Although papillary or follicular well-differentiated thyroid carcinoma usually has a good prognosis, a proportion of well-differentiated thyroid carcinomas show a more aggressive behavior with local recurrence and metastases, either at diagnosis (in less than 5% of cases) or over time. Although there are several scoring systems to assess prognosis of well-differentiated thyroid carcinoma, mainly based on clinical and pathological data, there is currently no valid criterion to define an adequate, differential treatment for patients with low risk carcinomas as compared to those with more aggressive tumors. Identification of patients with a high risk at the time of diagnosis would be essential to develop new therapeutic strategies and to improve follow-up, and molecular biomarkers could be a highly useful tool for this purpose.
Aunque el carcinoma diferenciado de tiroides, papilar o folicular, tiene habitualmente un buen pronóstico, existe un porcentaje de casos que presentan un comportamiento más agresivo con recurrencias locales y metastatización, ya sea en el momento del diagnostico (en menos de un 5% de los casos) ya en el seguimiento. A pesar de que existen diferentes sistemas de evaluación del pronóstico del carcinoma diferenciado de tiroides, basados especialmente en datos clínicos y patológicos, no hay en la actualidad un criterio válido que permita definir un tratamiento diferencial entre los pacientes con carcinomas de bajo riesgo y aquellos con carcinomas más agresivos. La identificación de los pacientes de riesgo en el momento del diagnóstico sería clave para desarrollar nuevas estrategias terapéuticas y mejorar el seguimiento, siendo en este sentido los biomarcadores moleculares una herramienta de gran valor.
Differentiated thyroid carcinoma is the most common endocrine neoplasm, with an increasing incidence in recent years.1,2 More than 90% of these tumors have their origin in follicular cells, and they are classified as well differentiated carcinoma (DTC), including the papillary (PTC) and follicular (FTC) variants, and undifferentiated or anaplastic carcinoma (ATC). The latter is one of the most aggressive tumors, with a mean 5-month survival rate from the time of diagnosis and a one-year survival rate of only 20%.3
Most well differentiated tumors have a favorable clinical course, with survival rates close to 90% 10 years after diagnosis.4–6 There is however a proportion of tumors with more aggressive behavior including local recurrence and metastases either at diagnosis (in less than 5% of cases) or follow-up, and it has been estimated that approximately 10–15% of patients with differentiated carcinoma will develop local or distant metastases.7,8 Survival rates ranging from 49% to 68% have been reported 10 years after the occurrence of cervical metastases, which are responsible for one third of deaths related to the disease. Distant metastases occur in lung (50%), bone (25%), lung and bone (20%), and other sites (5%). The survival rate following the diagnosis of distant metastases decreases to 25–42%.9
The treatment of low-risk differentiated thyroid carcinoma has not changed substantially over the past decades and is based on surgery, ablation with 131I, and suppressant treatment with levothyroxine,10 although there is an increasing trend to base both treatment and follow-up recommendations on individualized risk assessment.7,11 Most evaluation systems are based on clinical and pathological data, and different risk factors have been identified, including age at diagnosis, sex, size, the presence of metastasis, and the initial treatment used.6,12,13
The response rate to the standard treatment is high, with overall survival rates higher than 75%. However, patients with dedifferentiation processes (poorly differentiated thyroid carcinoma [PDTC]) who have iodine refractory disease (the presence of at least one lesion with no 131I uptake or progression within one year of 131I ablation) show a mean survival of 3–6 years after the diagnosis of distant metastases, and although growth is slow, most metastases progress. These patients are therefore candidates for other treatment modalities,14 and one of the main objectives of clinicians is to distinguish this subgroup from DTCs of good prognosis.
It is therefore essential to know the process of dedifferentiation or anaplastic transformation of these tumors. Our understanding of the molecular biomarkers related to this process, and thus with prognosis and survival, has increased in recent years, so allowing for new therapeutic targets to be established.
Biomarkers: DefinitionBiomarkers are defined as biological parameters that may be measured or detected and may in turn be correlated to a pathological process. However, for these parameters to be considered as true biomarkers, they must meet the following criteria, described by Herberman in 1977: (1) their measurement should be simple, reproducible, and easily available; (2) they should discriminate between normal and pathological states; (3) they should be highly sensitive and specific; and (4) they should be able to monitor recurrent disease processes.15
In recent years, the development of new technologies applied to the molecular understanding of disease has significantly contributed to the identification of new biomarkers, called molecular biomarkers. These new biomarkers have undoubtedly become highly relevant because they have made possible a deeper understanding of the etiopathogenesis of many diseases by identifying, at least partly, the molecular mechanisms involved, especially in neoplastic conditions, and have provided valuable diagnostic, prognostic, and therapeutic information. However, there are many factors, not only genetic or epigenetic, but also environmental, directly or indirectly involved in the development and metastatic progression of tumors, which often makes selection of these new biomarkers difficult. This is one of the most significant limitations in clinical practice. The different types of biomarkers in thyroid tumor disease are discussed below.
Serological markersIn epithelial thyroid tumors, thyroglobulin (TG), a glycoprotein produced by thyroid follicular cells, was one of the first tissue-specific biomarkers used. However, although this is a helpful serum marker for assessing the presence of residual or metastatic tumor in patients undergoing total thyroidectomy, many studies have reported that it is of no use in detecting populations at high risk of developing this type of tumor.16 In addition, TG tests may not be reliable in patients on suppressing levothyroxine therapies and in patients who develop some types of benign conditions such as thyroiditis, thyrotoxicosis, thyroid adenoma, or iodine deficiency. In these latter cases, false positive results are frequently seen because of increased serum TG levels.17
Genomic and transcriptomic markersIt is well known that the process of carcinogenesis results from the random accumulation of genetic and epigenetic aberrations in tissue. At least 10–20% of gene expression changes have been reported in tumor cells. These changes are directly induced by changes in cell DNA, significantly contributing to the oncogenetic process in virtually all human cancers.18,19 Early studies focused on the identification and subsequent analysis of either one or of several genes implicated in tumor start, development, and metastases. Thus, in the specific case of the thyroid gland and because of the discrepancies found by different authors in serum TG measurements in patients, one of the first choices proposed was the detection of the TG transcript in the blood of these patients instead of protein. However, the results obtained showed a great variability, and no differences in expression could be seen between the control subjects used in the studies and those with disease.17,20–22 The transcript of the thyroid-stimulating hormone (TSH) receptor was another marker proposed. This receptor acts as the main regulator, through TSH, of the differentiation and division process in thyroid follicular cells, but not in pathological follicular cells, which are insensitive or refractory to such stimulation. However, although different gene expression studies showed total or partial modification of gene expression in differentiated thyroid carcinomas, the results were not conclusive because neither TG nor TSH alone allowed for discrimination between the different degrees of malignancy of tumors or their progression to more aggressive tumors.23–25
The most relevant findings in terms of the expression of genomic and transcriptomic markers in tumors of different histological characteristics are discussed below.
Well differentiated tumors: Papillary and follicular variants of differentiated thyroid carcinomaEarly changes reported included mutations in RAS family oncogenes affecting both GTP in codons 12, 13 and the GTPase domain in codon 61 of the protein, with a similar prevalence in benign and malignant tumors. Some authors found these mutations in approximately 50% of FTCs analyzed, which may suggest their implication in associated early changes in the transformation process of thyroid follicular cells.26,27
The RET proto-oncogene located in chromosome 10q112 has been reported in 50% of PTCs diagnosed, and is called RET/PTC. Active forms of the RET proto-oncogene result from oncogenic rearrangements and fusions of the tyrosine kinase domain of the RET gene with the 5′ of the domain of different genes, leading to different variants (RET/PTC1, –2, –3, –4, and –5).28 However, while there are studies identifying RET/PTC as a good genetic marker in PTCs, others have found the mutations in benign nodules.29,30
Tyrosine kinase, which may be inadequately activated in PTCs, has also been studied. However, the molecular mechanism is unknown, and no validation exists in benign tumors.31
Angiogenic factors identified in DTC thyrocytes include VEGF (vascular endothelial growth factor), whose expression has been correlated to the degree of tumor dedifferentiation. It has therefore been proposed as a poor prognostic marker involved in metastatic processes in PTC.32,33 An additional growth factor implicated in tumorogenesis is EGF (epidermal growth factor), which in addition to promoting cell proliferation in the thyroid gland, acts by inhibiting specific thyroid functions such as iodine transport and organification and peroxidase and TG synthesis. That is to say, EGF promotes thyroid cell proliferation, but not differentiation. Finally, TGF-alpha (tumor growth factor-alpha) interacts with the EGF receptor (EGFR) and contributes with EGF to stimulate cell proliferation. However, the expression of both EGF and its receptor (EGFR) in the nucleus is not only detected in FTC, but also in Graves’ disease and adenomas.34
However, it was not until the advent of mass sequencing of human genoma and the subsequent technological advances, such as array and/or microarray techniques, that new molecular targets involved in key cell regulation processes (signaling pathways, transcription factors, changes in adhesion molecules as markers of tumor aggressiveness) and specific molecular profiles (also called genetic signatures) associated with a variety of clinical patterns could be identified. In many cases, advances made in the search for these specific genetic profiles have been crucial for improving our ability to ascertain differences in the biological behavior of closely related conditions which have no differential histological patterns but show major differences in their clinical course. In the thyroid gland, comparative tests performed in follicular adenomas or benign tumors and carcinomas have allowed for the identification of a number of differential genetic changes such as different galectin-3 gene expression, differences in telomerase activity, and detection of PAX8/PPARγ gene translocation, among others. However, none of these markers have proved sufficiently reliable to allow for the safe differentiation of benign and malignant tumors in cytological samples.35,36
These new diagnostic approaches were simultaneously used to study PTC.37–40 Thus, Huang et al. reported in 2001 one of the first studies using array techniques in this type of tumor. The authors were able to identify 220 differentially expressed genes. However, some of these could not subsequently be validated in a new PTC cohort, which is a critical point in the study.41
Subsequent studies by Giordano et al. in 2005 identified a genetic signature associated with mutations in BRAF, RAS, and RET/PTC genes in PTC. Testing for this signature allowed for the differentiation of tumors of epithelial origin which showed different histological patterns (classical papillary pattern and its different variants). This was thus the first study that identified gene expression patterns and their mutational correlation. This allows not only for the prediction of the potential success of the treatment used, but also for contemplating new cellular targets that allow for designing new treatment strategies.42 Thus, Capella et al.43 detected RAS gene mutations in different types of thyroid lesions, including ATC, thus confirming their role in tumorogenesis, although the relationship with prognosis could not be determined. Subsequent detection of hypermethylation of the PTEN promoter, especially in follicular tumors, suggested its potential role during tumor development or course.44
An increasing number of studies aimed at the identification of new clinically useful markers and genetic signatures were subsequently conducted on larger series. Recent studies including significant numbers of patients (n=1168) confirmed that BRAFv600E gene mutations were relevant in PTC and were associated with greater increases in recurrence, mortality, and loss of the capacity to take up 131I. They could therefore be used as a biomarker for these tumors. However, they had little prognostic power in sporadic tumors that lacked the mutation. Thus, their value as a diagnostic and prognostic test was not conclusive.45,46 A recent study examined the relationship of the presence of the BRAFT1799A mutation to PTC recurrence or persistence, and was unable to conclude that its presence confers a poorer prognosis.47
A meta-analysis by Griffith et al. of 21 published studies using the same Affimetrix technology found that among the 1785 differentially expressed genes, 39 were the most significant, including 12 candidate genes, of which six had previously been reported (MET, TFF, SERPINA1, TIMP1, FN1, and TPO) and six were candidate genes (TGFA, QPCT, CRABP1, FCGBP, EPS8, and PROS1) identified for the first time in the thyroid gland, which could represent, together with the previous ones, a valid diagnostic tool in a clinical setting.48
Dedifferentiated and anaplastic tumors (undifferentiated or anaplastic carcinoma and poorly differentiated carcinoma)Undifferentiated or anaplastic carcinomaFew gene expression studies have been conducted in more aggressive tumors, the etiology of which continues to be highly controversial. No key specific agent for the development of such tumors has been identified yet (a role for TSH and/or external irradiation in the promotion of the development of these tumors has been suggested).49
A correlation exists between the frequency of a loss of heterozygosity and a prognosis of thyroid carcinoma. A loss of heterozygosity occurs more frequently in ATC as compared to PTC and FTC, where it is found in 19p in up to 36% of cases.26 Localized loss in the short arm of chromosome 16 has also frequently been noted. This would suggest the probable lack of a suppressor gene at that location. This observation is supported by the fact that ATC cell lines have been seen to frequently have losses in 16p as compared to cell lines of differentiated carcinoma, which suggests the presence at this location of some gene that may be associated with the transformation from well differentiated to undifferentiated carcinoma.50
On the other hand, microarray studies in these tumors have identified groups of genes related to different signaling pathways, including transcription factors, mitosis, cell proliferation and differentiation, apoptosis, cell adhesion molecules, cytoskeleton, etc.,45,46,51–53 but have found no consistent genetic signature. As in differentiated tumors, mutations have been identified in RAS and BRAF genes, and this has led to the hypothesis that they could be the first steps in the dedifferentiation process and that they could allow in turn for the acquisition of new mutations (late mutations), including those identified in TP53, catenin (cadherin-associated protein), beta 1, and PIK3CA. It has been suggested that all these cumulative mutations in these types of tumors contribute to their extremely aggressive behavior. By contrast, rearrangements in the RET/PTC gene identified in childhood and in post-radiation PTC or fusion protein PAX8/PPARγ detected in FTC are not identified in poorly differentiated or anaplastic tumors.54 From this postmalignant transformation associated with genetic mutations arises the interesting but controversial concept of “anaplastic transformation”.55
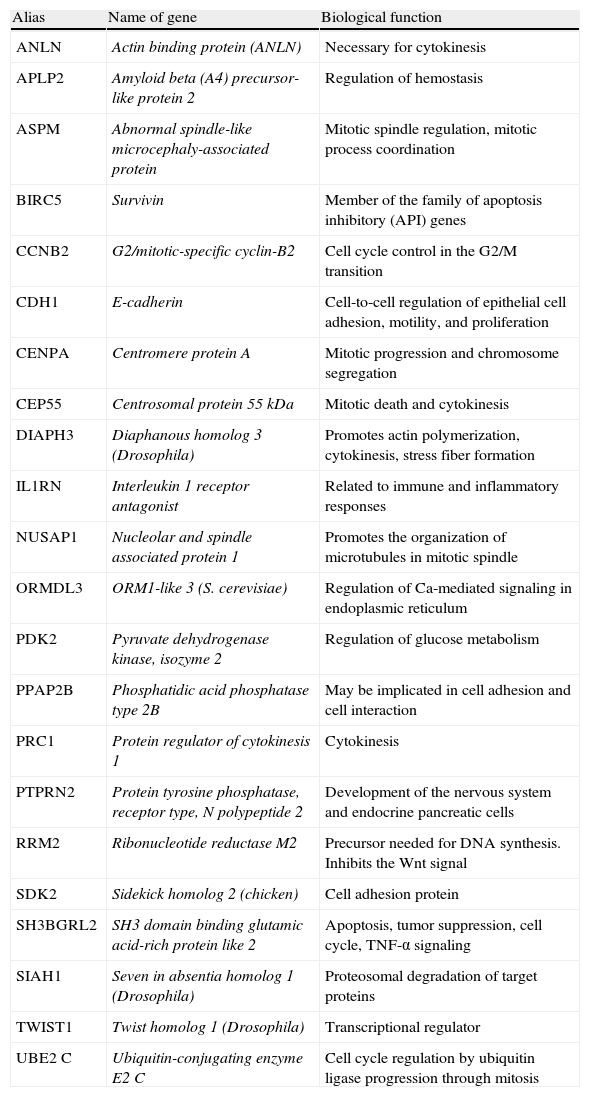
Poorly differentiated carcinomaPDTCs, recognized in 2004 by the World Health Organization as a separate pathological condition, have a differential molecular expression as compared to all other tumors described. Thus, our group56 studied in 2008 these types of tumors (n=6) and compared their differential expression from ATCs (n=6) and a group of well differentiated tumors (n=31). This study identified 1031 differential genes implicated in different cell-signaling pathways: MAPKinases, genes involved in cell cycle regulation, cell adhesion, cytoskeleton, and TGFβ pathways. Of all these genes, 23 represented a genetic signature conferring a poor prognosis (Table 1) which is currently being validated in a new patient cohort. To date, 22 of the 23 genes identified have been analyzed using low density array technology in a total of 19 well differentiated tumors at the time of diagnosis, of which 7 were FTCs and 12 PTCs. The data analyzed identified differential gene groups between the two types of tumors. Specifically, PTPRN2, TWIST1, ANLN, PRC1, and RRM2 were found in PTCs, and APLP2, PPAP2B, SIAH1, and CEP55 were found in FTCs.48 The next step will be a prospective analysis of the different expression of these gene groups in tumors which experience a dedifferentiation process during their course and those which do not.57
Genetic signatures identified by Montero et al. which are being analyzed in a new patient cohort.
| Alias | Name of gene | Biological function |
| ANLN | Actin binding protein (ANLN) | Necessary for cytokinesis |
| APLP2 | Amyloid beta (A4) precursor-like protein 2 | Regulation of hemostasis |
| ASPM | Abnormal spindle-like microcephaly-associated protein | Mitotic spindle regulation, mitotic process coordination |
| BIRC5 | Survivin | Member of the family of apoptosis inhibitory (API) genes |
| CCNB2 | G2/mitotic-specific cyclin-B2 | Cell cycle control in the G2/M transition |
| CDH1 | E-cadherin | Cell-to-cell regulation of epithelial cell adhesion, motility, and proliferation |
| CENPA | Centromere protein A | Mitotic progression and chromosome segregation |
| CEP55 | Centrosomal protein 55 kDa | Mitotic death and cytokinesis |
| DIAPH3 | Diaphanous homolog 3 (Drosophila) | Promotes actin polymerization, cytokinesis, stress fiber formation |
| IL1RN | Interleukin 1 receptor antagonist | Related to immune and inflammatory responses |
| NUSAP1 | Nucleolar and spindle associated protein 1 | Promotes the organization of microtubules in mitotic spindle |
| ORMDL3 | ORM1-like 3 (S. cerevisiae) | Regulation of Ca-mediated signaling in endoplasmic reticulum |
| PDK2 | Pyruvate dehydrogenase kinase, isozyme 2 | Regulation of glucose metabolism |
| PPAP2B | Phosphatidic acid phosphatase type 2B | May be implicated in cell adhesion and cell interaction |
| PRC1 | Protein regulator of cytokinesis 1 | Cytokinesis |
| PTPRN2 | Protein tyrosine phosphatase, receptor type, N polypeptide 2 | Development of the nervous system and endocrine pancreatic cells |
| RRM2 | Ribonucleotide reductase M2 | Precursor needed for DNA synthesis. Inhibits the Wnt signal |
| SDK2 | Sidekick homolog 2 (chicken) | Cell adhesion protein |
| SH3BGRL2 | SH3 domain binding glutamic acid-rich protein like 2 | Apoptosis, tumor suppression, cell cycle, TNF-α signaling |
| SIAH1 | Seven in absentia homolog 1 (Drosophila) | Proteosomal degradation of target proteins |
| TWIST1 | Twist homolog 1 (Drosophila) | Transcriptional regulator |
| UBE2 C | Ubiquitin-conjugating enzyme E2 C | Cell cycle regulation by ubiquitin ligase progression through mitosis |
Thyroid carcinoma is the most common endocrine tumor, accounting for 2% of all human cancers, and its incidence has constantly increased over the past three decades (3% annually), especially at the expense of papillary microcarcinoma.1 Ninety percent of thyroid carcinomas arise in follicular cells, and most of them are considered well differentiated and have an excellent prognosis. DTC usually has an indolent course, with a mean 10-year survival close to 90%. By contrast, ATC cause 50% of deaths related to thyroid cancer, with a mean survival of 6 months,3,58 and some patients with DTC have an unfavorable prognosis mainly related to dedifferentiation processes. An understanding of the different processes involved in carcinogenesis and in dedifferentiation processes would allow for a better understanding of the disease and of the potential uses of targeted therapies. In addition, the possibility of recognizing the genetic signatures associated with a poor prognosis among all the biomarkers identified would represent a tool of great diagnostic and prognostic value that would help us to better stratify the risks. This would in turn be very helpful for the application of adequate treatment strategies and for developing new pharmacological alternatives. In this regard, the availability of an animal model of undifferentiated/anaplastic carcinoma would be of great value for in vivo research of the biological mechanism of these tumors. Such a model would also allow for the testing of new treatment alternatives, such as the application of nanoparticles targeted to the tumor cell able to release drugs and gene therapies using cells carrying suicide genes. This work is currently being performed by our group in cooperation with other institutions within the CIBER-BBN project (Cell-Nano-Thyroid [website caber-bbn.es]).
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: González Blanco C, et al. Biomarcadores moleculares implicados en el proceso de desdiferenciación tumoral del carcinoma de tiroides de origen epitelial: perspectivas. Endocrinol Nutr. 2012;59(7):452–8.