We have described a novel Mahvash disease of hyperglucagonemia and pancreatic neuroendocrine tumors (PNETs) associated with an inactivating glucagon receptor mutation, and identified the glucagon receptor-deficient (Gcgr−/−) mice as its murine model. We aim to elucidate the natural history of the rare Mahvash disease by long-term observation of the Gcgr−/− mice.
Materials and methodWild type (WT) (n=52), heterozygous (n=127), and Gcgr−/− (n=56) mice living under standard vivarium conditions were observed without specific treatments over 22months. Autopsy was performed on dead animals.
ResultsThe WT and heterozygous mice did not exhibit any measurable differences. The Gcgr−/− mice became progressively lethargic and cachexic after 12months. Random glucose levels were stable in WT and heterozygous mice but decreased with age in the Gcgr−/− mice. At the end of observation, 28/56 Gcgr−/−, 7/52 WT, and 24/127 heterozygous mice died. The survival curve of Gcgr−/− mice began to separate from those of WT and heterozygous mice at 12months and the survival difference widened with age. At 18months, survival probability was 17% for Gcgr−/− mice but 77% for WT and 81% for heterozygous mice. Autopsy revealed numerous PNETs up to 15mm in diameter in most well-preserved Gcgr−/− pancreata (17/20) but none in WT or heterozygous ones. Four Gcgr−/− mice developed liver or subcutaneous metastasis.
ConclusionThe untreated Mahvash disease may cause cachexia, severe hypoglycemia, and early death. Patients with Mahvash disease need to undergo life-long surveillance for PNETs. Functional glucagon receptor is thus required for long-term survival.
Hemos descrito la nueva enfermedad de Mahvash en la que existen hiperglucagonemia y tumores neuroendocrinos pancreáticos (TNEP) asociados con una mutación inactivante del receptor de glucagón, e identificado a los ratones con déficit del receptor de glucagón (Gcgr−/−) como su modelo murino. Nuestro objetivo era desentrañar la historia natural de la rara enfermedad de Mahvash mediante observación a largo plazo de ratones Gcgr−/−.
Materiales y métodosSe observó durante 22 meses a ratones silvestres (WT) (n=52), heterocigotos (n=127) y Gcgr−/− (n=56) mantenidos en las condiciones habituales de un animalario sin recibir tratamientos específicos. Se practicaron autopsias a los animales muertos.
ResultadosNo se observaron diferencias apreciables entre los ratones WT y los heterocigotos. Los ratones Gcgr−/− mostraron letargia y caquexia progresiva al cabo de 12 meses. Los niveles de glucosa aleatorios eran estables en los ratones WT y heterocigotos, pero descendían con la edad en los ratones Gcgr−/−. Al final de la observación habían fallecido 28/56 ratones Gcgr−/−, 7/52 ratones WT y 24/127 ratones heterocigotos. La curva de supervivencia de los ratones Gcgr−/− empezaba a separarse de las de los WT y heterocigotos a los 12 meses, y la diferencia en la supervivencia se ampliaba con la edad. La probabilidad de supervivencia de los ratones Gcgr−/− a los 18 meses era del 175, en comparación con el 77% y el 81% en los ratones WT y heterocigotos, respectivamente. La autopsia reveló numerosos TNEP de hasta 15mm de diámetro en los páncreas mayoritariamente bien conservados de los ratones Gcgr−/− (17/20), pero ninguno en los ratones WT o heterocigotos. Cuatro ratones Gcgr−/− desarrollaron metástasis hepáticas o subcutáneas.
ConclusiónLa enfermedad de Mahvash no tratada puede causar caquexia, hipoglucemia intensa y muerte temprana. Los pacientes con enfermedad de Mahvash tienen que someterse a vigilancia de por vida en busca de TNEP. En consecuencia, es necesario un receptor de glucagón funcional para la supervivencia a largo plazo.
We have described a novel human disease (Mahvash disease) based on a patient who had extreme hyperglucagonemia but without glucagonoma syndrome, pancreatic α cell hyperplasia and nesidioblastosis, and pancreatic neuroendocrine tumors (PNETs), associated with an inactivating glucagon receptor mutation (P86S).1–3 The P86S mutant glucagon receptor exhibits abnormal subcellular localization and decreased glucagon binding; it also compromises glucagon-stimulated cAMP production and calcium signaling.2,4 After our initial report,1 similar pancreatic histology is also reported in a few other cases, but whether they are associated with glucagon receptor mutations is not clear.5,6 The molecular mechanisms for those patients with unknown genetic causes have not been studied. All patients with the unique pancreatic histology do not have clinical or genetic evidence of multiple endocrine neosplasia syndrome type 1 (MEN1) or von Hippel–Lindau disease (VHL) which can involve pancreatic neuroendocrine microadenomatosis.7,8 We further demonstrated that the glucagon receptor knockout (Gcgr−/−) mice are a faithful model of the human Mahvash disease.9,10 In the Gcgr−/− mice, hyperglucagonemia is severe but skin rash or diabetes is not present; islet hyperplasia and dysplasia become evident at 5–7months, and PNETs developed with 100% penetrance at 10–12months.
The natural history of the Mahvash disease is largely unknown. As the Mahvash disease is very rare, currently it is not possible to study the Mahvash disease in a large patient population. To study the natural history of the Mahvash disease, we have observed the Gcgr−/− mice over 22months. We demonstrate that the Gcgr−/− mice exhibit multiple abnormalities and die much earlier than the WT or heterozygous controls. PNETs continuously emerge and relentlessly grow in the Gcgr−/− mice. The natural history of the Mahvash disease shown in this murine model indicates that functional glucagon receptor is required for long-term survival.
Materials and methodsAnimalsThe Gcgr−/− mice generated and provided by Pfizer Global Research and Development were described previously.9 The colony propagated at Cedars-Sinai Medical Center was purebred DBA1/lacJ strain.10 Heterozygous parents were bred to produce all animals studied, and wild type (WT) and heterozygous littermates used as controls for the Gcgr−/− mice. Animals were raised in a 12-h dark/light cycle and fed with standard chow ad libitum. As there was no sexual dimorphism in the described abnormalities, both male and female mice were used, and data on them analyzed together. All animals were rounded daily by vivarium staff and carefully examined weekly by the investigators for health and signs of diseases after 12months. Periodically, animals were weighed and random glucose levels (defined as the glucose levels in tail-cut blood of free-feeding mice during the day time) measured by a glucometer. Minor lesions were monitored or treated symptomatically. Dead animals were stored at 4°C and autopsied as early as possible. Missing animals (2 WT, 6 heterozygous, and 4 Gcgr−/−) were presumed dead and the date on which they were found missing was used as date of death; the death was confirmed orally by vivarium staff in a few cases but the cadavers were disposed and not available for autopsy. When the vivarium staff and the investigators judged a mouse moribund or significantly suffering from non-lethal lesions, the animal was euthanized by CO2 asphyxiation after being weighed and random glucose levels measured. During autopsy, the visceral organs in the chest, abdomen, and pelvis were examined in detail and all organs with gross abnormalities were harvested and fixed in 10% neutral formalin. Four 18-month healthy-appearing WT and heterozygous mice were also euthanized to screen for pancreatic neuroendocrine tumors. Experiments were approved by the Cedars-Sinai Institutional Animal Care and Use Committee.
Histology, immunostaining, and microscopyTen 5-μm paraffin sections per pancreas, 100μm apart on the cross sections, were stained with hematoxylin and eosin (H&E) and histology examined. Dysplastic islets were identified by the blood islands and abundant stromal tissue and pancreatic neuroendocrine tumors (PNETs) by the trabecular cell growth pattern. For immunofluorescent staining, 5-μm pancreatic sections were deparaffinized, rehydrated, and incubated with rabbit anti-glucagon and guinea pig anti-insulin antibodies (Dako, Carpinteria, CA, USA), followed by FITC-labeled anti-rabbit and rhodamine-labeled anti-guinea pig secondary antibodies. All sections were counter-stained with Hoechst 33342. Stained pancreatic sections were examined with an Olympus IX2-SP microscope with both bright field capacity and fluorescence filters. Digital photographs were acquired with a MegnaFire camera (Olympus, Center Valley, PA, USA).
Data analysisThe changes of body weight and blood glucose levels over time were estimated by linear regression and the significance of the difference of the slopes was determined by a slope-by-group interaction term. Probability of survival was estimated by the Kaplan–Meier method and statistical significance calculated by the log-rank analysis. Student's t-test was used to compare the means of continuous parameters between 2 groups. SD was used to describe the variability of continuous data. Fisher's exact test was used to compare the frequencies of a parameter between 2 groups.
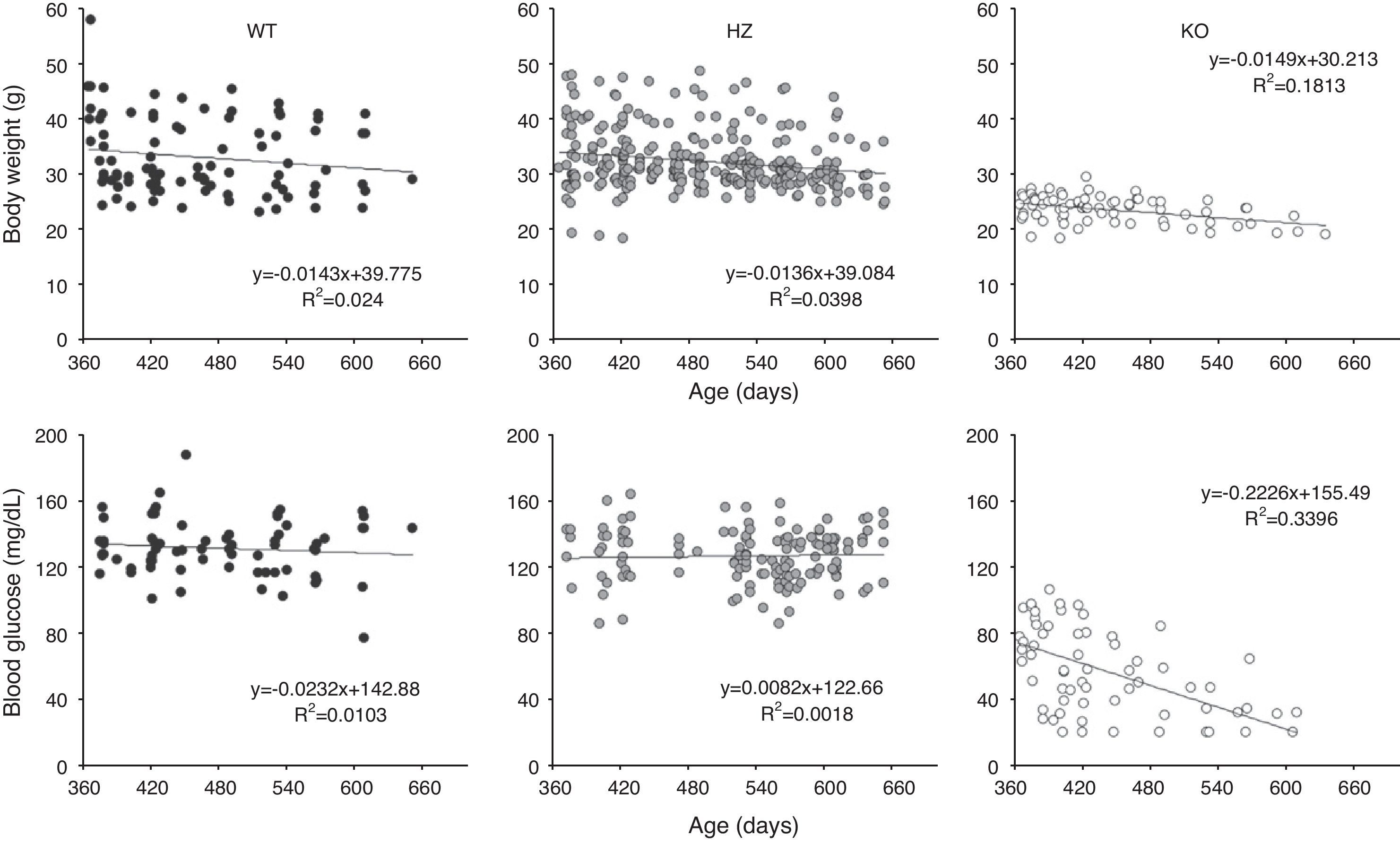
ResultsGrowth and glycemia of old Gcgr−/− miceFifty-two WT, 127 heterozygous, and 56 Gcgr−/− mice were observed for a maximum of 22months (Table 1). Some animals were observed for shorter than 22months. We have previously described that the Gcgr−/− mice fail to gain weight after 3months but the WT and heterozygous mice continue to grow until 12months.10 After 12months, all 3 groups of mice gradually lost weight at about the same rate. The Gcgr−/− mice remained lighter throughout life; at 18months and later, they were 33% lighter than the WT and heterozygous mice (body weight 21.9±2.1g for Gcgr−/−, 32.7±6.8g for WT, and 31.1±4.3g for heterozygous mice, p<0.001) (Fig. 1). Random blood glucose levels were stable in the WT or heterozygous group after 12months but decreased over time in the Gcgr−/− mice (0.22mg/dL/day, p<0.0001) (Fig. 1). The random glucose levels were very low in the Gcgr−/− mice after 18months (33.6±14.3mg/dL vs. 127.2±20.5mg/dL in the WT and 123.8±15.2mg/dL in the heterozygous mice, p<0.0001). The lowest random blood glucose levels in the WT or heterozygous mice were 77mg/dL at any time. Interestingly, although most Gcgr−/− mice with blood glucose levels <20mg/dL died within days, a few mice remained alive for up to 3months.
Fate of all animals at the time of writing.
| WT | Heterozygous | Gcgr−/− | |
| Numbers observed, n (F/M) | 52 (24/28) | 127 (67/60) | 56 (28/28) |
| Euthanized for experimentsa, n (F/M) | 19 (9/10) | 36 (20/16) | 15 (8/7) |
| Euthanized for non-lethal lesions or birth traumas, n (F/M) | 6 (0/6) | 7 (3/4) | 1 (1/0) |
| Euthanized for non-lethal lesions or birth traumas, mean age (SD), months | 8.8 (2.1) | 10.7 (3.8) | 7.4 |
| Died naturally or euthanized for moribund appearanceb, n (F/M) | 7 (4/3) | 24 (8/16) | 28 (14/14) |
| Died naturally or euthanized for moribund appearance, mean age (SD), months | 13.8 (4.0) | 14.3 (5.5) | 14.0 (3.4) |
| Probable causes of death, n | 3 (1 diabetes, 1 pelvic tumor, 1 abnormal kidney) | 6 (2 pelvic tumor, 1 adrenal tumor, 1 abnormal liver, 1 anasarca, 1 ascites) | 17 (all PNET). 13 mice with PNET also had hypoglycemia (<60mg/dL) |
| Causes of death unclear, n | 4 | 18 | 11 |
| Alive, n (F/M) | 20 (11/9) | 60 (36/24) | 12 (5/7) |
| Alive, mean age (SD), months | 16.9 (3.8) | 17.4 (4.1) | 12.6 (2.7)c |
WT, wild type; F, female; M, male; n, number.
Growth and glycemia of older Gcgr−/− mice. Body weight and blood glucose levels were measured periodically in 52 wild type (WT), 127 heterozygous (HZ), and 56 Gcgr−/− mice (KO) over 22months. (Upper panels) Body weight from 12 to 22months. (Lower panels) Random blood glucose levels from 12 to 22months. The straight lines stand for the results of linear regression of body weight or blood glucose levels versus time. The linear regression equation and R squared value are shown for each graph.
After 12months, the Gcgr−/− mice became progressively lethargic and cachexic and some died, while the WT and heterozygous mice mostly remained active and maintained body weight. During the 22-month period, 7 of 52 WT, 24 of 127 heterozygous, and 28 of 56 Gcgr−/− mice died or were euthanized due to moribund appearance during the observation period (Table 1). Kaplan–Meier analysis showed that while WT and heterozygous mice did not exhibit difference in survival, the Gcgr−/− mice died significantly earlier (Fig. 2). The survival curve of the Gcgr−/− mice began to separate from those of WT and heterozygous mice at 12months (survival probability 79%, 96%, and 92%, respectively) and the survival difference became progressively larger with age. At 18months, the survival probability was 17% for the Gcgr−/− mice but 77% for the WT and 81% for heterozygous mice. Thus, most deaths in the Gcgr−/− mice occurred between 12 and 18months. No Gcgr−/− mice survived beyond 22months; in contrast, the WT and heterozygous mice had >60% surviving probability beyond 22months. As expected, the mean age of surviving Gcgr−/− mice was ∼4.5months younger than that of surviving WT or heterozygous animals (Table 1). Univariate analysis of the relationship between hypoglycemia and Gcgr−/− mice mortality showed that the mortality was much higher in mice with random glucose levels <60mg/dL than in those with ≥60mg/dL (83% vs. 47%, p<0.05).
Kaplan–Meier survival curve of wild type (WT) (green), heterozygous (HZ) (red), and Gcgr−/− (KO) (blue) mice. The overall log-rank p<0.0001 when all 3 groups were calculated simultaneously; p=0.804 comparing WT and HZ survival; p<0.0001 comparing WT and KO survival or comparing HZ and KO survival separately.
The cadavers were available for autopsy in 5 WT, 16 heterozygous, and 24 Gcgr−/− mice. The cadavers were severely autolysed and no discerning gross features could be examined in 1 WT, 5 heterozygous, and 4 Gcgr−/− mice. Overall, 4 WT, 11 heterozygous, and 20 Gcgr−/− mice gave satisfactory autopsy results (Table 2). The cause of death was apparent on anatomical grounds in 2 WT, 6 heterozygous, and 17 Gcgr−/− mice; in the remainder of animals, no gross abnormalities or only small pancreatic tumors (in Gcgr−/− mice) were seen (Tables 1 and 2). One WT mouse had severe diabetes for unclear reason which was likely the cause of death. There was no evidence of diabetes in the remaining 51 WT mice. In all cases, gross pancreatic lesions were not found in any WT or heterozygous mice at autopsy but most (17/20, 85%) discernable Gcgr−/− pancreata harbored numerous pancreatic tumors of various sizes, some of which were so large (e.g. 15mm) that the normal pancreas structure was severely distorted (Fig. 3). The total tumor mass comprised ∼10–20% of body weight in some cadavers. Gross liver metastasis was found in 3 Gcgr−/− mice with larger pancreatic neuroendocrine tumors (PNETs) (Table 2, Fig. 3); a bulky subcutaneous metastasis was seen in another. All other visceral organs of the Gcgr−/− mice appeared normal at autopsy. Thirteen of the 17 mice with gross PNETs also had hypoglycemia (<60mg/dL) (Table 1), which was associated with higher mortality (see above).
Summary of autopsy findings of 20 Gcgr−/− mice that died naturally or were euthanized due to moribund appearance.
| ID | Age at death (months) | Sex | Autopsy findings | 3 Largest PTs (mm) | Metastasis |
| 33 | 10.3 | M | Multiple PTs | 5/2/2 | No |
| 79a | 10.4 | M | No gross abnormality | NA | No |
| 27a | 10.5 | M | No gross abnormality | NA | No |
| 44 | 11.3 | M | 1 PT | 1/NA/NA | No |
| 39 | 11.5 | M | Multiple PTs | 7/3/2 | Liver (miliary) |
| 93 | 12.2 | F | 1 PT | 4.1/NA/NA | No |
| 168 | 13.2 | M | Multiple PTs | 5/3/3 | No |
| 189 | 14.5 | F | Numerous PTs | 4/3/3 | No |
| 97 | 14.7 | F | 1 PT | 5/NA/NA | No |
| 89 | 14.7 | F | Multiple PTs | 5/3/2 | Chest wall (10-mm) |
| 155 | 15.1 | M | Multiple PTs | 3/1/1 | No |
| 100 | 15.2 | F | Multiple PTs | 12/5/3 | No |
| 184 | 15.5 | M | Multiple PTs | 15/3/3 | No |
| 188 | 15.7 | F | Multiple PTs | 15/3/2 | Liver (numerous, largest one 5-mm) |
| 67 | 16.4 | M | Multiple PTs | 10/3/3 | No |
| 147 | 17.1 | M | Numerous PTs | 6/4/2 | No |
| 113 | 17.9 | M | Numerous PTs | 7/5/4 | No |
| 118 | 19.5 | M | Multiple PTs | 5/4/3 | No |
| 111 | 20.5 | F | Multiple PTs | 15/10/7 | Liver (numerous, largest one 6-mm) |
| 91 | 21.6 | F | Numerous PTs | 6/5/4 | No |
Mice 33, 27, 44, and 39 were partly reported in Ref. 10. PT, pancreatic tumors; F, female; M, male; NA, not applicable.
Autopsy findings of Gcgr−/− mice that died naturally or were euthanized due to moribund appearance. (A) Normal wild type (WT) and heterozygous (HZ) pancreata at 18months are shown for comparison. (B) Gcgr−/− (KO) pancreata at 17months (left) and 18months (right). Note the multiple pancreatic neuroendocrine tumors (PNETs) of various sizes dotting the pancreata. (C–E) Multiple bulky pancreatic tumors from 3 Gcgr−/− mice. (F) A large, metastatic subcutaneous PNET with central degeneration. (G) Numerous metastatic PNETs in the liver. Large arrows, large PNETs; and small arrows, small PNETs. Ruler: in cm.
To examine whether older WT and heterozygous mice harbor abnormal islets or grossly inapparent PNETs, 4 WT and 4 heterozygous healthy-appearing 18-month-old mice (2 females and 2 males in each group) were euthanized and their pancreata compared with those of 4 Gcgr−/− mice at 15-month (2), 17-month (1), and 18-month (1). Compared with their Gcgr−/− counterparts (815±309mg), the WT (314±24mg) and heterozygous (297±57mg) pancreata were much smaller (∼2.6-fold, p<0.01). Multiple sections of the 18-month-old WT and heterozygous pancreata showed normal endocrine and exocrine components without any evidence of islet dysplasia or micro-PNETs (Fig. 4). In comparison, similar sections of the Gcgr−/− pancreata showed numerous dysplastic islets, microadenomas, or PNETs (Fig. 4), which suggests that new PNETs continuously develop even in older animals. Immunostaining of 17 PNETs of various sizes from 8 randomly picked older Gcgr−/− mice showed that most PNETs were glucagonomas (14/17) and 2/17 were negative for either glucagon or insulin. No pure insulinomas were found but 1 small PNET was a mixed glucagonoma/insulinoma (expressing both glucagon and insulin in the same tumor cells).
Histology of pancreas and pancreatic tumors. (A–C) Islet histology of wild type (WT) and heterozygous (HZ) mice at 18months, and Gcgr−/− mice (KO) at 15months. Sections were stained with hematoxylin and eosin. (D–F) Histology of each of the gross lesions shown in Fig. 3D–F, respectively. Note the typical trabecular pattern of neuroendocrine tumor cells. Bar, 100μm.
The role of animal models in the study of human diseases, especially rare diseases, is increasingly recognized. The Gcgr−/− mice were originally created to examine the effect of glucagon signaling on glycemia regulation.9 There was no literature on similar human diseases available to us when we first reported the syndrome of the Mahvash disease.1 The striking similarities between the phenotypes of the Mahvash disease and the Gcgr−/− mice prompted us to sequence the glucagon receptor gene in a patient with the Mahvash disease which led to the discovery of a homozygous inactivating P86S mutation in the glucagon receptor.2 Further investigation of the Gcgr−/− mice confirms that they develop pancreatic neuroendocrine tumors (PNETs) with 100% penetrance at middle age.10 In the current work, we have performed a natural history study in the Gcgr−/− mice, a model of the Mahvash disease, to understand the natural course of untreated Mahvash disease.
Our results show that the Gcgr−/− mice are less healthy and die prematurely. As all animals were littermates, lived in standard vivarium conditions, were fed with standard chows, and did not receive any specific treatments, their health and survival likely reflect the natural effects of deficient or inactive glucagon receptor, the cause of the Mahvash disease. The cachexia is probably a consequence of both decreased oral intake, as previously reported,11 and pancreatic PNET tumor burden which may compromise nutrient absorption by disrupting the normal pancreas structure and may consume large amounts of nutrients themselves. Hypoglycemia is another prominent abnormality of the Gcgr−/− mice which is associated with higher mortality. The mechanisms for the progressive hypoglycemia in older animals are not clear. The random glucose levels of young Gcgr−/− mice are somewhat normal but lower than those in WT or heterozygous mice,10 consistent with the loss of hyperglycemic function of glucagon. It appears that some aspects of aging worsen hypoglycemia in the Gcgr−/− mice. The remarkable hypoglycemia is unlikely caused by insulinoma, as hypoglycemia was present in all old animals but only 1 had a mixed glucagonoma/insulinoma. In patients with the Mahvash disease, it might be advisable to maintain normal body weight and monitor blood glucose levels to avoid cachexia and hypoglycemia.
The most important abnormality exhibited by the Gcgr−/− mice is the much reduced survival manifesting after 12months. The reduced survival must be caused by glucagon receptor deficiency as the absence or presence of glucagon receptor was the only genetic difference between the Gcgr−/− mice and the WT and heterozygous littermates, and all the mice lived in the same environment. What are the underlying mechanisms for the reduced survival? Besides cachexia and hypoglycemia, the Gcgr−/− mice are known to exhibit other gross abnormalities such as placental insufficiency, increased in utero mortality, decreased fetal weight, and poor vision, some or all of which could contribute to the reduced survival.12–14 In addition, the Gcgr−/− mice also have several metabolic abnormalities such as maladaptive metabolic adjustment to fasting, increased circulatory levels of amino acids, cholesterol, and bile acid, and high risks of fatty liver.15–17 Those abnormalities need to be confirmed in patients with the Mahvash disease before their clinical significance is assessed.
We show that PNETs continuously develop and relentlessly grow as the Gcgr−/− mice age. The mechanisms for PNET pathogenesis are not clear but may involve abnormal subcellular localization of menin.10 As the Gcgr−/− mice do not have glucagon receptor, hyperglucagonemia and hyperactivity of glucagon signaling unlikely contribute to PNET pathogenesis. Several lines of evidence suggest that PNETs contribute to the early death of Gcgr−/− mice. First, gross PNETs were found at autopsy in most Gcgr−/− mice but not in any WT or heterozygous ones. Second, the PNET tumor burden was significant as evidenced by the large numbers and sizes of PNETs in the pancreas found at autopsy of most Gcgr−/− mice. Third, the extensive liver metastasis in some Gcgr−/− mice likely cause abnormal liver function and contribute to the mortality in those animals. Fourth, untreated human sporadic PNETs are lethal.18,19 Last, no other gross visceral abnormalities were found in the dead or moribund Gcgr−/− mice that can explain their death. As it is not practical to do detailed histological studies of all visceral organs and we did not examine the central nervous system, we cannot rule out that potential lesions in those organs or systems also contribute to the early death of Gcgr−/− mice. In addition, hypoglycemia was present in most mice with gross PNETs and associated with higher mortality; the specific contribution of PNETs in the death of Gcgr−/− mice is thus unclear and requires further study. Interestingly, transdifferentiation from glucagonoma to insulinoma does not develop in old Gcgr−/− mice, in contrast to that in the mice with α cell specific deletion of menin, which also exhibit α cell hyperplasia and glucagonomas but the glucagonomas transdifferentiate into insulinomas as the animals grow older.20 The continuous emergence and growth of PNETs in the Gcgr−/− mice indicate that patients with the Mahvash disease need to undergo imaging surveillance for early detection of clinically significant PNETs. All the findings from the Gcgr−/− mice need to be tested in patients with the Mahvash disease. Currently, detailed natural history of the Mahvash disease cannot be satisfactorily studied in humans due to its recent discovery and slow clinical progression.
Finally, our data suggest that glucagon antagonism as an experimental means of diabetes treatment needs long-term study to confirm its safety. Glucagon antagonism is a reasonable approach to treat diabetes but has been associated with reversible hyperglucagonemia and pancreatic α cell hyperplasia, a phenotype resembling that in young Gcgr−/− mice, raising concerns for the risk of PNETs after long-term use.21,22 It should be emphasized that glucagon receptor antagonism starting at adulthood is different from glucagon receptor deficiency since conception and pharmacological inhibition of glucagon signaling may prove to be safe after long-term studies.
In summary, the Gcgr−/− mice help delineate the natural history of the Mahvash disease and suggest that untreated Mahvash disease entails cachexia, hypoglycemia, large PNET tumor burden, and early death. These findings may guide clinical management of patients with the Mahvash disease. Our study also demonstrates that functional glucagon receptor is indispensable for health and life.
FundingThis study was supported by NIH grant DK071870 (R.Y.) and Cedars-Sinai Medical Center.
Conflicts of interestThe authors have no conflicts of interest to declare.
The authors thank Pfizer Global Research and Development for providing the Gcgr−/− mice.