The aim of this study was to examine the quality of life, anxiety and affectivity in children and adolescents with type 1 diabetes (T1D) and in their parents after participating in a diabetes summer camp.
MethodA total of 20 children and adolescents with T1D, aged 8–14, and their parents participated. The study design was quasi-experimental longitudinal with an intra-subject factor with two measurements (pre/post), and an inter-group factor (child/parent).
ResultsAfter attending camp, a significantly increased quality of life, demonstrated by the emotional well-being and self-esteem scores, was found in the children but not in the parents. Less negative affectivity and an improvement in positive affectivity was seen in the parents, but not in the children. Differences in anxiety were found in both the children and the parents.
ConclusionsThis research presents empirical evidence of the benefits of participation in a diabetes camp in both children and their parents.
El objetivo de este estudio fue examinar la calidad de vida, la ansiedad y la afectividad en niños y adolescentes con diabetes mellitus tipo 1 (DM1), y en sus padres después de participar en un campamento de verano de diabetes.
MétodoParticiparon un total de 20 niños y adolescentes con DM1 de 8 a 14 años, además de sus padres. El diseño del estudio fue longitudinal cuasi-experimental con un factor intra-sujeto con 2 mediciones (pre/post), y un factor intergrupo (niño/padre).
ResultadosDespués de asistir al campamento se observa un aumento significativo en la calidad de vida, demostrado en las puntuaciones de bienestar emocional y autoestima en los niños. Los padres mostraron menos afectividad negativa y más positiva. Se encontraron diferencias en ansiedad en niños y sus padres.
ConclusionesSe muestra evidencia empírica sobre los beneficios de un campamento de diabetes tanto en niños como en sus padres.
Type 1 diabetes (T1D) is a chronic disease that affects more than just physical health. The impact of the diagnosis, due to changes imposed by treatment, can generate a high level of stress, alter emotions and decrease quality of life (QoL) in the child and in the family.1 Accordingly, the relationship between the patient, the healthcare team and the family is critical to achieving good adaptation to the disease.2
The International Society for Pediatric and Adolescent Diabetes (ISPAD) in its 2018 statement3 reports that adolescents with diabetes have a higher incidence of depression, anxiety, psychological distress and eating disorders compared to their peers without T1D. Depression and anxiety are related to fewer glucose controls and poor glycemic control. In addition, depression is also associated with poor treatment adherence and poor QoL.3 Improved QoL in adolescents is associated with increased self-efficacy, less depression and better metabolic control.4 The entire family system suffers considerable stress, and some authors report that the psychological adaptation of children to the disease is governed by the reactions of their parents to this stress,5 which forces the family to modify their lifestyle6 and affects their QoL. Thus, 48% of parents report poor QoL and 81% feel overwhelmed by their child's diabetes, at times associating this feeling with anxiety and depression.7
Although there are many factors influencing a child's adherence to treatment regimen and glycemic control,3–8 parental mood plays an important role in controlling the child's diabetes. Mothers with a high anxiety level tend to take greater responsibility for managing their child's diabetes, perceiving their teenagers as not capable of doing so, while their children perceive a high maternal control of their diabetes and a high level of overprotection by their parents. Parental psychological well-being (especially the mother), however, is associated with the metabolic outcomes of the child,1 finding worse glycemic control the poorer the psychological well-being of the parents.9 The protective factors are higher QoL in children with T1D and reduced depressive symptoms and stress in their parents.10
Several studies have examined the effect of diabetes camp participation on psychological variables without a consensus. There is no agreement regarding anxiety11 since, while Briery and Rabian12 reported a significant decrease in anxiety levels from pre-camp to post-camp, Török et al.13 found no significant differences in these levels, nor did García-Pérez et al.14 Additionally, no improvement was found in QoL,11,15,16 although there was improvement in the Self-perception subscale.17 Improvement was seen, however, in distress, self-esteem, self-efficacy and attitudes toward the child's disease after diabetes camp.12,13,16 Although the ISPAD3 reports that adolescents with diabetes have a higher incidence of depression, no studies have analyzed the emotional state of children who have participated in a diabetes camp.
Diabetes camp has become a common part of diabetes care worldwide. Although a patient's knowledge and self-management of diabetes may improve after camp,18 improvements in psychological variables have not been consistently demonstrated. Studies examining the role of psychological variables in children and adolescents who participate in diabetes camp are scarce and even nonexistent in some of these variables.
Consequently, the aim of this study was to investigate both the outcome effectiveness of a diabetes summer camp program for children and adolescents with diabetes, and the expected changes in QoL, anxiety and affectivity in both children and their parents.
MethodsParticipantsInformed consent was obtained from 21 children with T1D who participated in a summer camp and from their parents. The final sample consisted of 20 children with T1D and their parents. One child was excluded after failing to complete the full evaluation (post-test). Sociodemographic characteristics were: 10 (50%) boys and 10 (50%) girls, mean age 10.4 years (95% CI: 9.54–11.26) with a standard deviation (SD) 1.85 (range: 8–14, median: 10 years). All children had completed primary school. The mean disease duration was 3.03 years (95% CI: 1.67–4.40) with an SD 2.92 (range: 0.13–10.05, median: 1.69 years). Parental participation comprised 18 mothers (90%) and two fathers (10%), with a mean age of 40.65 (95% CI: 37.8–43.5) in the 29–52 age range with a median age of 41 years. Once the parents signed the informed consent, interviews were conducted by psychologists with research experience. The children/adolescents and parents then completed the questionnaire independently. The psychological evaluation was conducted two weeks before the camp and one month following its completion. The study protocol was approved by the joint Ethics Research Committee of the Regional University Hospital of Malaga (Spain).
InstrumentsQuality of Life: (a) Psychological screens in parents: Health Questionnaire SF-12.19 The short version of the SF-36 was used to assess QoL in the parents of children with T1D. The SF-12 consists of 12 items, with two measures: physical (PCS-12) and mental (MCS-12) components. Higher scores indicate higher QoL. (b) Psychological screens in Children: the KINDLR20 is a general instrument that evaluates QoL in children and adolescents, with a version for parents. The corresponding version was used for each age: Kid-Kindl: 8–12 years; Kiddo-KINDL: 13–16 years; and the version for parents of children aged 8–16 years. The specific areas of evaluation are: physical well-being, emotional well-being, self-esteem, family, friends, and school (6 modules, each comprising 4 items). There is a seventh module (Hospital stay) with six items which are only answered by those who have had a recent hospital admission or were diagnosed a long time ago. Typically, only the 24 items corresponding to the first 6 modules (physical well-being, emotional well-being, self-esteem, family, friends, and school) are administered and provide a total score (see Tables 1 and 2: TOTAL score without module 7). However, when necessary, module 7 (Hospital stay: 6 items) is also administered, thus enabling a total score to be obtained for all 7 modules (see Tables 1 and 2: TOTAL score with module 7). The answers are recorded on a five-category Likert scale (1=never and 5=always). The questions refer to the week before the interview and the scores obtained from the means for each dimension indicate that a higher score represents better QoL.
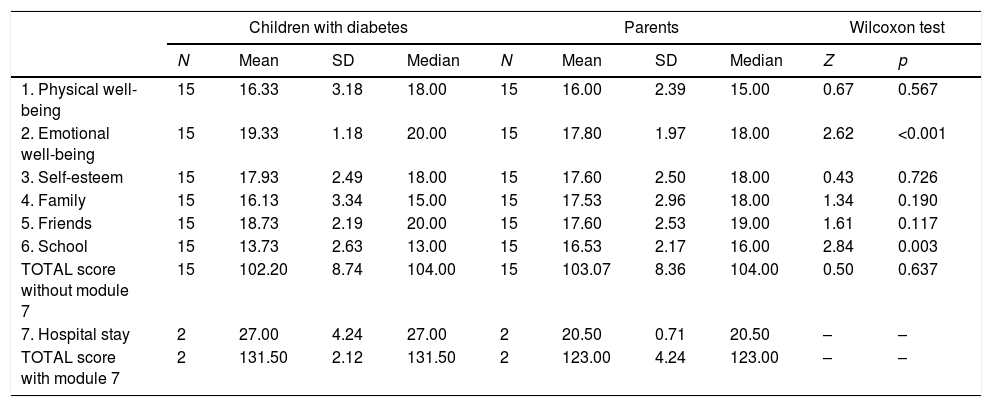
Comparative analysis inter-group post-test KINDL/KINDL parents’ version.
| Children with diabetes | Parents | Wilcoxon test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Median | N | Mean | SD | Median | Z | p | |
| 1. Physical well-being | 15 | 16.33 | 3.18 | 18.00 | 15 | 16.00 | 2.39 | 15.00 | 0.67 | 0.567 |
| 2. Emotional well-being | 15 | 19.33 | 1.18 | 20.00 | 15 | 17.80 | 1.97 | 18.00 | 2.62 | <0.001 |
| 3. Self-esteem | 15 | 17.93 | 2.49 | 18.00 | 15 | 17.60 | 2.50 | 18.00 | 0.43 | 0.726 |
| 4. Family | 15 | 16.13 | 3.34 | 15.00 | 15 | 17.53 | 2.96 | 18.00 | 1.34 | 0.190 |
| 5. Friends | 15 | 18.73 | 2.19 | 20.00 | 15 | 17.60 | 2.53 | 19.00 | 1.61 | 0.117 |
| 6. School | 15 | 13.73 | 2.63 | 13.00 | 15 | 16.53 | 2.17 | 16.00 | 2.84 | 0.003 |
| TOTAL score without module 7 | 15 | 102.20 | 8.74 | 104.00 | 15 | 103.07 | 8.36 | 104.00 | 0.50 | 0.637 |
| 7. Hospital stay | 2 | 27.00 | 4.24 | 27.00 | 2 | 20.50 | 0.71 | 20.50 | – | – |
| TOTAL score with module 7 | 2 | 131.50 | 2.12 | 131.50 | 2 | 123.00 | 4.24 | 123.00 | – | – |
KINDL: questionnaire for evaluating QoL in children and adolescents and the KINDL parents’ version. The mean scores correspond to the sum of the items in each module and the total score (with or without module 7).
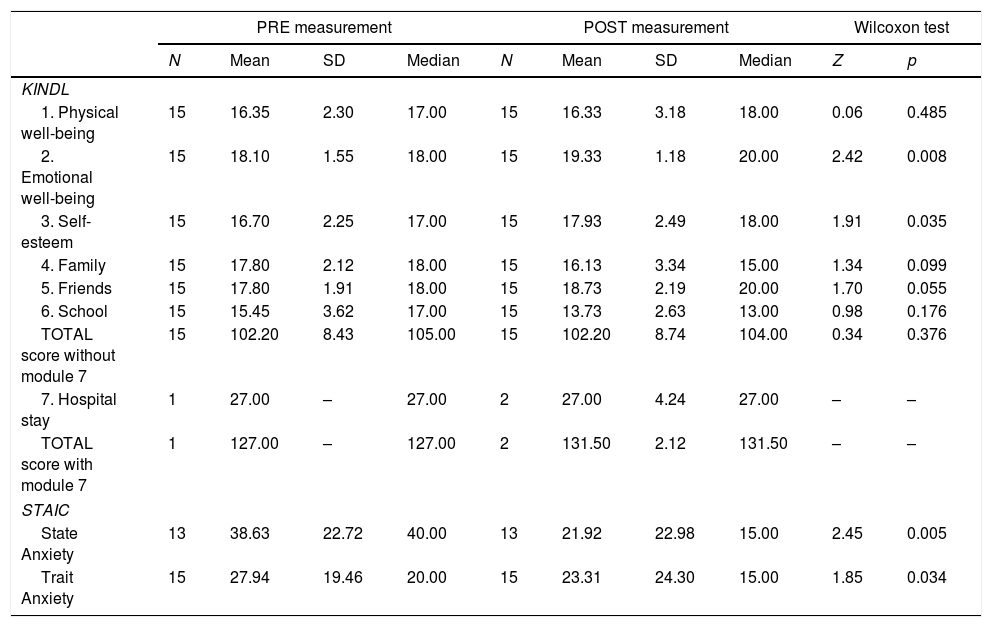
Comparative analysis intra-group pre-post children: KINDL and STAIC.
| PRE measurement | POST measurement | Wilcoxon test | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | SD | Median | N | Mean | SD | Median | Z | p | |
| KINDL | ||||||||||
| 1. Physical well-being | 15 | 16.35 | 2.30 | 17.00 | 15 | 16.33 | 3.18 | 18.00 | 0.06 | 0.485 |
| 2. Emotional well-being | 15 | 18.10 | 1.55 | 18.00 | 15 | 19.33 | 1.18 | 20.00 | 2.42 | 0.008 |
| 3. Self-esteem | 15 | 16.70 | 2.25 | 17.00 | 15 | 17.93 | 2.49 | 18.00 | 1.91 | 0.035 |
| 4. Family | 15 | 17.80 | 2.12 | 18.00 | 15 | 16.13 | 3.34 | 15.00 | 1.34 | 0.099 |
| 5. Friends | 15 | 17.80 | 1.91 | 18.00 | 15 | 18.73 | 2.19 | 20.00 | 1.70 | 0.055 |
| 6. School | 15 | 15.45 | 3.62 | 17.00 | 15 | 13.73 | 2.63 | 13.00 | 0.98 | 0.176 |
| TOTAL score without module 7 | 15 | 102.20 | 8.43 | 105.00 | 15 | 102.20 | 8.74 | 104.00 | 0.34 | 0.376 |
| 7. Hospital stay | 1 | 27.00 | – | 27.00 | 2 | 27.00 | 4.24 | 27.00 | – | – |
| TOTAL score with module 7 | 1 | 127.00 | – | 127.00 | 2 | 131.50 | 2.12 | 131.50 | – | – |
| STAIC | ||||||||||
| State Anxiety | 13 | 38.63 | 22.72 | 40.00 | 13 | 21.92 | 22.98 | 15.00 | 2.45 | 0.005 |
| Trait Anxiety | 15 | 27.94 | 19.46 | 20.00 | 15 | 23.31 | 24.30 | 15.00 | 1.85 | 0.034 |
KINDL: questionnaire for evaluating QoL in children. The mean scores correspond to the sum of the items in each module and the total score (with or without module 7).
STAIC: questionnaire for evaluating state/trait anxiety in children. The mean scores correspond to the direct scores transformed into percentiles according to age and sex (from 1% to 99%).
Anxiety: (a) Psychological screens in parents: STAI: State-Trait Anxiety Inventory21 consisting of two self-report scales measuring State Anxiety (STAI-S) and Trait Anxiety (STAI-T); (b) Psychological screens in children: STAIC, self-report State-Trait Anxiety Inventory for Children (between the ages of 9 and 15). The direct scores on these scales are transformed into percentiles, with highers scores indicating, greater anxiety (state or trait).
Affectivity: (a) Psychological screens in parents: The PANAS Positive and Negative Affect Schedule22 evaluates positive (10 items) and negative (10 items) emotional states in two ways: during the past week and as a general status. (b) Psychological screens in children: PANASN, version for children and adolescents. High scores on the Positive Affect subscale indicate greater positive affectivity (e.g., enthusiastic, energetic, active, etc.) while high scores on the Negative Affect subscale indicate greater negative affectivity (e.g., fearful, nervous, hostile, etc.).
ProcedureThe summer camp lasted 10 days and the intervention team comprised 10 physicians and one diabetes educator (nurse). The non-medical personnel included the president of the diabetes association of Málaga, 10 free-time monitors with diabetes, each monitor caring for five children to support glycemic control (performing checks and insulin administration) and five free-time monitors without T1D in charge of the leisure and sports activities for all 50 children who attended the camp. To ensure that their responses were not biased by previous experience, only those who attended the camp for the first time were included in this study thus fulfilling the principle of homogeneity. Accordingly, the differences found in the post-test evaluation could not be attributed to the previous state (experience) of the participants. The camp structure, contents, and educational program followed the recommendations of the American Diabetes Association23 for summer camp organization and management. Education consisted of two parts: lecture and small group discussion with 10–15 children per group. Sessions lasted one hour each day and were directed by a pediatric or adult endocrinologist. Lecture topics included disease etiology and symptoms, insulin therapy and injection techniques, the importance of diabetes control, blood glucose monitoring, exercise and diabetes. At each meal (breakfast, lunch, snack and dinner) workshops were conducted on counting carbohydrate servings, conveniently adapted to present-day meals, and insulin dose was calculated in collaboration with the physician–monitor–child, analyzing the insulin administered at the same meal the previous day. The rest of the time the children participated in sports, painting, ceramics, and other activities. Among the special activities, one highlight was a motivational talk by an astronaut with diabetes who told his life story.
Data analysisThe study design was quasi-experimental longitudinal with an intra-subject factor and an inter-group factor (child/parent) with two measurements (pre/post). Thus, four groups were statistically defined: children pre-post intervention and parents pre-post intervention. Therefore we compared:
- -
Inter-group analysis before and after the summer camp: children with their parents at baseline (pre-test) and post-test.
- -
Intra-group analysis before and after the summer camp: children between their baseline and post-test measurements.
- -
Intra-group analysis before and after the summer camp: parents between their baseline (pre-test) and post-test measurements.
Of the 20 children initially evaluated (pre-test), 15 were measured post-test (six girls and nine boys) representing a loss of 25% (five cases) for the longitudinal analyses. Their parents were also evaluated post-test (15). For STAIC analysis, three children were removed due to their age (8 years) as the STAIC is administered between the ages of 9 and 15 years. As shown in the results, the N of the analyses varied among variables because not all parents could be fully evaluated. Thus, when presenting the results as pairs, the data from the unmatched case was lost.
The non-parametric Wilcoxon test was used to perform the intra-subject repeated measures analysis for both children and parents. As the cases were matched (parent/child), the inter-group analysis must be considered a situation of related samples and thus the Wilcoxon test was applied. The description was performed with the mean and the median, parallel to the comparisons. All statistical analyses were carried out with SPSS Statistics, version 22.
ResultsQuality of life- -
Inter-group analysis (pre/post-test children versus parents): The groups were homogeneous at baseline, with no statistically significant differences between the children and their parents in QoL. Significant differences were found in two of the areas evaluated by the KINDL. In emotional well-being after the summer camp, children scored higher than their parents. In the school area, parents scored higher than their children, tending to better perceive the situation of their children in school. In the remaining areas no significant differences were found. Nor were there differences in the total score (Table 1).
- -
Intra-group analysis (pre/post-test children): Of the seven KINDL questionnaire subscales, significant differences were found in two: self-esteem (p=0.035) and emotional well-being (p=0.008), with a significant improvement in the emotional well-being and self-esteem of children who participated in the camp (Table 2). However, in the total score, no significance (p=0.376) was detected.
- -
Intra-group analysis (pre/post-test parents): No statistically significant differences were found in any of the areas evaluated with KINDL (parent version). Nor did we find significant differences in the SF-12 (physical or mental component).
- -
Inter-group analysis (pre/post-test children versus parents): No significant differences were seen in the scores on state and trait anxiety between the children and their parents at baseline or after the children's participation in the camp; thus, the groups were homogeneous.
- -
Intra-group analysis (pre/post-test children): Table 2 shows a significant decrease in both state anxiety and trait anxiety scores in children post-camp.
- -
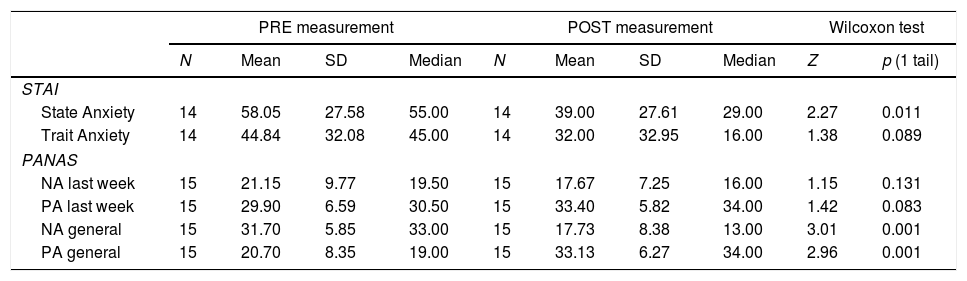
Intra-group analysis (pre/post-test parents): Following the camp, a statistically significant decrease was seen in the state anxiety scores in the parents of children with T1D. No significant differences were seen in trait anxiety scores (Table 3).
Table 3.Comparative analysis intra-group pre-post parents: STAI and PANAS.
PRE measurement POST measurement Wilcoxon test N Mean SD Median N Mean SD Median Z p (1 tail) STAI State Anxiety 14 58.05 27.58 55.00 14 39.00 27.61 29.00 2.27 0.011 Trait Anxiety 14 44.84 32.08 45.00 14 32.00 32.95 16.00 1.38 0.089 PANAS NA last week 15 21.15 9.77 19.50 15 17.67 7.25 16.00 1.15 0.131 PA last week 15 29.90 6.59 30.50 15 33.40 5.82 34.00 1.42 0.083 NA general 15 31.70 5.85 33.00 15 17.73 8.38 13.00 3.01 0.001 PA general 15 20.70 8.35 19.00 15 33.13 6.27 34.00 2.96 0.001 STAI: State/Trait anxiety questionnaire (adults). The mean scores correspond to the direct scores transformed into percentiles according to age and sex (from 1% to 99%).
PANAS: Affectivity Questionnaire (adults). The mean scores correspond to the sum of the items of each of the subscales: NA or PA (from 10 to 50 points).
NA=negative affect; PA=positive affect.
- -
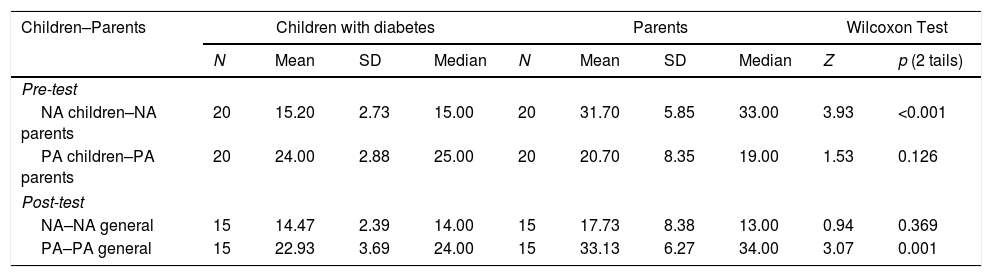
Inter-group analysis (pre/post-test children versus parents): At baseline, no significant differences were seen in positive affect (PA). However, in negative affect (NA) highly significant differences were found between the scores of children and those of their parents, with the parents scoring higher in NA than their children (Table 4).
Table 4.Comparative analysis inter-group pre-test and post-test PANASN/PANAS.
Children–Parents Children with diabetes Parents Wilcoxon Test N Mean SD Median N Mean SD Median Z p (2 tails) Pre-test NA children–NA parents 20 15.20 2.73 15.00 20 31.70 5.85 33.00 3.93 <0.001 PA children–PA parents 20 24.00 2.88 25.00 20 20.70 8.35 19.00 1.53 0.126 Post-test NA–NA general 15 14.47 2.39 14.00 15 17.73 8.38 13.00 0.94 0.369 PA–PA general 15 22.93 3.69 24.00 15 33.13 6.27 34.00 3.07 0.001 PANAS: Affectivity Questionnaire. The mean scores correspond to the sum of the items of each of the subscales: NA or PA (from 10 to 50 points).
PANASN: Version for children and adolescents.
NA=negative affect; PA=positive affect.
- -
Following the camp we found highly significant differences between PA in children and their parents (Table 4), with parents scoring higher.
- -
Intra-group analysis (pre/post-test children): Although a slight decrease was observed in the mean scores of the children in both NA and PA post-camp, no significant differences were found.
- -
Intra-group analysis (pre/post-test parents): Considering affectivity in the past week, we found no significant differences in the pre-post scores of the parents. However, post-camp, highly significant differences (p<0.01) were seen in general affectivity (trait); NA scores were lower, while PA scores were higher (Table 3).
The main goal of modern diabetes care in children and adolescents has evolved from a purely medical approach to one seeking optimal glycemic control, normal psychological development and maximum QoL.24 Camp researchers strive to provide empirical evidence that disease specific camps are beneficial for children with chronic diseases. Some studies report the effectiveness of these camps on QoL, apart from medical and physiological impacts,25 although others provide conflicting data. Studies examining psychological variables in this field, however, are rare. The literature yields no results on affective symptoms (such as depression) and the results on anxiety are contradictory.11 Therefore, we must delve deeper into this topic to provide new empirical evidence in a field little studied and hence the motivation for this study.
Concerning QoL, after children attended summer camp they had greater emotional well-being than their parents, while parents tended to be more aware of their child's situation in school. This data is in line with the scientific literature. Parents reported poorer QoL than their children and this may indicate the burden of diabetes on parents.26 Children had better QoL (emotional well-being dimension) than their parents. However, the diabetes camp enabled parents to perceive their child's school situation differently, perhaps due to the gain they observed in autonomy and overall management of diabetes.
This is much more evident in the intra-group comparison of children, whose levels of self-esteem and emotional well-being were significantly increased after camp participation, which is not observed in the intra-group analysis of the parents (with the SF-12). This improved QoL (particularly emotional well-being) supports data from other authors.11–15 Improvement in the self-esteem of children after diabetes camp has also been reported previously.12,13
The findings on anxiety in children after attending camp show significantly decreased scores in both state and trait anxiety. These results support studies in which camps had a positive impact on anxiety in the child.12 Moreover, considering depression and anxiety in this population and its impact on adherence and changes in glycemic control,3 a decrease in anxiety provides empirical evidence of the benefit of summer camps, and can be considered a positive experience for children with T1D.
A significant decrease in anxiety was also found in the parents following their child's attendance at camp, although only in state anxiety. Nonetheless, it should be noted that, initially, parents scored higher in anxiety (state and trait) than their children. Despite improvements in parental anxiety after camps, their scores were higher than those of their children. These results are consistent as parents are the primary caregivers for their children and although their anxiety decreased, they may still have felt overwhelmed by the demands of T1D experience by their children.7
Although at baseline there were no statistically significant differences between children and their parents in PA, there were differences in NA, with parents scoring higher than their children. However, there were no significant intra-group (pre-post) differences, in the children or their parents. Differences were seen in the general affectivity of the parents after their child's attendance at the camp, which improved significantly with decreasing NA scores (general) and increasing PA scores. Hence, this research provides evidence that while the parents initially had a higher negative affectivity than their children, the child's attendance at summer camp had beneficial effects on the parents who then showed more positive affectivity (general). These data support the literature that shows a high percentage of parents are overwhelmed by their child's diabetes,8 which is related to NA (such as depressed mood) and anxiety.6 Thus, the evidence presented indicates that camp attendance by the children who participated in this study was a protective factor for the mood of their parents.6 These results may have a wider scope considering that the well-being of parents is positively associated with the metabolic outcomes of their children.1
Overall, this study provides empirical evidence of the benefits of participation in a diabetes camp not only in children who have participated in this study but also in their parents. Improvements were seen in the QoL of the children (mainly self-esteem and emotional well-being) and anxiety in the children and parents, as well as in the general affectivity of the parents. We therefore suggest summer camp as a highly beneficial option for children with T1D that also provides benefits for parents. Nonetheless, given the small sample size (main limitation of this study), these results cannot be generalized. More studies are needed27 to advance knowledge and to improve the methodological deficiencies seen in previous studies28–30 to enable firm conclusions to be drawn regarding the psychosocial impact of camps on both children and adolescents with T1D and their parents. Future research is recommended to identify the protective factors that contribute to empowerment in both children and their parents as well as to analyze the effects on adherence and metabolic control, which could have an impact on improved control in children and adolescents attending these camps.
Contribution of authorsMTA, ILG, and JPLS contributed to the design of the study. MC, EV, MMA and ALG contributed to the collected the data. MTA contributed to the analysis of data. MTA, ILG, EV, MC and MMA contributed to the interpretation of the data, wrote and revised the manuscript. All authors read and approved the submitted final version of the paper.
Conflict of interestsThe authors declare that they have no conflict of interests.
The English translation of this study was funded by the University of Malaga (Spain). Project Ref.: Proyecto Puente (B.5). Universidad de Málaga (2018–2019).
We want to thank the parents and children who have collaborated in this study disinterestedly.