To provide practical recommendations for evaluation and treatment of osteoporosis associated to endocrine diseases and nutritional conditions.
ParticipantsMembers of the Bone Metabolism Working Group of the Spanish Society of Endocrinology, a methodologist, and a documentalist.
MethodsRecommendations were formulated according to the GRADE system (Grading of Recommendations, Assessment, Development, and Evaluation) to describe both the strength of recommendations and the quality of evidence. A systematic search was made in MEDLINE (Pubmed), using the following terms associated to the name of each condition: AND “osteoporosis”, “fractures”, “bone mineral density”, and “treatment”. Papers in English with publication date before 18 October 2011 were included. Current evidence for each disease was reviewed by two group members, and doubts, related to the review process or development of recommendations were resolved by the methodologist. Finally, recommendations were discussed in a meeting of the Working Group.
ConclusionsThe document provides evidence-based practical recommendations for evaluation and management of endocrine and nutritional diseases associated to low bone mass or an increased risk of fracture. For each disease, the associated risk of low bone mass and fragility fractures is given, recommendations for bone mass assessment are provided, and treatment options that have shown to be effective for increasing bone mass and/or to decreasing fragility fractures are listed.
Proporcionar unas recomendaciones prácticas para la evaluación y tratamiento de la osteoporosis asociada a diferentes enfermedades endocrinas y alteraciones nutricionales.
ParticipantesMiembros del Grupo de Metabolismo Mineral de la Sociedad Española de Endocrinología y Nutrición, un metodólogo y un documentalista.
MétodosLas recomendaciones se formularon de acuerdo al sistema Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) para establecer tanto la fuerza de las recomendaciones como el grado de evidencia. Se realizó una búsqueda sistemática en Medline de la evidencia disponible para cada patología usando las siguientes palabras clave asociadas al nombre de cada patología: AND osteoporosis, fractures, bone mineral density, bone markers y treatment. Se revisaron artículos escritos en inglés con fecha de inclusión hasta 18 de octubre de 2011, y cada tema fue revisado por dos personas del Grupo. Un metodólogo resolvió las diferencias que surgieron durante el proceso de revisión de bibliografía y formulación de recomendaciones. Tras la formulación de las recomendaciones estas se discutieron en una reunión conjunta del Grupo de Trabajo.
ConclusionesEl documento establece unas recomendaciones prácticas basadas en la evidencia acerca de la evaluación y tratamiento de la osteoporosis en las enfermedades endocrinas y nutricionales que asocian baja masa ósea o aumento del riesgo de fractura. Para cada patología, se señala el riesgo de osteoporosis y fracturas asociado, se formulan recomendaciones en cuanto a la evaluación de masa ósea y se enumeran las opciones terapéuticas que han demostrado eficacia en aumentar la densidad mineral ósea y/o reducir el riesgo de fractura.
Many diseases in the field of endocrinology and nutrition are associated with osteoporosis and an increased risk of fracture. However, for many of these conditions there are no specific recommendations available for bone mass evaluation and management.
In this setting, the Working Group on Mineral Metabolism of the Spanish Society of Endocrinology and Nutrition (SEEN) decided to prepare practical recommendations for the evaluation and treatment of osteoporosis associated with different endocrine diseases and nutritional disorders. The objective was to establish evidence-based recommendations with regard to the risk of low bone mass and fracture associated with each condition, the diagnostic tests required for their assessment, and treatments that have been shown to increase bone mass and/or decrease the risk of fracture. When poor or no evidence was available, members of the Working Group made recommendations based on their experience and understanding of these diseases.
Development of evidence-based recommendationsRecommendations were made based on the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) system to establish the strength of the recommendations and the level of evidence.1 This system gives a graphic description of the quality of the available evidence and the strength of the recommendations made based on that evidence. Thus, in terms of strength a distinction is made between strong recommendations, expressed as “We recommend” at number 1, and weak recommendations, expressed as “We suggest” at number 2. The quality of evidence is expressed in symbols: ⊕OOO indicates very low evidence; ⊕⊕OO, low evidence; ⊕⊕⊕O, moderate evidence and ⊕⊕⊕⊕, high evidence. After each recommendation, the evidence supporting it is provided.
A systematic search was made in Medline for the evidence available for each condition using the following keywords associated with their names: AND osteoporosis, fractures, bone mineral density, and treatment. Articles in English included up to October 18, 2011 were reviewed. Each subject was reviewed by two members of the Working Group. A methodologist resolved any differences which arose during the process of the literature review and the formulation of recommendations. Once the recommendations were formulated, they were discussed at a joint meeting of the Working Group.
For each condition, the following aspects were reviewed based on the available evidence: the need for evaluation of bone mass by dual X-ray densitometry (DXA) and the presence of asymptomatic vertebral fractures using X-rays of the thoracic and lumbar spine, as well as recommendations on preventive measures and treatment.
In addition to the measures specified under each heading, the Working Group on Mineral Metabolism generally recommends that adequate calcium intake and adequate vitamin D levels are ensured in all conditions associated with decreased bone mass and/or increased fracture risk (1⊕OOO).
Type 1 diabetes mellitusEvaluationRecommendations- -
We recommend the evaluation of bone mass using DXA and of the risk of non-vertebral fracture in patients with type 1 diabetes mellitus (T1DM) (1⊕⊕OO).
- -
We suggest the evaluation of vertebral fracture using conventional X-rays in patients with T1DM (2⊕⊕OO).
Most studies have demonstrated that T1DM has a negative impact on bone mineral density (BMD) at both lumbar and femoral levels. This effect appears to be independent of the bone mass index (BMI), disease duration, and the extent of metabolic control, and has been reported in both women and men.2–5 Involvement exists from the time of diagnosis6 and improves with intensive insulin therapy.7 Microvascular complications and smoking are in turn associated with a decreased bone mass in this patient group.8–10 However, other authors found no significant differences in BMD between patients with T1DM and a control population.11–13
Two meta-analyses have shown that patients with T1DM have a 6- to 8-fold increase in the risk of hip fracture. This increase is higher than expected based on the BMD decrease seen, independently from HbA1c,2,14 and greater in the presence of microvascular and macrovascular complications.15 A higher number of all non-vertebral fractures have in turn been reported in patients with T1DM.16,17
Fracture risk evaluation may be made using the FRAX tool, available at: http://www.shef.ac.uk/FRAX/tool.jsp?country=4, which considers T1DM as secondary osteroporosis.
The results for vertebral fractures (VFs) are not consistent. Thus, while a cross-sectional study found no significant differences,18 Vestergaard et al., using a case–control design, found an increased risk in both males and females with T1DM.19 On the other hand, several studies in non-diabetic populations demonstrated that the presence of VFs, both clinical and morphometric, was an independent risk factor for the occurrence of new episodes of fragility fracture.
TreatmentRecommendations- -
We suggest that patients with T1DM who have osteoporosis and/or fragility fracture should follow the same general and pharmacological recommendations as the non-diabetic population (2⊕⊕OO).
- -
Deficient osteoblastic function in this condition makes the use of anabolic drugs attractive in high-risk or secondary prevention patients (2⊕OOO).
Although hyperglycemia has been associated with a low level of bone remodeling, no study to date has specifically analyzed the effect of the different treatment options on BMD in patients with T1DM and osteoporosis. As regards fracture prevention, only a recently published study which showed that the presence of T1DM did not decrease the anti-fracture effectiveness of bisphosphonates or raloxifene is available.20
Type 2 diabetes mellitusEvaluationRecommendations- -
We suggest BMD measurement and VF evaluation using plain X-rays in patients with type 2 diabetes mellitus (T2DM) (2⊕⊕OO).
- -
We recommend the evaluation of non-vertebral fracture risk in patients with T2DM, especially in those with vascular complications, insulin therapy, or glitazone therapy (1⊕⊕OO).
Although different studies have provided variable results, it may be stated that, overall, patients with T2DM have increased BMD at both lumbar and femoral level as compared to non-diabetic subjects in the different study populations.2,21–26 This increase correlates positively with BMI and negatively with disease duration at femoral level and with glitazone treatment. It appears to be independent of age and HbA1c.2,27
However, despite this increase in BMD, two recent meta-analyses have demonstrated that patients with T2DM experience a greater number of non-vertebral fractures (wrist, foot, hip) despite the fact that no significant increase has been shown in VF risk. Specifically in the hip, patients with T2DM have a relative fracture risk ranging from 1.4 to 1.7 as compared to the non-diabetic population.2,14 By contrast, Yamamoto et al., in a cross-sectional study, found an increased risk of vertebral fractures in this patient group.28
Fracture risk has been associated with the presence of cataract, microangiopathy (neuropathy, retinopathy), insulin therapy, and stroke, all of them related to the increased risk of falls characteristic of this patient group.29–34 Femoral BMD and FRAX score in turn predict the risk of hip fracture and all non-vertebral fractures, but they appear to underestimate the actual risk in these patients.35 On the other hand, conflicting results have been reported for the effect of oral antidiabetics on fracture risk. Thus, while the use of metformin and sulfonylureas has been associated with a lower risk,19,33 treatment with glitazones increases risk, particularly in postmenopausal women, but also in men.36–38
The risk of fracture in patients with T2DM may be assessed using the QFracture scale (http://www.qfracture.org), which considers the presence or absence of T2DM as a risk factor, although it has not been validated for the Spanish population.
TreatmentRecommendations- -
We suggest that in patients with T2DM, the same recommendations should be followed for osteoporosis and fracture prevention as in the non-diabetic population (2⊕⊕OO).
- -
Deficient osteoblastic function in this condition makes the use of anabolic drugs attractive in high-risk or secondary prevention patients (2⊕OOO).
Few studies have specifically analyzed the prevention and treatment of osteoporosis in patients with T2DM. In a post hoc analysis of the Fracture Intervention Trial, treatment with alendronate resulted in similar BMD gains in postmenopausal women with or without T2DM.39 By contrast, in a retrospective case–control study, Dagdelen et al. noted that the presence of T2DM was associated with a decreased response to alendronate treatment at cortical bone level (femur, radius).40 As regards fracture prevention, and as occurs in T1DM, anti-fracture efficacy of bisphosphonates and raloxifene does not appear to decrease in patients with T2DM.
Primary hyperparathyroidismEvaluationRecommendation- -
We recommend the evaluation of bone mass and lateral X-rays of the thoracic and lumbar spine to assess the presence of vertebral fractures in all patients with primary hyperparathyroidism (PHPT) (1⊕⊕⊕O).
One out of every three patients has BMD loss from 10 years of disease onset. Cortical bone is predominantly affected, while trabecular bone is preserved. These changes are reversed with parathyroidectomy. Twenty-one percent of patients experience bone mass loss (T-score<2.5 SD) at 10 years of follow-up,41 for which baseline calcium levels, menopause, and younger age are determinant factors.42 After 10 years, cortical BMD does not remain stable, but is impaired in most patients.43
Observational studies in groups with asymptomatic PHPT show an increased risk of fractures, mainly cortical fractures in the axial skeleton. Fracture risk increases are 1.8 for all fractures, 3.2 for VFs, 2.3 for distal radius fractures, and 1.4 for hip fractures.44,45
TreatmentRecommendations- -
We recommend parathyroidectomy in most patients because it increases bone mass and decreases fracture risk (1⊕⊕⊕O).
- -
If surgery is contraindicated or refused, we suggest combined treatment with cinacalcet (to control PTH and calcium levels) and an anticatabolic (bisphosphonates or denosumab) to prevent bone mass loss, although no data are available for fractures (2⊕OOO).
In patients with PHPT, parathyroidectomy improves pulsatile PTH secretion and increases bone remodeling, mineralization, and bone formation. Following parathyroidectomy, a BMD increase ranging from 4% to 12% occurs, which is greater in the lumbar spine, followed by the femur and radius.41,46–49 Bone mass improves in 100% of patients (8–12% in 3 years), and this improvement is maintained at 15 years, particularly in trabecular bone. Most, but not all, studies show that fracture risk is decreased by 50% after parathyroidectomy.43,50–52
The criteria for parathyroidectomy in asymptomatic patients are as follows: a T-score of −2.5 SD or less in the lumbar spine, femoral neck, hip, or distal third of the radius in postmenopausal women and men over 50 years of age (3th International Workshop).41 In premenopausal women or men under 50 years of age, the International Society for Clinical Densitometry considers as a criterion a Z-score less than −2.5 SD in the same locations.53
Bisphosphonates, of which alendronate is the most widely studied, decrease bone resorption and increase lumbar and femoral BMD. They are recommended in patients with PHPT with no surgical criteria and low BMD, although they do not modify calcium or PTH levels. In postmenopausal women, estrogens–progestogens decrease bone resorption and increase BMD to an extent similar to that seen in normocalcemic subjects or after parathyroidectomy in PHPT. Cinacalcet decreases calcium and PTH levels, but neither decreases bone turnover nor increases BMD.54–56
Endogenous hyperthyroidismEvaluationRecommendation- -
We suggest the evaluation of bone mass and the presence of fractures in patients with a history of hyperthyroidism. A history of hyperthyroidism should be considered a risk factor for hip fracture (2⊕⊕OO).
Hyperthyroidism decreases bone mass57–59 regardless of its cause in both men and pre- and postmenopausal women,60,61 and the decrease is greater with age.57 There is an greater risk of hip fracture.57,58,62 An overall increase in risk of Colles’ or vertebral fracture has not been shown,63 but does exist in women over 65 years of age.64 Bone mass loss is due to increased bone resorption with an increase in remodeling markers, which normalize upon returning to a euthyroid state,65 although a part of the bone mass loss is irreversible according to histomorphometric studies.66
TreatmentRecommendations- -
Treatment of hyperthyroidism with rapid achievement of euthyroidism causes at least partial improvement in bone mass and a decrease in fracture risk (1⊕⊕OO).
- -
We recommend that adequate calcium and vitamin D intake be ensured (1⊕OOO).
- -
We suggest considering individualized treatment with anticatabolic drugs (aminobisphosphonates or denosumab) in postmenopausal women and elderly patients of both sexes at a high risk of osteoporotic fractures. Patients with postmenopausal osteoporosis and a history of hyperthyroidism may benefit from anabolic treatment (PTH or teriparatide) before receiving antiresorptive drugs (2⊕OOO).
Bone loss caused by hyperthyroidism is usually reversible, at least partially, from the first year of treatment with antithyroid drugs and for a variable time according to various studies.57,63,67,68 Radioiodine therapy also causes BMD improvement.57,69 There are no studies assessing BMD alone in patients undergoing surgery, but a decreased fracture risk has been shown in these patients.58 Some studies show BMD improvement but not complete recovery, a bone mass lower than expected being maintained.70,71
Subclinical endogenous hyperthyroidismEvaluationRecommendation- -
We suggest the evaluation of bone mass and the presence of fractures in patients with subclinical hyperthyroidism, especially in postmenopausal women and patients over 65 years of age (2⊕⊕OO).
While the harmful effect of endogenous hyperthyroidism on BMD and fracture risk is well established, the risk in patients with untreated subclinical hyperthyroidism is more controversial. Increased levels of bone remodeling markers, both formation and resorption markers, have been shown in patients with subclinical hyperthyroidism.72 Bone mass assessment studies have mainly been conducted in postmenopausal women, showing decreases in BMD in those with endogenous subclinical hyperthyroidism, regardless of its cause.73–76 Results in premenopausal women are conflicting, and BMD losses are in any case lower than those seen in postmenopausal patients.73,76 As regards fracture risk, this was recently assessed in a cohort study showing hip fracture rate in males over 65 years of age with subclinical hyperthyroidism of 13.65 per 1000 patient-years and a high risk of hip fracture: RR 4.91, 95% CI 1.13–21–27; no significant results were found in women.75 On the other hand, an increased fracture risk has been shown in patients with subclinical hyperthyroidism and undetectable TSH,77 and a prospective study in women older than 65 years showed undetectable TSH to be a risk factor for vertebral and hip fractures after 4 years of follow-up.64 There are also studies showing no harmful effect of subclinical hyperthyroidism upon bone metabolism, but they were conducted on premenopausal patients with a younger mean age.78–80
TreatmentRecommendations- -
Treatment of subclinical hyperthyroidism to rapidly achieve euthyroidism causes bone mass improvement (1⊕⊕OO).
- -
We suggest that individualized antiresorptive treatment be considered for postmenopausal women and elderly patients with osteoporosis to rapidly improve fracture risk (2⊕OOO).
Two prospective studies in postmenopausal women with subclinical hyperthyroidism showed mild improvement or stabilization of BMD in patients treated with radioiodine or antithyroid drugs, while untreated patients experienced BMD impairment.81,82
HypothyroidismEvaluationRecommendation- -
Specific measures other than ensuring adequate calcium and vitamin D intake are not recommended for this patient group (1⊕OOO).
Untreated hypothyroidism is associated with greater BMD due to decreased bone remodeling.66 Some studies have shown a greater risk of fracture in both men and women with primary hypothyroidism, with a maximum peak at diagnosis.58,63,83 Various explanations have been given for this increased fracture risk, including that an initial increase in remodeling, followed by normalization, occurs after treatment with levothyroxine has been started, or that stress fractures accumulate because of low bone remodeling or an increased number of falls in patients with hypothyroidism due to the effect of this at neuromuscular level.
TreatmentRecommendations- -
Although the treatment of clinical hypothyroidism causes an initial loss of bone mass, it does not appear to increase fracture risk. Specific measures are therefore not recommended if TSH levels remain within normal limits. (1⊕⊕OO).
- -
We recommend that adequate calcium and vitamin D intake be ensured (1⊕OOO).
Conflicting results have been reported on the effect of replacement therapy for hypothyroidism on BMD. Thus, several studies showed no negative effect of replacement therapy with levothyroxine on BMD,63,84,85 while a meta-analysis of cross-sectional studies did show a decreased BMD in premenopausal women, but not in postmenopausal women or in men.86 A recent study in elderly people over 70 years of age found an increased fracture risk in those currently using levothyroxine as compared to those who had taken it in the past (OR 1.88, 95% CI 1.71–2.05). Patients receiving higher cumulative and mean doses had a greater risk, but TSH levels and the reason for the indication were not analyzed.87
Subclinical hypothyroidismEvaluationRecommendation- -
No specific measures are recommended (1⊕OOO).
Little evidence is available on the effects of subclinical hypothyroidism on bone mass.88,89 As regards the risk of fracture, a recent study showed an increased hip fracture incidence in men with subclinical thyroid dysfunction, while no association was found in women.75
TreatmentRecommendations- -
Treatment of subclinical hypothyroidism with levothyroxine at replacement doses does not appear to increase fracture risk. Specific measures are therefore not recommended if TSH levels remain within the normal range (1⊕OOO).
- -
We recommend that adequate calcium and vitamin D intake be ensured (1⊕OOO).
No data are available on the effects of levothyroxine treatment on bone mass or risk of fracture in patients with subclinical hypothyroidism, but increases in both formation and resorption markers have been shown in women treated with levothyroxine as compared to placebo.90
Suppressive therapy with levothyroxineEvaluationRecommendations- -
We recommend BMD monitoring every 1–2 years in patients on suppressive therapy with levothyroxine, especially postmenopausal women and patients older than 65 years (1⊕⊕OO).
- -
We recommend the use of the lowest possible suppressive dose, and that adequate calcium and vitamin D intake be ensured (1⊕OOO).
- -
We suggest that individualized treatment with potent antiresorptive drugs (aminobisphosphonates, denosumab) be considered in postmenopausal women and elderly patients with average fracture risk, estimated from models which do not take the history of subclinical hyperthyroidism into account (2⊕OOO).
Multiple studies are available concerning patients receiving suppressive therapy with levothyroxine as part of their treatment for differentiated thyroid cancer and as treatment for non-toxic multinodular goiter. The results of such studies are conflicting, with significant BMD decreases in both men and women in some of them91–97 and no differences in BMD in others.98–107 This may reflect differences in the use of the minimum suppressive dose in clinical practice.
A population study of 17,684 patients treated with levothyroxine showed that patients with suppressed TSH had double the risk of osteoporotic fracture as compared to those with normal TSH levels, although no data were available on FT4 levels.108
Female hypogonadismEvaluationRecommendation- -
We recommend the evaluation of bone mass with DXA and fractures using lateral X-rays of the thoracic and lumbar spine at diagnosis of hypogonadism and every 3–5 years thereafter (1⊕⊕⊕⊕).
In women, decreased estrogen levels increase the risk of low bone mass and fragility fractures.109,110 This risk is greater when hypogonadism starts in early age.
TreatmentRecommendations- -
We recommend treatment for the cause of hypogonadism. When this is not possible, hormone replacement therapy must be given (1⊕⊕⊕⊕).
- -
We recommend maintenance of an optimum calcium and vitamin D intake, and regular physical activity (1⊕⊕⊕⊕).
- -
Treatment with bisphosphonates is not recommended in adolescent and premenopausal women (1⊕OOO).
- -
If pregnancy is not planned in the short term and fracture risk is high, we suggest starting treatment with denosumab (2⊕OOO).
- -
In patients with very high fracture risk or prevalent fracture and low BMD, anabolic treatment is suggested (2⊕OOO).
Etiological treatment is indicated when hypogonadism is secondary to prolactinoma, anorexia nervosa, or hypothalamic functional amenorrhea.111,112 In all other situations, hormone replacement therapy should be started with combined oral contraceptive pills (estrogens combined with progestogens) in young patients. Transdermal formulations should be used in the event of obesity, smoking, or hypertension. In women with osteopenia, the use of oral ethinyl estradiol or high-dose conjugated estrogens is recommended.111 Estrogen replacement should be performed until approximately 50 years of age, and risk/benefit should be assessed taking age, osteoporosis, smoking, and risk of thrombosis into consideration.111
Hormone-treated breast cancerEvaluationRecommendation- -
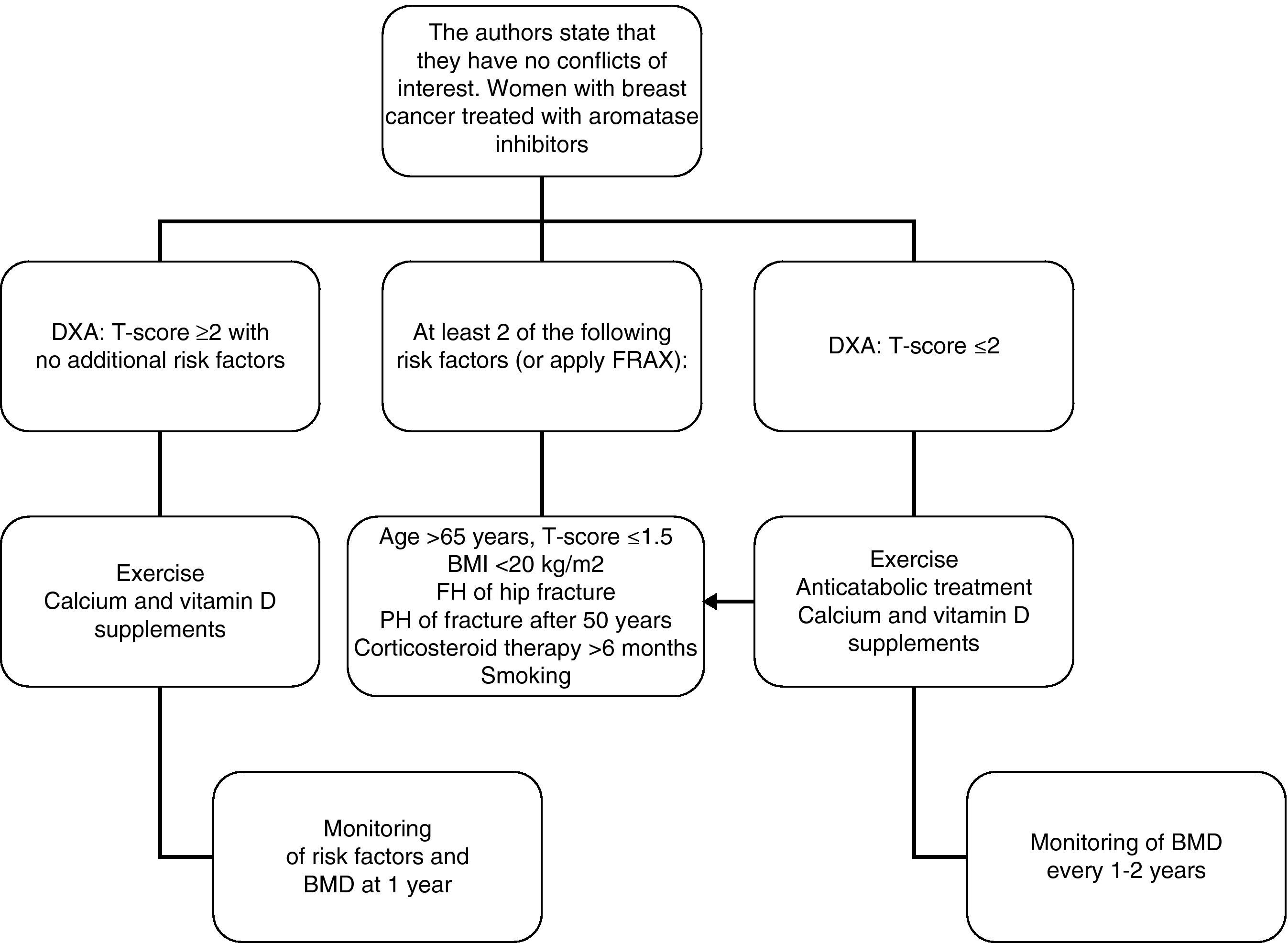
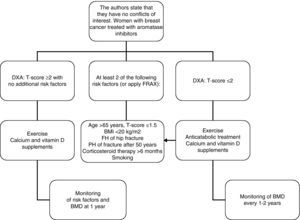
In women with breast cancer receiving treatment with GnRH agonists and/or aromatase inhibitors, we recommend that bone mass be evaluated (1⊕⊕⊕O) and that the presence of vertebral fractures be ruled out (1⊕⊕OO).
- -
We recommend the administration of calcium and vitamin D supplements and that advice on physical activity be given to all patients (1⊕⊕OO).
- -
In patients in whom anticatabolic treatment is not indicated (Fig. 1), we recommend the use of bisphosphonates such as zoledronate (1⊕⊕⊕⊕), risedronate (1⊕⊕OO), ibandronate (1⊕⊕OO), or denosumab (1⊕⊕OO).
In women treated with aromatase inhibitors, zoledronate (4mg/6 months IV) has been shown to increase bone mass between 4.4% and 6.2% in the lumbar spine and between 1.2% and 2.6% in the hip.116,117 There is also evidence from large clinical trials suggesting antitumor benefits from zoledronate, derived from a positive impact on the bone marrow microenvironment.
In 2-year studies in women with breast carcinoma treated with anastrozole, risedronate (35mg PO/week) has been shown to induce increases in lumbar (0.4–2.2%) and femoral (0.9–1.8%) bone mass compared to placebo.118,119
Ibandronate (150mg PO/month) has also been shown to prevent bone mass loss in this patient group.120 Finally, treatment with denosumab (60mg SC/6 months) for 24 months induces BMD to increase by 7.6% in the lumbar spine and 4.7% as compared to placebo.121
Male hypogonadismEvaluationRecommendations- -
In young males, decreased testosterone levels are associated with low bone mass and an increased risk of fracture (1⊕⊕⊕⊕).
- -
We recommend that DXA be performed on diagnosis of hypogonadism and every 3–5 years thereafter (1⊕⊕OO).
Studies conducted on adult patients with Klinefelter syndrome showed a 25–48% reduction in BMD as compared to healthy adult males, and osteoporosis in 6–15%.122–124
TreatmentRecommendations- -
To increase bone mass and decrease risk of fracture, we recommend the restoration of testosterone levels (1⊕⊕OO), the maintenance of adequate calcium and vitamin D intake, and regular physical activity (1⊕⊕⊕⊕).
- -
We recommend treatment with bisphosphonates in males with osteoporosis and/or fragility fractures (1⊕⊕⊕⊕). In the event of severe osteoporosis, very low bone mass (<3 SD), or no response to bisphosphonates, the use of teriparatide is recommended (1⊕⊕OO).
Testosterone replacement therapy may be given by intramuscular or transdermal (patch or gel) administration. The potential adverse effects of treatment should always be monitored.125 The addition of bisphosphonates is recommended in older patients with osteoporosis,126–128 and teriparatide should be used in the event of severe osteoporosis and/or no response to bisphosphonates.124
Prostate cancer treated with androgen deprivation therapyEvaluationRecommendation- -
We recommend DXA and X-rays to search for vertebral fractures at the start of treatment with GnRH agonists or following orchidectomy and every 12 months thereafter (1⊕⊕⊕⊕).
GnRH agonists (goserelin, triptorelin, leuprolide) used in advanced prostate carcinoma induce bone mass loss and an increased fracture incidence. Both effects are related to treatment time and the dose of GnRH agonists administered.126–130 Annual bone mass loss ranges from 0.6% to 4.5%, and percent loss is even greater (4–10%) in the first 6–12 months after the start of treatment.131,132
TreatmentRecommendations- -
We recommend that all patients treated with GnRH or orchidectomy be given calcium (1000–1500mg) and vitamin D (800IU), cease smoking, and practice regular physical activity(1⊕⊕⊕⊕).
- -
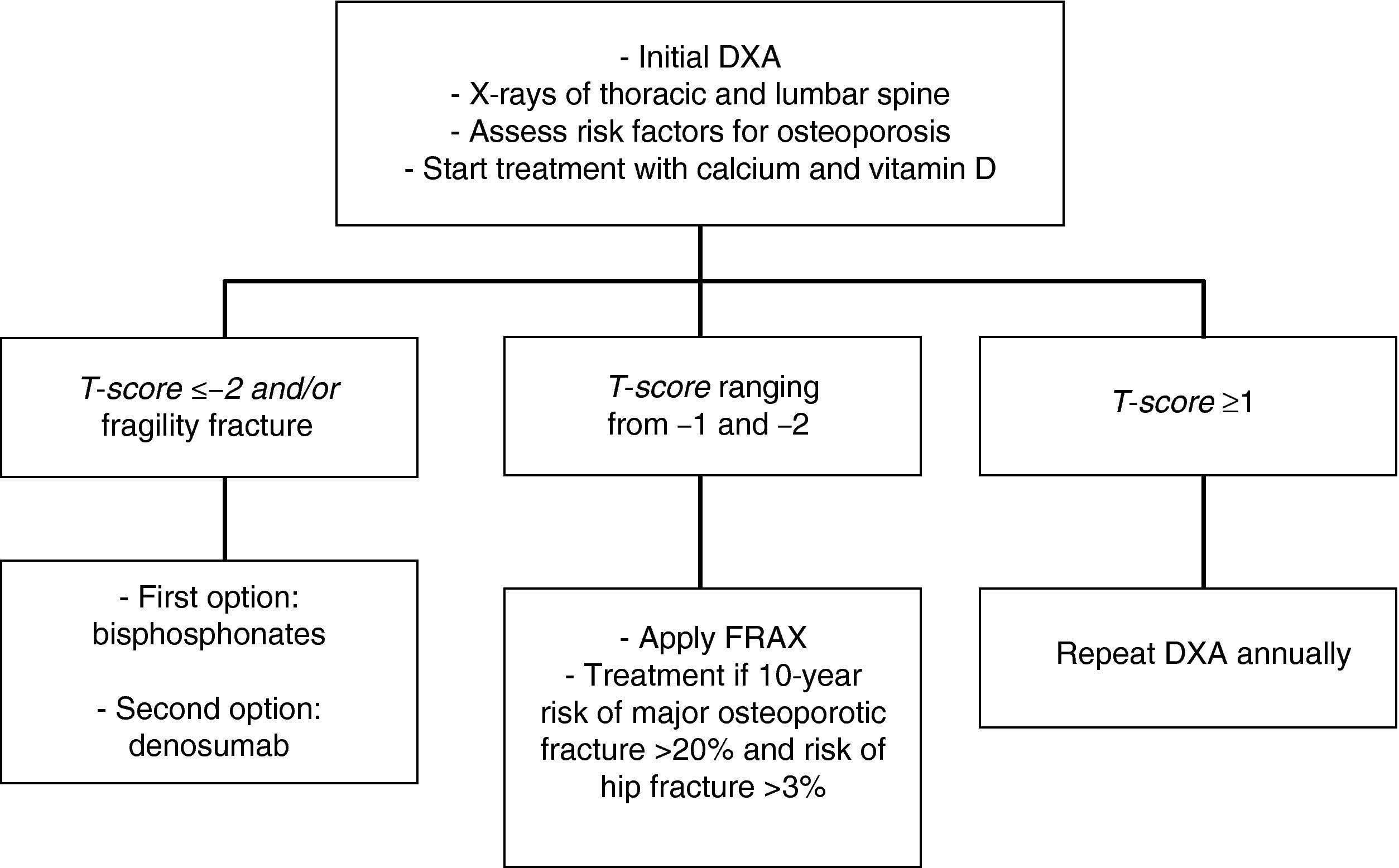
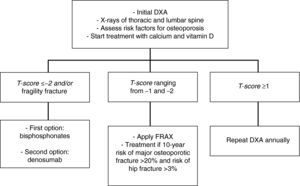
In patients with a T-score lower than −2 and/or a history of osteoporotic fracture, we recommend that treatment be started with bisphosphonates [zoledronic acid (1⊕⊕⊕⊕) as first option, or denosumab (1⊕⊕⊕⊕)].
- -
In patients with T-scores ranging from −1 and −2, we recommend the assessment of other risk factors for osteoporosis (1⊕⊕⊕O).
In patients with prostate cancer given androgen deprivation therapy, zoledronate has been shown to increase bone mass as compared to placebo (6.7–7.8% in lumbar spine and 2.6–3.9% in total hip), but no data are available concerning the reduction of new fractures.131,133 Although it does not prevent the development of bone metastases, it does decrease the skeletal complications associated with them by 36%.134 In patients with prostate cancer, alendronate treatment induces bone mass gain (3.7% in the spine and 1.6% in the hip). However, fracture data are not available here, either.135
Denosumab is the only drug that has been shown to decrease the incidence of new fractures in patients with prostate carcinoma. After 36 months of treatment, the risks of new vertebral fractures or any new fractures were reduced by 62% and 28% respectively.136 Denosumab also decreases skeletal complications associated with bone metastases.137 Treatment with teriparatide is not recommended for patients with bone metastases, including micrometastases or hidden disease. Strontium ranelate is not recommended in patients with prostate cancer.
In intermediate risk patients (T-score ranging from −1 and −2), drug treatment is recommended when there are other risk factors for osteoporosis, such as smoking, low weight (<70kg), alcohol consumption, or the use of certain drugs (anticoagulants, omeprazole, corticosteroids). In this situation, fracture risk may also be calculated using the FRAX tool and 10-year fracture risk may be assessed, with treatment being started in patients with major osteoporotic fracture risk >20% and hip fracture risk >3% at 10 years138 (Fig. 2).
Adult GH deficiencyEvaluationRecommendation- -
We suggest the evaluation of bone mass and fractures in patients with severe GH deficiency (2⊕⊕OO).
Severe GH deficiency (GHD), alone or with other pituitary hormone deficiencies, causes a bone mass decrease139 whose magnitude depends on age and other factors. Many cross-sectional studies with low numbers of patients show bone mass decreases by approximately one standard deviation in adults with severe GHD. However, somewhat larger retrospective studies show the effect of GHD on bone mass to be dependent on age at the start of GHD and to be greater in younger patients. Thus, patients under 30 years show the most severe involvement, those aged 30–45 years have intermediate involvement,140 and involvement at more advanced ages is reduced, with no differences from controls in patients over 55–60 years of age.140–142
Only three studies have assessed the risk of fracture in GHD, showing a 2- to 5-fold increase.143–145 No good correlation exists between bone mass and fracture risk, because up to 50% of patients with vertebral deformities have a normal bone mass.146
TreatmentRecommendations- -
Treatment with GH of adults with GHD improves bone mass (2⊕⊕OO).
- -
After the first years of treatment (4–5 years), the addition of alendronate induces an additional increase in bone mass (2⊕OOO).
- -
We recommend continued replacement therapy in persistent GHD after adult height is reached to achieve complete bone maturation during the transition period from adolescence to adult age (1⊕⊕OO).
- -
We suggest treatment with GH to decrease the risk of vertebral fracture in patients with GHD (2⊕OOO).
- -
If treatment response is inadequate, we suggest the start of anabolic treatment followed by anticatabolic treatment (2⊕OOO).
GH treatment exerts an anabolic effect. No effect on BMD and even a decrease in BMD may be seen in the first year of treatment,147–151 but after 18–24 months most studies show BMD increases ranging from 4% to 10%, with a greater effect on the lumbar spine. Although bone mass increase has been reported up to 10 years after treatment start, a plateau effect is usually seen at 5 years.152 When this occurs, the addition of alendronate induces a greater increase in bone mass as compared to GH alone.153–155
In patients with persistent severe GHD al the end of the growth period, replacement therapy continuation or restart for up to 2 years has been shown to result in a significantly greater BMD as compared to no treatment.156–158
There are no prospective studies assessing the effect of replacement therapy on fracture risk. A lower prevalence of vertebral deformities has been seen in patients treated with GH (54% versus 78%), especially if treatment is started early after diagnosis.146
Cushing's syndromeEvaluationRecommendation- -
Cushing's syndrome decreases bone mass and increases fracture risk (2⊕⊕OO).
- -
In these patients, we recommend the evaluation of bone mass and the presence of fractures, particularly asymptomatic vertebral fractures (1⊕⊕OO).
Cushing's syndrome causes a decrease in bone mass, mainly trabecular mass.159–162 It has not been confirmed that the etiology of Cushing's syndrome influences the prevalence of osteoporosis and the risk of fracture,159–162 except in ectopic Cushing's syndrome, where the risk of fracture may be greater.161
TreatmentRecommendations- -
Etiological treatment is suggested, because it results in bone mass increase (2⊕⊕OO).
- -
We suggest that treatment be started with alendronate, because it may promote a greater bone mass increase, although its effect on fractures has not been assessed (2⊕⊕OO).
- -
Because of the deficient osteoblastic function characteristic of this condition, we recommend consideration of anabolic treatment if very there are low bone mass, prevalent fractures, or no response to anticatabolic treatment, especially in younger patients (1⊕OOO). In premenopausal women, we recommend the use of denosumab as an anticatabolic (1⊕OOO).
BMD increase has been reported following etiological treatment for Cushing's syndrome.163,164 As regards drug treatment, a single observational study on 39 patients showed that treatment with alendronate for 12 months induced a greater BMD increase as compared to no treatment.165
Subclinical hypercortisolism secondary to adrenal incidentalomaEvaluationRecommendation- -
In patients with adrenal incidentaloma and subclinical hypercortisolism, we suggest the evaluation of bone mass and vertebral fractures (2⊕⊕OO).
Conflicting evidence is available as to whether or not bone mass is decreased in subclinical hypercortisolism caused by an adrenal incidentaloma.166–170 As regards the risk of vertebral fracture, most cross-sectional studies,166,169–171 but not all,168 report an increased incidence of vertebral fractures. However, two recent prospective studies on larger patient samples have confirmed an increased risk of vertebral fracture in these patients.172,173
TreatmentRecommendations- -
Treatment with anticatabolic drugs may promote a bone mass increase in premenopausal women with subclinical hypercortisolism, but its effect on fractures has not been evaluated (2⊕⊕OO).
- -
We recommend that anabolic treatment be considered if very there are low bone mass, prevalent fractures, or no response to anticatabolic treatment, especially in younger patients (1⊕OOO). In premenopausal women, we recommend the use of denosumab as an anticatabolic (1⊕OOO).
A single study has assessed the effects of treatment with bisphosphonates on bone mass in premenopausal women with subclinical hypercortisolism due to adrenal incidentaloma, showing that weekly clodronate administration for 1 year increases BMD as compared to calcium and vitamin D.174
Addison's diseaseEvaluationRecommendations- -
Primary hypercortisolism may be associated with bone mass decrease and a greater prevalence of osteoporosis related to steroid replacement therapy and adrenal androgen deficiency. It is not known whether the risk of fracture is increased (1⊕OOO).
- -
We suggest that BMD is only analyzed in patients with long-standing disease or on higher steroid doses, and in premenopausal or amenorrheal women (2⊕⊕OO).
There are studies which report no general differences in bone mass between patients with Addison's disease and controls.175,176 Bone involvement has mainly been shown in postmenopausal women,177,178 in whom a high prevalence of osteoporosis178,179 has been reported, mainly in trabecular bone.179–181 A single study examined the presence of morphometric vertebral fractures by simple X-rays and found no differences from a control group.182
TreatmentRecommendations- -
We suggest reduction of steroid replacement therapy dosage to minimize the effects on bone (2⊕⊕OO).
- -
For replacement therapy we suggest a short-acting corticosteroid (hydrocortisone) instead of those with intermediate or long action (2⊕⊕OO).
- -
Treatment with DHEA improves hip BMD compared to placebo (2⊕⊕OO).
There are no studies which assess the effect of standard antiosteoporotic drugs on BMD in these patients. Two studies suggest a reduction of standard replacement doses181,183 because of the finding of a greater prevalence of low bone mass in these patients, but their effect was not assessed prospectively. Moreover, in a recent study, patients treated with prednisone had a lower bone mass than those treated with hydrocortisone at equivalent doses. The authors therefore suggested that hydrocortisone should preferentially be used for treatment.176 A single double-blind clinical trial assessing the effect on BMD of DHEA 50mg for 12 months versus placebo has been found. Significant positive changes in the hip were reported.183
Bariatric surgeryEvaluationRecommendation- -
We recommend bone mass evaluation before surgery (1⊕OOO).
- -
In patients undergoing malabsorptive procedures (Roux-en-Y gastric bypass, gastric band, biliopancreatic diversion), we recommend annual/biannual DXA until bone mass has been stabilized (1⊕⊕OO).
Bariatric surgery leads to increased bone resorption and decreased bone formation,184,185 greater than that occurring after weight loss through medical treatment186,187 and which may vary depending on the surgical procedure performed.185 Postmenopausal patients have a greater risk of bone mass and require closer monitoring.188,189
The actual risk of fracture in these patients is unknown, but does not appear to be highly based on risk predictions using the FRAX algorithm.189,190
TreatmentRecommendations- -
Before surgery, the maintenance of calcium intake similar to the general population and the normalization of vitamin D levels are recommended (2⊕⊕OO).
- -
After surgery, we recommend routine calcium and vitamin D supplementation when malabsorptive procedures have been performed (1⊕⊕⊕O).
- -
In the event of osteoporosis, we suggest that recommendations in standard clinical practice guidelines be followed, after correcting calcium and vitamin D deficiency to prevent episodes of severe hypocalcemia (2⊕OOO).
- -
To treat vitamin D deficiency, we suggest the use of calcifediol (25 OH D) 50,000IU (half a vial of Hidroferol 0.266mg) one to three times weekly. In severe cases, daily administration of this same dose or administration of calcitriol may be required (2⊕OOO).
After surgery, routine calcium and vitamin D supplementation is recommended when malabsorptive procedures have been performed. Dosage should be adjusted, based on biochemical measurements.191–193 Calcium citrate is preferred over carbonate because of its greater bioavailability and greater efficacy for the normalization of bone markers.194 In addition, it is metabolized to bicarbonate which, as a neutralizing effect in urine, decreases the risk of nephrolitiasis.195 Weekly use of 50,000IU of vitamin D in addition to a daily supplement of 8000+1500mg of calcium citrate has been shown to decrease bone loss following gastric bypass.196 No adequate data are available to indicate routine magnesium supplementation in addition to that included in the routinely used multivitamin complex.191
When osteoporosis is treated with bisphosphonates, potential oral intolerance and the risk of stomal ulcer should be taken into account. The use of intravenous formulations or other treatment alternatives such as denosumab is suggested in these cases.191,195
Celiac diseaseEvaluationRecommendations- -
We recommend the assessment of bone mass and the presence of fractures in the typical presentation of celiac disease (CD) in adults (1⊕⊕⊕O).
- -
In CD with an atypical or silent presentation, we recommend that bone mass and fracture risk be assessed using criteria for the general population, paying special attention to patients with poor compliance with gluten-free diet, low weight (BMI<20kg/m2), weight loss >10%, and older than 70 years (1⊕⊕OO).
- -
In any form of presentation, we recommend the measurement of vitamin D, PTH, and calcium levels (1⊕⊕OO).
- -
Screening for CD is not recommended in patients with osteoporosis (2⊕⊕OO).
CD causes bone mass impairment.197–201 Osteoporosis mainly occurs in patients with the typical presentation or with a greater treatment adherence. Controversy exists as to whether the prevalence of osteoporosis is increased in atypical or silent presentations.197–203 It has been estimated that the relative risk of fracture is increased by 43% in symptomatic disease, while the risk associated with atypical or silent presentations is not significantly different from that of the general population.203–208
Despite the high prevalence of CD (0.3–1% of the population) and the fact that most cases remain undiagnosed,197,198 screening for CD is not recommended in patients with osteoporosis.209–212
No agreement exists about the pathogenetic process, but two pathways are considered to be involved.199,200,213 Nutrient malabsorption predominates in symptomatic CD, while the production of proinflammatory cytokines predominates in asymptomatic and silent CD.198,199 Calcium malabsorption occurs in both types.199,200,213–215 In addition, there is evidence associating low bone density in CD with genetic predisposition,216 decreased IGF-1 levels, and positive autoantibodies against osteoprotegerin.217
TreatmentRecommendations- -
In patients with CD diagnosed in childhood, we recommend a gluten-free diet because no other specific treatment or monitoring is required for the prevention of osteoporosis provided the patient adequately adheres to such a diet (1⊕⊕⊕O).
- -
In patients diagnosed in adulthood, the supplementation of a gluten-free diet with vitamin D and calcium according to general recommendations, with adjustment based on the degree of malabsorption, is suggested (2⊕⊕OO).
- -
If anticatabolic treatment is required, it should be started after the completion of a 1 year gluten-free diet period (1⊕⊕OO).
- -
We recommend that the general indications for prescribing drugs for osteoporosis in CD be followed (1⊕⊕OO).
- -
Monitoring for hypocalcemia is recommended if treatment with bisphosphonates is given, particularly in subjects with poor diet adherence (1⊕OOO).
When CD is diagnosed in childhood, a gluten-free diet is the only treatment needed, and good diet adherence allows for achieving normal bone mass.218–221 In adults, a gluten-free diet is the mainstay of treatment and improves bone mineral density (usually by 5% in the first year and by up to 7% at 2–3 years) even in patients without total mucosal recovery,222,223 although there are studies which show that diet alone does not achieve bone mass normalization in all subjects and that fracture risk remains increased.224–227
No randomized studies demonstrating the efficacy of standard treatments for osteoporosis in patients with celiac disease are available.228 It is therefore assumed that the same treatment recommendations as for the general population should be followed in patients with CD.200,202,213
Inflammatory bowel diseaseEvaluationRecommendations- -
Although inflammatory bowel disease (IBD) is associated with low bone mass and increased fracture risk, we do not recommend routine bone mass evaluation (1⊕OOO).
- -
We recommend risk fracture assessment using the FRAX tool in the remission phase of IBD (1⊕⊕OO).
- -
We recommend assessment with DXA in patients at intermediate or high risk using the FRAX tool, in patients treated with corticosteroids, or if two or more of the following risk factors exist: persistent active disease, BMI<20kg/m2, weight loss>10%, and age>70 years (1⊕⊕OO).
- -
We recommend the measurement of serum levels of vitamin D, PTH, and calcium (1⊕OOO).
- -
Depending on fracture risk, we suggest repeat assessment by DXA every 2–3 years or every year in the event of corticosteroid treatment (2⊕OOO).
In the published studies, IBD is associated with an increased risk of osteopenia and osteoporosis which varies according to the inflammatory process itself (activity, site involved, and prior surgery), age at diagnosis, and duration. The pathogenesis of osteoporosis in IBD is multifactorial. In addition to inflammatory cytokines, age, corticosteroid treatment, malnutrition, and calcium and vitamin D deficiency also have an influence.200,213
The prevalence of osteopenia ranges from 22% to 55% in Crohn's disease and from 32% to 65% in ulcerative colitis depending on the study. The prevalence of osteoporosis ranges from 3% to 57% in Crohn's disease and from 4% to 50% in ulcerative colitis.229–232 The risk of fracture is 40–60% higher as compared to the general population.233–237
Several studies show that bone mineral density itself does not predict the risk of fracture in patients with IBD,234,238 and screening with DXA is generally not recommended in these patients.239,240
IBD is one of the causes of secondary osteoporosis included in the FRAX tool. To date, a single retrospective cohort study has shown its value in this condition.241 It should be noted that the FRAX tool has not been validated for IBD in populations younger than 40 years, that sudden BMI changes limit its accuracy in active disease phases, and that it does not take into consideration the cumulative corticosteroid dose.
The FRAX tool allows for rating absolute fracture risk as low, intermediate, and high. Current guidelines and the studies conducted support the idea of DXA assessment in subjects at intermediate and high risk and in patients treated with corticosteroids.138,241,242 It is also recommended that DXA assessment be performed in patients with IBD having two or more of the following risk factors: BMI<20kg/m2, weight loss>10%, or corticosteroid treatment.200,238,239,243
TreatmentRecommendations- -
We recommend that inflammation be controlled by diet or non-steroidal drugs, because remission or improvement of the inflammatory process results in bone mass improvement (1⊕⊕OO).
- -
To prevent fractures in IBD patients, we recommend an improvement of nutritional status and calcium and vitamin D supplementation, particularly in young patients and patients treated with glucocorticoids (1⊕OOO).
- -
We recommend regular physical activity as a measure to prevent bone mass loss (1⊕OOO).
- -
The use of oral or IV bisphosphonates improves bone mass in IBD, and their prescription should be adjusted to the general recommendations. Their effect on fracture risk is unknown (1⊕⊕⊕O).
In patients in remission, bone mass increases proportionally to time in remission,200,213,239,242 and treatment of IBD with azathioprine or anti-TNF-alpha improves bone mass.242,244–246 There is also evidence to show that polymeric diet is an alternative to corticosteroids for the control of mild disease.247
Treatment with bisphosphonates (alendronate, risedronate, and ibandronate IV) has been shown to be effective as compared to placebo for the prevention and treatment of osteoporosis in patients with IBD with and without glucocorticoid treatment.248–250 This effect has not been shown with pamidronate IV.251 Although no data supporting a decrease in the risk of fracture in patients with IBD treated with bisphosphonates are available, it is assumed that the general therapeutic indications and recommendations for osteoporosis are applicable.239,252
Anorexia nervosaEvaluationRecommendations- -
Anorexia nervosa decreases bone mass and increases fracture risk (2⊕⊕OO).
- -
In these patients, we recommend the evaluation of bone mass and the presence of fractures (1⊕⊕OO).
- -
The diagnosis of osteoporosis in children and adolescents should not be based on densitometric criteria alone (Z-score of −2.0 or less), but also requires a history of clinically significant fractures, including long bone fractures in the lower limbs, compression vertebral fractures, or two or more long bone fractures in the upper limbs (2⊕OOO).
Bone mass loss exists in anorexia nervosa (AN), particularly at trabecular level.253–257 Most studies have been conducted in adult women and show that 38–50% of patients already have osteoporosis at the time of diagnosis.253–261
There is also an impaired microarchitecture which, combined with low bone mass, increases fracture risk. Thus, cumulative incidence of any fracture was 57% in women with AN older than 40 years, as compared to 42% in an age- and sex-matched population.259 Other authors state that more than 50% of women with a prior history of AN will experience a fracture at 40 years of age and that these women have triple the risk of fracture as compared to those with no history of AN. Fracture location is similar to postmenopausal osteoporosis (spine, distal radius, and proximal femur).260
The pathophysiology of bone changes is attributed to various factors: amenorrhea, deficient calcium absorption, extreme physical exercise, 1.25 (OH) vitamin D deficiency, low creatinine clearance, excess serum and urinary cortisol levels, and high GH levels.257,259
The main predictors of bone mass loss in this group usually include low weight, disease duration, duration of amenorrhea, and inadequate calcium consumption in adolescence.257,259 Moreover, the occurrence of anorexia nervosa during adolescence is associated with a lower bone mass peak.262–264
TreatmentRecommendations- -
We recommend the normalization of weight and menstrual cycles to increase bone mass (1⊕⊕OO).
- -
We suggest the provision of a daily calcium intake of 1300 to 1500mg/day and vitamin D 400U/day or more in patients with vitamin D levels less than 30ng/dL (2⊕⊕OO).
- -
We suggest that treatment with bisphosphonates (alendronate and risedronate) should not be generally used to increase bone mass in patients with AN (2⊕OOO).
- -
We suggest individualized assessment of bisphosphonate treatment in adult patients with very low bone mass and fragility fractures (2⊕OOO).
- -
We suggest that hormone therapy should not be used to prevent bone mass loss in patients with persistent amenorrhea and AN (2⊕OOO).
- -
We suggest that anabolic treatment be started if there are fragility fractures (2⊕OOO).
- -
As anticatabolic treatment we suggest denosumab, which may have advantages in young patients because of its reversible effect (2⊕OOO).
Spontaneous recovery of menses has been shown to be the treatment causing the greatest increase in bone mass, with a 19% increase in BMD.265 In this same study, estrogen therapy did not induce significant changes in bone mass as compared to untreated patients, although patients treated with estrogens who also gained weight experienced a 4% increase in BMD.
Several factors have been proposed to explain the lack of effect of hormone treatment on bone mass in adolescents with AN: that the estrogen dose effective for treatment in menopausal women is inadequate in the young population; treatment noncompliance; and that estrogen treatment is not sufficient to correct the multiple factors involved in bone mass loss.260,261,265,266
Few data are available on bisphosphonate treatment in AN. Issues related to long-term safety in adolescence and the potential teratogenic effects of bisphosphonates should also be taken into account. Treatment with these drugs should therefore be individualized. In this regard, a randomized, double-blind study compared alendronate (10mg/day) to placebo in 32 adolescents with AN and osteopenia for 1 year and concluded that although weight recovery is the most important determinant of BMD, treatment with alendronate slightly increases bone mass in the lumbar spine and femoral neck.267 In the second study, risedronate (5mg/day) induced a slight increase in BMD in the spine. No data are available on fractures.268
Home parenteral nutritionEvaluationRecommendations- -
We suggest that BMD is assessed when a patient is included in a home parenteral nutrition (HPN) program if so warranted by vital prognosis of the patient (2⊕⊕OO).
- -
We suggest differential diagnosis between osteopenia/osteoporosis and osteomalacia in patients with low BMD, especially when planning therapy (2⊕⊕OO).
- -
We suggest regular evaluation (every 1–2 years) of BMD in patients on HPN if warranted by patient prognosis (2⊕OOO).
- -
We suggest evaluation of 25 OH vitamin D levels in patients included in a HPN program (2⊕⊕OO).
Various cross-sectional and cohort studies have found a high prevalence of bone involvement (30–60%) at the start of HPN.269–276 Involvement included osteomalacia, osteopenia, and osteoporosis. Few comparisons with healthy controls are available, and only the Tjllesen et al. study suggests that BMD could be lower in patients on HPN than in age- and sex-matched subjects.277 Cohort studies report heterogeneous results in terms of BMD evolution in patients given HPN.269,274,278 In patients with malnutrition or intestinal failure occurring before peak bone mass is reached, the administration of HPN may increase BMD, probably because of an improved nutritional status.275,279 Although patients receiving HPN have high fracture prevalence (10–40%), there is no evidence to suggest that patients on HPN have an increased risk of fracture as compared to sex- and age-matched subjects.
TreatmentRecommendations- -
We suggest that drug treatment be considered in patients on HPN with bone involvement if warranted by vital prognosis (2⊕OOO).
- -
We suggest that adequate provision of oral or parenteral vitamin D be ensured, because of the high prevalence of vitamin D deficiency and the usual coexistence of malabsorption in these patients (2⊕OOO).
- -
In patients on HPN with osteoporosis, we suggest the consideration of intravenous bisphosphonates (2⊕⊕OO) or denosumab (2⊕OOO) as a therapeutic option.
- -
In patients with a long life expectancy, we suggest treatment with anabolic drugs in the event of fragility fractures or poor response to anticatabolic treatment (2⊕OOO).
The different studies276,280 show a high prevalence of vitamin D deficiency (60–100%) according to the criteria most commonly used today (<30ng/mL). As regards drug treatment, a single randomized, controlled, double-blind study281 has assessed the efficacy of treatment with bisphosphonates in patients on HPN. In this study, the administration of clodronate (1500mg/3 months IV for 1 year) reduced bone resorption markers in patients on HPN with a T-score less than −1; BMD increases were found in the hip, spine, and radius in women, and in the radius only in men. There was no difference in fracture incidence. An additional uncontrolled study reported an increase in T-score with intravenous pamidronate in patients previously receiving glucocorticoids.282
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Reyes García R, et al. Guías de práctica clínica para la evaluación y tratamiento de la osteoporosis asociada a enfermedades endocrinas y nutricionales. Endocrinol Nutr. 2012;59:174–96.