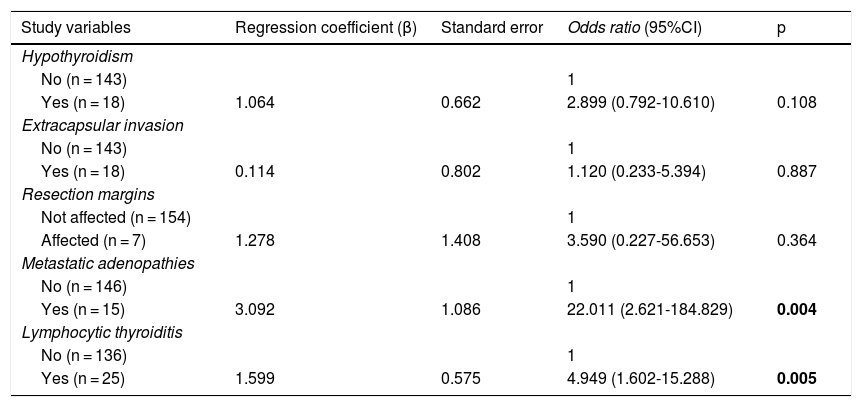
In the multivariate analysis (Table 4), the presence of lymphocytic thyroiditis in the histopathological study (OR: 4.949, 95%CI 1.602-15.288; p = 0.005) and the presence of metastatic adenopathies (OR: 22.011, 95% CI 2.621-184.829; p = 0.004) were independent risk factors associated with the clinical diagnosis of PTMC.
Multivariate analysis of factors associated with a clinical diagnosis of PTMC.
| Study variables | Regression coefficient (β) | Standard error | Odds ratio (95%CI) | p |
|---|---|---|---|---|
| Hypothyroidism | ||||
| No (n = 143) | 1 | |||
| Yes (n = 18) | 1.064 | 0.662 | 2.899 (0.792-10.610) | 0.108 |
| Extracapsular invasion | ||||
| No (n = 143) | 1 | |||
| Yes (n = 18) | 0.114 | 0.802 | 1.120 (0.233-5.394) | 0.887 |
| Resection margins | ||||
| Not affected (n = 154) | 1 | |||
| Affected (n = 7) | 1.278 | 1.408 | 3.590 (0.227-56.653) | 0.364 |
| Metastatic adenopathies | ||||
| No (n = 146) | 1 | |||
| Yes (n = 15) | 3.092 | 1.086 | 22.011 (2.621-184.829) | 0.004 |
| Lymphocytic thyroiditis | ||||
| No (n = 136) | 1 | |||
| Yes (n = 25) | 1.599 | 0.575 | 4.949 (1.602-15.288) | 0.005 |
Statistically significant differences in bold.
Over a mean follow-up of 119.8 ± 65.1 months (range 24-271), no persistence was detected, though disease relapse was recorded in a patient with clinically diagnosed PTMC (0% vs. 1.4%; p = 0.460). No patient died because of the disease.
DiscussionAn incidental diagnosis of PTMC is established in 1.3-21.6% of all thyroidectomies performed for presumably benign disease, according to the published series.3–7 In the present study, PTMC as an incidental diagnosis accounted for 54% of cases. Most of these tumors were identified in the histopathological study after lobectomy or total thyroidectomy for presumably benign thyroid disease.
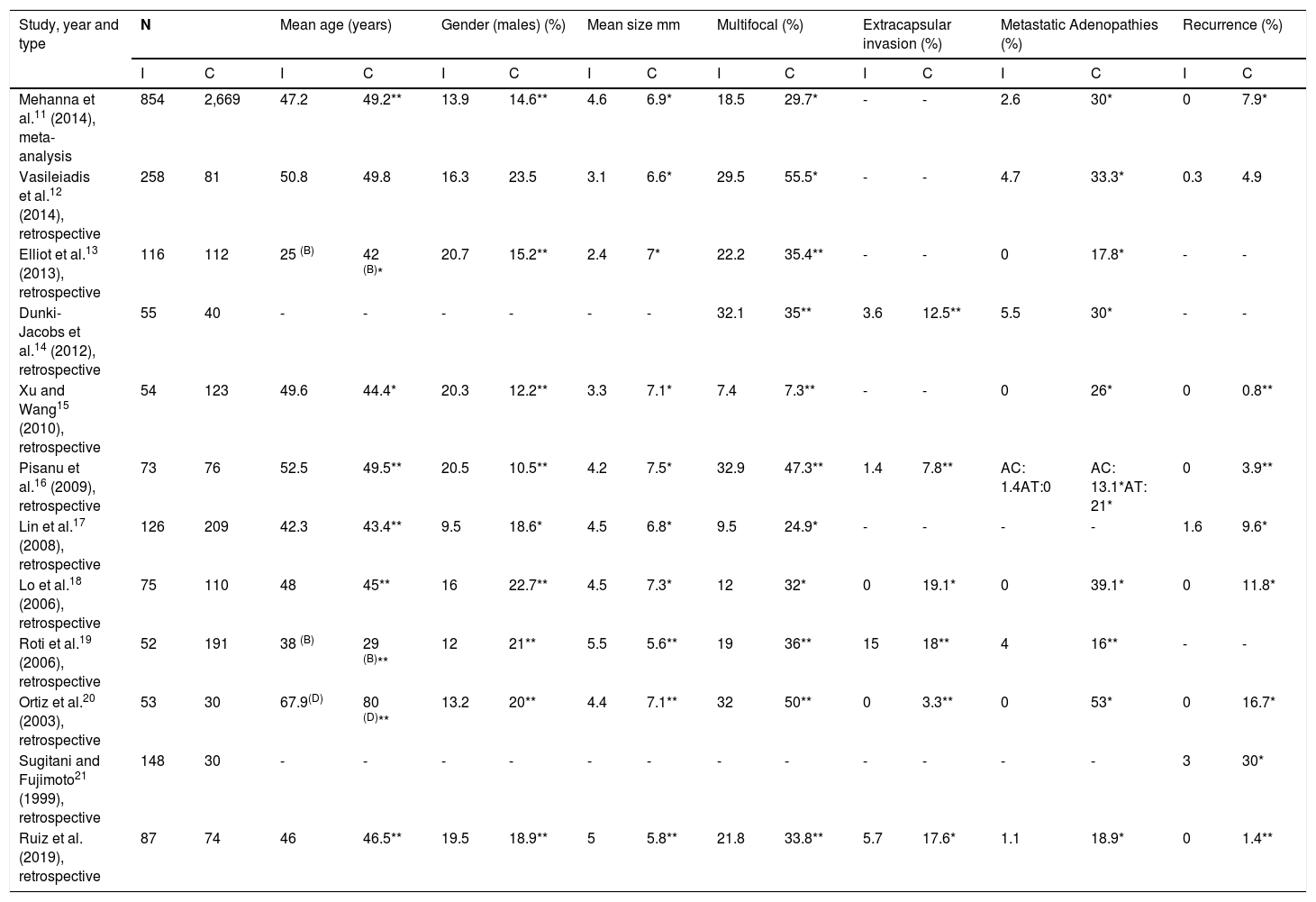
A number of studies have compared PTMC as an incidental diagnosis versus PTMC as a clinical diagnosis11–21 (Table 5).
Differences between incidentally diagnosed PTMC and clinically diagnosed PTMC documented in the literature.
| Study, year and type | N | Mean age (years) | Gender (males) (%) | Mean size mm | Multifocal (%) | Extracapsular invasion (%) | Metastatic Adenopathies (%) | Recurrence (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | C | I | C | I | C | I | C | I | C | I | C | I | C | I | C | |
| Mehanna et al.11 (2014), meta-analysis | 854 | 2,669 | 47.2 | 49.2** | 13.9 | 14.6** | 4.6 | 6.9* | 18.5 | 29.7* | - | - | 2.6 | 30* | 0 | 7.9* |
| Vasileiadis et al.12 (2014), retrospective | 258 | 81 | 50.8 | 49.8 | 16.3 | 23.5 | 3.1 | 6.6* | 29.5 | 55.5* | - | - | 4.7 | 33.3* | 0.3 | 4.9 |
| Elliot et al.13 (2013), retrospective | 116 | 112 | 25 (B) | 42 (B)* | 20.7 | 15.2** | 2.4 | 7* | 22.2 | 35.4** | - | - | 0 | 17.8* | - | - |
| Dunki-Jacobs et al.14 (2012), retrospective | 55 | 40 | - | - | - | - | - | - | 32.1 | 35** | 3.6 | 12.5** | 5.5 | 30* | - | - |
| Xu and Wang15 (2010), retrospective | 54 | 123 | 49.6 | 44.4* | 20.3 | 12.2** | 3.3 | 7.1* | 7.4 | 7.3** | - | - | 0 | 26* | 0 | 0.8** |
| Pisanu et al.16 (2009), retrospective | 73 | 76 | 52.5 | 49.5** | 20.5 | 10.5** | 4.2 | 7.5* | 32.9 | 47.3** | 1.4 | 7.8** | AC: 1.4AT:0 | AC: 13.1*AT: 21* | 0 | 3.9** |
| Lin et al.17 (2008), retrospective | 126 | 209 | 42.3 | 43.4** | 9.5 | 18.6* | 4.5 | 6.8* | 9.5 | 24.9* | - | - | - | - | 1.6 | 9.6* |
| Lo et al.18 (2006), retrospective | 75 | 110 | 48 | 45** | 16 | 22.7** | 4.5 | 7.3* | 12 | 32* | 0 | 19.1* | 0 | 39.1* | 0 | 11.8* |
| Roti et al.19 (2006), retrospective | 52 | 191 | 38 (B) | 29 (B)** | 12 | 21** | 5.5 | 5.6** | 19 | 36** | 15 | 18** | 4 | 16** | - | - |
| Ortiz et al.20 (2003), retrospective | 53 | 30 | 67.9(D) | 80 (D)** | 13.2 | 20** | 4.4 | 7.1** | 32 | 50** | 0 | 3.3** | 0 | 53* | 0 | 16.7* |
| Sugitani and Fujimoto21 (1999), retrospective | 148 | 30 | - | - | - | - | - | - | - | - | - | - | - | - | 3 | 30* |
| Ruiz et al. (2019), retrospective | 87 | 74 | 46 | 46.5** | 19.5 | 18.9** | 5 | 5.8** | 21.8 | 33.8** | 5.7 | 17.6* | 1.1 | 18.9* | 0 | 1.4** |
AC: central metastatic adenopathies; AL: lateral metastatic adenopathies; B: percentage in patients under 45 years; C: clinical diagnosis; D: percentage in patients under 50 years; I: incidental diagnosis.
Hypothyroidism was identified as a factor associated with the clinical diagnosis of PTMC in the bivariate analysis, but not in the multivariate analysis. This could be because 64.3% of the patients with a clinical diagnosis of PTMC and hypothyroidism presented associated chronic lymphocytic thyroiditis, the latter being an independent factor associated with the clinical diagnosis of PTMC. Chronic lymphocytic thyroiditis, which is the most common cause of hypothyroidism,22 is increasing in prevalence among patients with PTMC.23 Since lymphocytic thyroiditis has been associated with the development of PTMC, particularly in relation to the clinical diagnosis of the disease,16 it is believed that there may be an immunological relationship between the two conditions,22 and several immunopathological mechanisms have been proposed to explain this relationship.24
On the other hand, the presence of hyperthyroidism was not associated with clinically diagnosed PTMC, but has been related to incidental diagnosis of the disease,17 as in our own observations. In turn, Graves’ disease, which accounts for 50-80% of all cases of hyperthyroidism,25 was also more common in patients with incidentally diagnosed PTMC. This is because hyperthyroidism was the surgical indication in all cases, and FNAB of the possible nodules revealed preoperatively was not performed.
In most published studies, a larger tumor size has been associated with the clinical diagnosis of PTMC.11–13,15–18 This could be due to the greater aggressiveness of the lesions.26,27 Although one might assume that larger PTMC lesions would be easier to detect clinically, either upon palpation if superficial or by ultrasound, thus offering a more feasible approach for FNAB, there have been other studies19,20 in which PTMC size was not associated with the type of diagnosis.
Clinically diagnosed PTMC has also been associated with an increased presence of multifocality in different studies.11,12,17,18 By contrast, in our study, as in other publications,13–16,19,20 no association was seen between multifocality and the type of diagnosis, though it might be thought that a clinical diagnosis would be easier to establish if the tumor had multiple foci.
Extracapsular invasion has not been associated with a clinical diagnosis of PTMC in most published studies.14,16,19,20 By contrast, in our study, as in some other publications,18,28 extracapsular invasion was associated with clinically diagnosed PTMC in the bivariate analysis, but not in the multivariate analysis. Since extracapsular invasion of PTMC was microscopic and therefore difficult to assess in the context of a clinical diagnosis, it could not form part of the independent risk factors associated with a clinical diagnosis of PTMC.
The presence of metastatic adenopathies, as in most studies,11–16,19,20 was associated with a clinical diagnosis of PTMC. This could have been because over one-half of the patients with clinically diagnosed PTMC and metastatic adenopathies were diagnosed by FNAB evaluation of an adenopathy.
Although clinically diagnosed PTMC has more aggressive histopathological characteristics, such as an increased presence of extracapsular invasion and metastatic adenopathies, the long-term prognosis is excellent. These patients had few relapses, with no differences with respect to the group of patients with incidentally diagnosed PTMC, in contrast to other studies in which a clinical diagnosis was associated with a higher percentage of relapses.11,17,18,20,21,29,30 It should be taken into account that patients with clinically diagnosed PTMC had a greater frequency of more aggressive treatments, i.e., more cervical lymphadenectomies and a greater administration of 131I. Although mortality associated with incidentally and clinically diagnosed PTMC is 0-2.4% and 0.1-3.8%, respectively,11,17 no deaths were associated with the disease in our study.
A limitation of the present study is its retrospective nature. Regarding the assessment of the presence of metastatic adenopathies, as cervical lymphadenectomy was not performed in all cases, the presence of subclinical micrometastases may have been overlooked. Furthermore, since total thyroidectomy was not performed in all cases, the presence of multifocality in the remaining thyroid lobe may have gone unnoticed.
In conclusion, although clinically diagnosed PTMC has more aggressive histopathological characteristics (extracapsular invasion and the presence of metastatic adenopathies), the long term prognosis is similar to that of incidentally diagnosed PTMC, with no persistence, few recurrences and no mortality associated with the disease. Nevertheless, more aggressive treatments were used in clinically diagnosed PTMC, such as cervical lymphadenectomy and the administration of 131I. The independent factors associated with clinically diagnosed PTMC were the presence of metastatic adenopathies and chronic lymphocytic thyroiditis revealed by the histopathological study.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ruiz A, Ríos A, Rodríguez JM, Paredes M, Soriano V, Oviedo MI, et al. Diagnóstico incidental versus clínico del microcarcinoma papilar de tiroides. Pronóstico a largo plazo. Endocrinol Diabetes Nutr. 2020;67:317–325.