Gastroenteropancreatic neuroendocrine tumors (GEP-NETs) are the second most prevalent group of advanced gastrointestinal tract tumors. Resources invested in research on this patient population have exponentially increased in recent years, and this has become one of the most attractive fields for oncological research. Several proangiogenic proteins have been found to be overexpressed in GEP-NETs, including vascular endothelial growth factor (VEGF) and its receptors and the more closely related intracellular signaling pathways such as the epidermal growth factor pathway, type I insulin-like growth factor receptor (IGFR), and the PI3K-(PTEN)-AKT-mTOR pathway. The recent results of the three most important Phase III studies in GEP-NETs have allowed for approval of two targeted agents, sunitinib and everolimus, for the treatment of patients with pancreatic neuroendocrine tumors after decades of minimal advances in this population.
Los tumores neuroendocrinos gastroenteropancreáticos (TNE-GEP) constituyen el segundo tumor avanzado más prevalente del tracto digestivo. En los últimos años, los recursos invertidos para la investigación en esta población de pacientes se han visto aumentados exponencialmente convirtiéndose en uno de los escenarios más atractivos para la investigación oncológica. Varias proteínas proangiogénicas han sido identificadas como sobreexpresadas en los TNE-GEP, incluyendo el factor de crecimiento del endotelio vascular y sus receptores, y las vías de señalización intracelular más relacionadas como la del receptor del factor de crecimiento epidérmico, el receptor tipo i del factor de crecimiento similar a la insulina y la vía de PI3K-(PTEN)-AKT-mTOR. Los resultados recientes de los 3 estudios fase iii más importantes en TNE-GEP han permitido la aprobación de 2 terapias dirigidas, sunitinib y everolimus, para el tratamiento de los pacientes con tumores neuroendocrinos pancreáticos después de décadas de mínimos avances en esta población.
GEP-NETs are a heterogeneous group of neoplasms derived from Kultchitzky cells of the diffuse neuroendocrine system located in the gastrointestinal tract and pancreatic islet cells. Although their overall incidence is low, less than 2% of all gastrointestinal tumors, they have a high prevalence because of their natural history, representing the second most common advanced gastrointestinal tumors after colorectal cancer.1 These tumors typically have the capacity to produce several types of hormones and amines, causing a variety of hormonal syndromes such as carcinoid syndrome.
The available therapeutic approaches are also multiple, ranging from curative surgery to palliative procedures such as hepatic locoregional treatments (embolization or radio frequency), palliative surgery, radionuclide therapies, or systemic treatments including hormone, immune or cytotoxic therapy, or the new targeted therapies.2 However, despite the different therapeutic options available, there is a clear deficiency of systemic treatment options for these tumors. Hormone and immune therapies, despite providing control of the symptoms related to hormone secretion in a high proportion of cases, have a limited antitumor effect.3 Standard cytotoxic agents have been shown to have a limited activity in GEP-NETs.4–6 This lack of activity has been related to the typical histological features of these tumors, such as a low proliferation rate as measured by degree of differentiation, Ki 67 expression or mitotic index, and also with the expression of biological markers related to resistance to chemotherapy (such as Akt overexpression).7 The limited efficacy of these standard drugs has led to new therapeutic agents which attempt to exploit the phenotypical characteristics of GEP-NETs being investigated. More detailed understanding of the molecular mechanisms related to cell growth, apoptosis, angiogenesis, and tumor invasion has allowed new targeted therapies in the field of oncology to be developed. Unfortunately, the development of new targeted therapies in the field of neuroendocrine tumors has been limited by several factors, including the great heterogeneity of those tumors, the limitations of in vitro and in vivo models for preclinical research into new drugs, and limited resources, which have had a special impact on groups of uncommon tumors that require an international effort for clinical study design and implementation.
One of the main characteristics of neuroendocrine tumors is their rich vascularization, associated with the high expression of proangiogenic molecules such as VEGF and its receptors (VEGFR1–3), which has been the basis for research into multiple therapies targeted against the tumor angiogenesis process in GEP-NETs (Table 1). PI3K-(PTEN)-AKT-mTOR is another of the main metabolic pathways involved in the pathogenesis of GEP-NETs. There are several hereditary syndromes in which constitutive activation of this metabolic pathway occurs, such as tuberous sclerosis, neurofibromatosis type I, or multiple endocrine neoplasia type I, in which the incidence of GEP-NETs is significantly higher as compared to the general population. There have also been reports of an overexpression of cell surface receptors with tyrosine kinase activity, such as epidermal growth factor receptor (EGFR) or IGFR, which have the mTOR pathway as their main intracellular activating pathway.
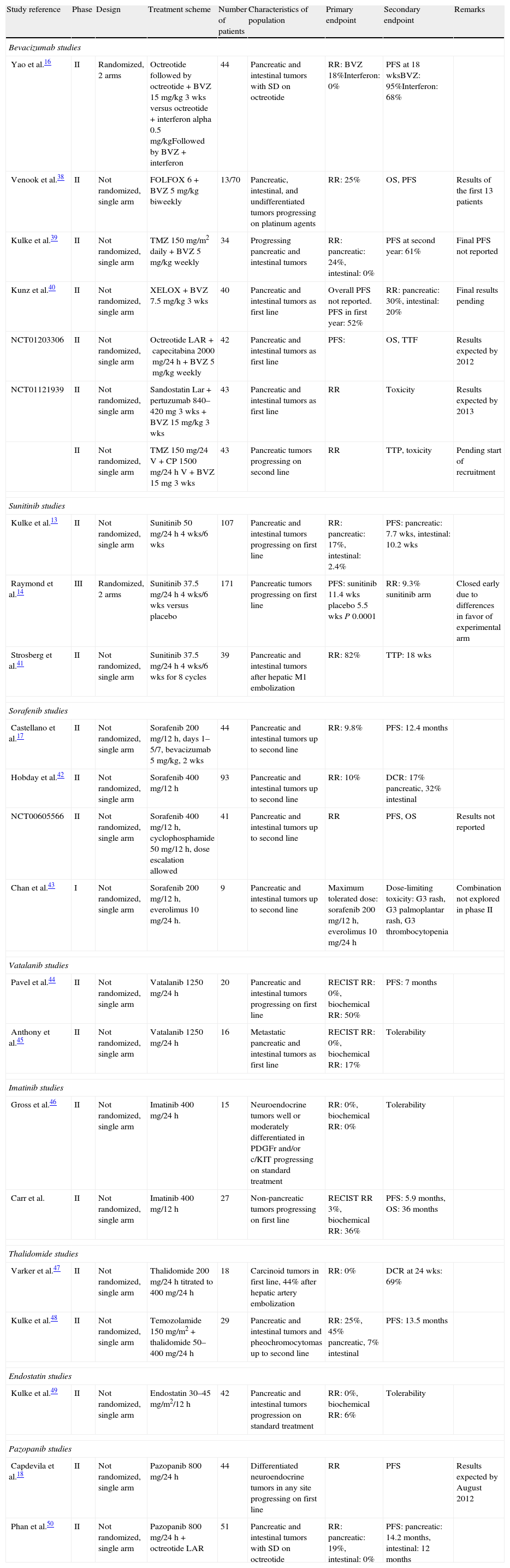
Clinical trials with antiangiogenic drugs in gastroenteropancreatic neuroendocrine tumors.
| Study reference | Phase | Design | Treatment scheme | Number of patients | Characteristics of population | Primary endpoint | Secondary endpoint | Remarks |
| Bevacizumab studies | ||||||||
| Yao et al.16 | II | Randomized, 2 arms | Octreotide followed by octreotide+BVZ 15mg/kg 3 wks versus octreotide+interferon alpha 0.5mg/kgFollowed by BVZ+interferon | 44 | Pancreatic and intestinal tumors with SD on octreotide | RR: BVZ 18%Interferon: 0% | PFS at 18 wksBVZ: 95%Interferon: 68% | |
| Venook et al.38 | II | Not randomized, single arm | FOLFOX 6+BVZ 5mg/kg biweekly | 13/70 | Pancreatic, intestinal, and undifferentiated tumors progressing on platinum agents | RR: 25% | OS, PFS | Results of the first 13 patients |
| Kulke et al.39 | II | Not randomized, single arm | TMZ 150mg/m2 daily+BVZ 5mg/kg weekly | 34 | Progressing pancreatic and intestinal tumors | RR: pancreatic: 24%, intestinal: 0% | PFS at second year: 61% | Final PFS not reported |
| Kunz et al.40 | II | Not randomized, single arm | XELOX+BVZ 7.5mg/kg 3 wks | 40 | Pancreatic and intestinal tumors as first line | Overall PFS not reported. PFS in first year: 52% | RR: pancreatic: 30%, intestinal: 20% | Final results pending |
| NCT01203306 | II | Not randomized, single arm | Octreotide LAR+capecitabina 2000mg/24h+BVZ 5mg/kg weekly | 42 | Pancreatic and intestinal tumors as first line | PFS: | OS, TTF | Results expected by 2012 |
| NCT01121939 | II | Not randomized, single arm | Sandostatin Lar+pertuzumab 840–420mg 3 wks+BVZ 15mg/kg 3 wks | 43 | Pancreatic and intestinal tumors as first line | RR | Toxicity | Results expected by 2013 |
| II | Not randomized, single arm | TMZ 150mg/24V+CP 1500mg/24h V+BVZ 15mg 3 wks | 43 | Pancreatic tumors progressing on second line | RR | TTP, toxicity | Pending start of recruitment | |
| Sunitinib studies | ||||||||
| Kulke et al.13 | II | Not randomized, single arm | Sunitinib 50mg/24h 4 wks/6 wks | 107 | Pancreatic and intestinal tumors progressing on first line | RR: pancreatic: 17%, intestinal: 2.4% | PFS: pancreatic: 7.7 wks, intestinal: 10.2 wks | |
| Raymond et al.14 | III | Randomized, 2 arms | Sunitinib 37.5mg/24h 4 wks/6 wks versus placebo | 171 | Pancreatic tumors progressing on first line | PFS: sunitinib 11.4 wks placebo 5.5 wks P 0.0001 | RR: 9.3% sunitinib arm | Closed early due to differences in favor of experimental arm |
| Strosberg et al.41 | II | Not randomized, single arm | Sunitinib 37.5mg/24h 4 wks/6 wks for 8 cycles | 39 | Pancreatic and intestinal tumors after hepatic M1 embolization | RR: 82% | TTP: 18 wks | |
| Sorafenib studies | ||||||||
| Castellano et al.17 | II | Not randomized, single arm | Sorafenib 200mg/12h, days 1–5/7, bevacizumab 5mg/kg, 2 wks | 44 | Pancreatic and intestinal tumors up to second line | RR: 9.8% | PFS: 12.4 months | |
| Hobday et al.42 | II | Not randomized, single arm | Sorafenib 400mg/12h | 93 | Pancreatic and intestinal tumors up to second line | RR: 10% | DCR: 17% pancreatic, 32% intestinal | |
| NCT00605566 | II | Not randomized, single arm | Sorafenib 400mg/12h, cyclophosphamide 50mg/12h, dose escalation allowed | 41 | Pancreatic and intestinal tumors up to second line | RR | PFS, OS | Results not reported |
| Chan et al.43 | I | Not randomized, single arm | Sorafenib 200mg/12h, everolimus 10mg/24h. | 9 | Pancreatic and intestinal tumors up to second line | Maximum tolerated dose: sorafenib 200mg/12h, everolimus 10mg/24h | Dose-limiting toxicity: G3 rash, G3 palmoplantar rash, G3 thrombocytopenia | Combination not explored in phase II |
| Vatalanib studies | ||||||||
| Pavel et al.44 | II | Not randomized, single arm | Vatalanib 1250mg/24h | 20 | Pancreatic and intestinal tumors progressing on first line | RECIST RR: 0%, biochemical RR: 50% | PFS: 7 months | |
| Anthony et al.45 | II | Not randomized, single arm | Vatalanib 1250mg/24h | 16 | Metastatic pancreatic and intestinal tumors as first line | RECIST RR: 0%, biochemical RR: 17% | Tolerability | |
| Imatinib studies | ||||||||
| Gross et al.46 | II | Not randomized, single arm | Imatinib 400mg/24h | 15 | Neuroendocrine tumors well or moderately differentiated in PDGFr and/or c/KIT progressing on standard treatment | RR: 0%, biochemical RR: 0% | Tolerability | |
| Carr et al. | II | Not randomized, single arm | Imatinib 400mg/12h | 27 | Non-pancreatic tumors progressing on first line | RECIST RR 3%, biochemical RR: 36% | PFS: 5.9 months, OS: 36 months | |
| Thalidomide studies | ||||||||
| Varker et al.47 | II | Not randomized, single arm | Thalidomide 200mg/24h titrated to 400mg/24h | 18 | Carcinoid tumors in first line, 44% after hepatic artery embolization | RR: 0% | DCR at 24 wks: 69% | |
| Kulke et al.48 | II | Not randomized, single arm | Temozolamide 150mg/m2+thalidomide 50–400mg/24h | 29 | Pancreatic and intestinal tumors and pheochromocytomas up to second line | RR: 25%, 45% pancreatic, 7% intestinal | PFS: 13.5 months | |
| Endostatin studies | ||||||||
| Kulke et al.49 | II | Not randomized, single arm | Endostatin 30–45mg/m2/12h | 42 | Pancreatic and intestinal tumors progression on standard treatment | RR: 0%, biochemical RR: 6% | Tolerability | |
| Pazopanib studies | ||||||||
| Capdevila et al.18 | II | Not randomized, single arm | Pazopanib 800mg/24h | 44 | Differentiated neuroendocrine tumors in any site progressing on first line | RR | PFS | Results expected by August 2012 |
| Phan et al.50 | II | Not randomized, single arm | Pazopanib 800mg/24h+octreotide LAR | 51 | Pancreatic and intestinal tumors with SD on octreotide | RR: pancreatic: 19%, intestinal: 0% | PFS: pancreatic: 14.2 months, intestinal: 12 months | |
BVZ: bevacizumab; SD: stable disease; M1: metastasis; RECIST: response evaluation criteria in solid tumors; OS: overall survival; wks: weeks; PFS: progression-free survival; DCR: disease control rate; TTF: time to treatment failure; TMZ: temozolamide; RR: response rate.
Based on this molecular rationale, multiple phase II studies have been conducted with therapies targeted to these metabolic pathways, and the recently published early results of the three most important phase III studies in GEP-NETs have allowed for the approval of the first drugs directed to specific targets in this population.
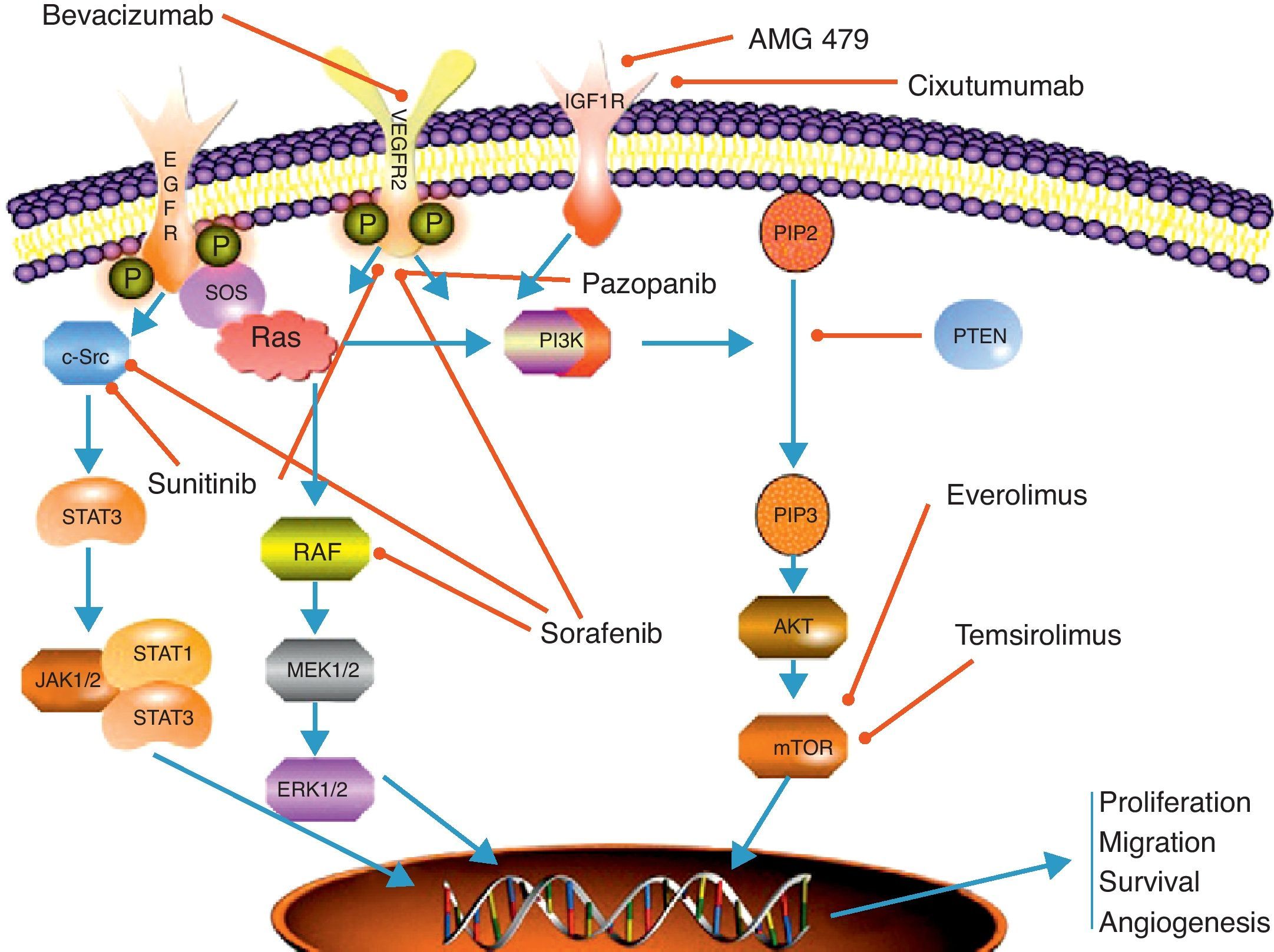
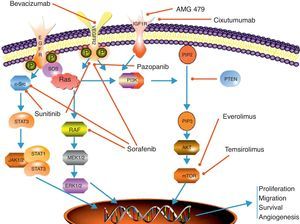
Antiangiogenic therapyAngiogenesis plays a crucial role in the process of tumor growth and systemic dissemination of tumor cell clones. The regulation of angiogenesis is a complex process which results from a dynamic balance between proangiogenic and antiangiogenic factors.8 VEGF and its receptors are among the most significant regulatory factors. VEGF has been seen to have a multiple effect on angiogenesis control, stimulating endothelial cell proliferation and migration and increasing microvascular permeabillity.9 The effects of VEGF are mediated by its binding to different receptors, of which the most important include VEGFR-1 (also known as Flt-1), VEGFR-2 (or KDR/Flk-1), and VGFR-3 (or Flt-4). The VEGF transduction signal is mediated through the domain with tyrosine kinase activity in the intracellular portion of the receptor8 (Fig. 1).
Scheme depicting the most relevant signaling pathways in neuroendocrine carcinomas and the main mechanisms of action of targeted therapies. EGFr: epithelial growth receptor; ERK 1/2: extracellular signal regulated kinase; IGFr: insulin-like growth factor receptor; JAK 1/2: Janus kinases 1 and 2; MEK 1/2: mitogen-activated protein kinase; mTOR: mammalian target of rapamycin: PIP2: phosphoinositol biphosphate; PIP3: phosphoinositol triphosphate; PI3 K: phosphoinositol 3-kinase; PTEN: phosphatase and tensin homolog protein; RAF: RAS-associated factor; RAS; protein associated with rat sarcoma; SOS: son of sevenless protein; STAT 1/3: signal transducer and activator of transcription proteins 1 and 2; VEGFr: vascular endothelial growth factor receptor.
Because of the central role played by VEGF in the angiogenesis process, this ligand and its receptors have become a very attractive target for cancer research. In GEP-NETs, as in most solid tumors, VEGF overexpression has been related to a more advanced stage of the disease and a poorer prognosis.10 Based on these findings, therapies targeted to VEGF and its receptors are being widely studied for the treatment of GEP-NETs, including monoclonal antibodies directed against VEGF and small molecules able to inhibit VEGF receptors with tyrosine kinase activity (Table 1).
SunitinibSunitinib malate (SU-11248, Sutent®) is a potent ATP-competitive inhibitor of multiple cell surface receptors with tyrosine kinase activity primarily involved in cell proliferation and angiogenesis processes, such as VEGFR1-3, PDGFRa-b, FLT-3, c-KIT, and RET.
The development program of sunitinib in GEP-NETs over the past 5 or 6 years has allowed for the progression from the early preclinical studies to final approval by the regulatory authorities for the treatment of advanced pancreatic NETs. The effects of the inhibition of VEGFR and PDGFR (platelet-derived growth factor receptor) on the RIP-TAG xenograft model of pancreatic islet carcinoma were clearly shown in early in vivo studies, and clinically documented in the phase I sunitinib study by the occurrence of radiographic tumor response in two patients with GEP-NETs.11,12 A phase II study of 107 patients showed sunitinib activity to be superior in NETs of a pancreatic origin as compared to small bowel NETs, with a radiographic response rate up to 16.7% based on RECIST criteria (response evaluation criteria in solid tumors) (Table 2), although a clinical benefit was shown in 84% of patients, including stabilizations and minor responses.13 Improved results achieved in pancreatic NETs guided the objective of a phase III international, placebo-controlled study of sunitinib 37.5mg/day as a continuous regimen in patients with well or moderately differentiated, metastatic or locally advanced, non-surgically curable pancreatic NETs progressing during the previous 12 months of follow-up.14 The study was designed to recruit 340 patients, but patient enrolment was stopped when half the sample had been recruited on the recommendation of an independent monitoring committee because the primary objective of an increase in progression-free survival (PFS) had already been achieved and an increased death rate had been found in the placebo arm. The study finally randomized 169 patients, and found a 6-month increase in PFS with sunitinib as compared to placebo (5.5 vs 11.4 months, HR: 0.48, p=0.0001). A clinical benefit of sunitinib was also seen in more than 70% of patients, with a 9% response rate using RECIST criteria. The toxicity profile was similar to that of prior sunitinib studies, and quality of life tests found no differences between the two treatment arms. In addition to data from the phase III sunitinib study, retrospective clinical experiences with the drug in standard clinical practice which show data reproducibility outside the clinical trial setting have been reported.15
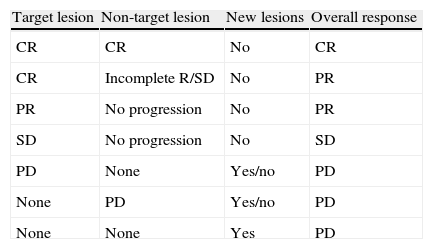
RECIST criteria.
| Target lesion | Non-target lesion | New lesions | Overall response |
| CR | CR | No | CR |
| CR | Incomplete R/SD | No | PR |
| PR | No progression | No | PR |
| SD | No progression | No | SD |
| PD | None | Yes/no | PD |
| None | PD | Yes/no | PD |
| None | None | Yes | PD |
CR: complete response (disappearance of target and non-target lesions); RECIST: Response evaluation criteria in solid tumors; PR: partial response (at least 30% decrease from baseline in the sum of the greater diameter of target lesions); PD: progressive disease (at least 20% increase in the sum of diameters of target lesions of the study with lowest values, occurrence of new lesions or increase in non-target lesions); SD: stable disease (changes in lesion size between those stated for PR and PD).
In the coming years, sunitinib development in GEP-NETs will focus on the evaluation of its activity in combination with somatostatin analogues (lanreotide) in patients with NETs arising in the small bowel (www.clinicaltrials.gov).
BevacizumabBevacizumab is a humanized monoclonal antibody directed against the VEGF ligand that has shown activity in GEP-NETs in a randomized phase II study versus pegylated interferon α-2b. Study results showed that bevacizumab achieved a higher response rate (18% vs 0%) and a lower disease progression rate (5% vs 27%), and improved PFS at 18 weeks (95% vs 68%).16 These good results led to the design of a phase III study of bevacizumab with octreotide LAR as compared to pegylated interferon α-2b plus octreotide LAR in patients with advanced NETs of intestinal origin (SWOG S0518, www.clinicaltrials.gov).
Bevacizumab is also being investigated in combination with other cytotoxic drugs, and also in combination with other targeted therapies such as sorafenib or everolimus, the early results of which have recently been reported.
Many other targeted therapies with antiangiogenic effects are being developed for GEP-NETs, essentially multiple receptor tyrosine kinase inhibitors such as pazopanib or sorafenib. The preliminary results of some phase II studies are already available, and other studies are considering combinations of therapies targeted to different intracellular signaling pathways or sequential treatments in order to achieve optimal disease control17,18 (Table 1).
mTOR inhibitorsThe mammalian target of rapamycin (mTOR) is a serine-threonine kinase of the PI3 K (phosphoinositol-3-kinase)–AKT intracellular signaling pathway. This signal transduction pathway plays a primary role in the regulation of cell growth, proliferation, motility, and survival, as well as in protein synthesis and transcription.19,20 mTOR integrates the signaling of multiple stimuli such as insulin-like growth factors or epidermal growth factor (IGF-1/2, EGF) and mitogens. It also acts as a sensor of cell nutrient and energy levels and oxidation/reduction status. mTOR is also involved in the antiangiogenesis process, regulating the translation and activity of hypoxia-inducible factor (HIF1α), which is related to VEGF expression in cell hypoxia states21 (Fig. 1).
Two mTOR inhibitors have been developed in GEP-NETs with disparate results: temsirolimus and everolimus (Table 3).
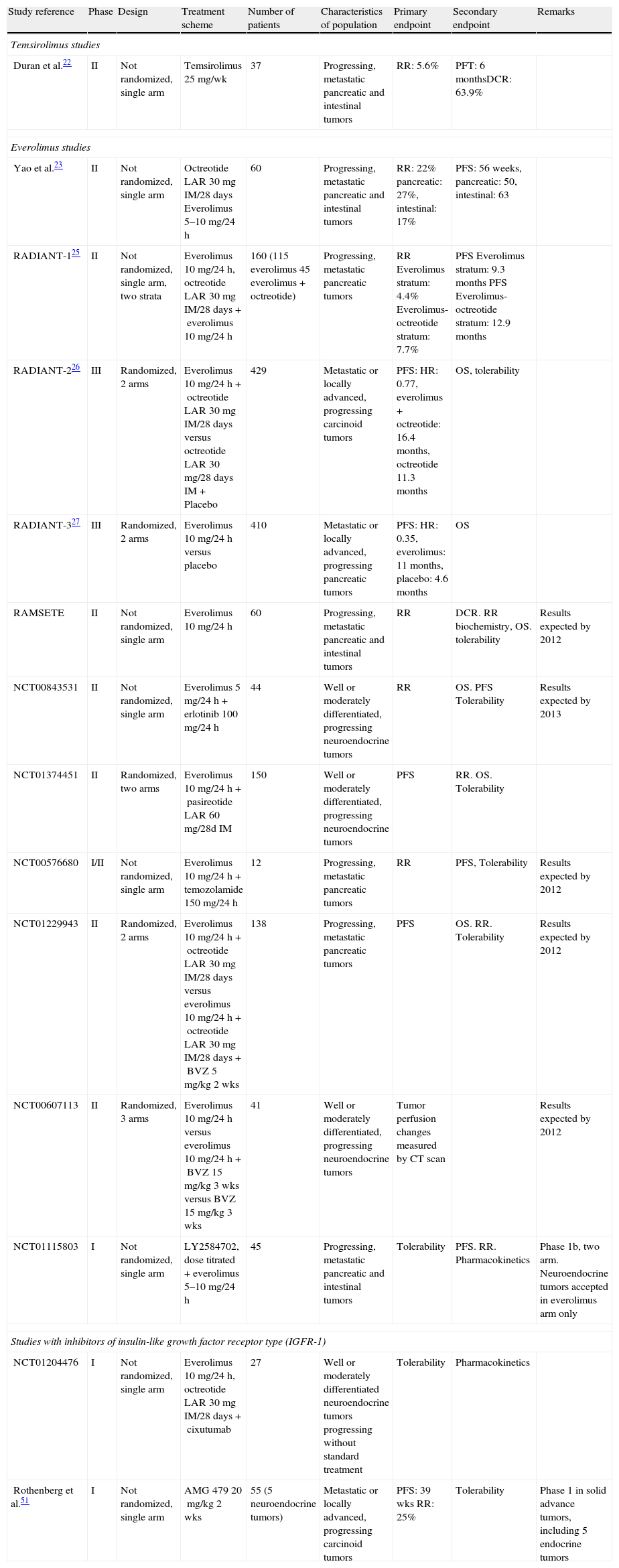
Studies with inhibitors of the PI3K-AKT-MTOR pathway in gastroenteropancreatic neuroendocrine tumors.
| Study reference | Phase | Design | Treatment scheme | Number of patients | Characteristics of population | Primary endpoint | Secondary endpoint | Remarks |
| Temsirolimus studies | ||||||||
| Duran et al.22 | II | Not randomized, single arm | Temsirolimus 25mg/wk | 37 | Progressing, metastatic pancreatic and intestinal tumors | RR: 5.6% | PFT: 6 monthsDCR: 63.9% | |
| Everolimus studies | ||||||||
| Yao et al.23 | II | Not randomized, single arm | Octreotide LAR 30mg IM/28days Everolimus 5–10mg/24h | 60 | Progressing, metastatic pancreatic and intestinal tumors | RR: 22% pancreatic: 27%, intestinal: 17% | PFS: 56 weeks, pancreatic: 50, intestinal: 63 | |
| RADIANT-125 | II | Not randomized, single arm, two strata | Everolimus 10mg/24h, octreotide LAR 30mg IM/28 days+everolimus 10mg/24h | 160 (115 everolimus 45 everolimus+octreotide) | Progressing, metastatic pancreatic tumors | RR Everolimus stratum: 4.4% Everolimus-octreotide stratum: 7.7% | PFS Everolimus stratum: 9.3 months PFS Everolimus-octreotide stratum: 12.9 months | |
| RADIANT-226 | III | Randomized, 2 arms | Everolimus 10mg/24h+octreotide LAR 30mg IM/28days versus octreotide LAR 30mg/28days IM+Placebo | 429 | Metastatic or locally advanced, progressing carcinoid tumors | PFS: HR: 0.77, everolimus+octreotide: 16.4 months, octreotide 11.3 months | OS, tolerability | |
| RADIANT-327 | III | Randomized, 2 arms | Everolimus 10mg/24h versus placebo | 410 | Metastatic or locally advanced, progressing pancreatic tumors | PFS: HR: 0.35, everolimus: 11 months, placebo: 4.6 months | OS | |
| RAMSETE | II | Not randomized, single arm | Everolimus 10mg/24h | 60 | Progressing, metastatic pancreatic and intestinal tumors | RR | DCR. RR biochemistry, OS. tolerability | Results expected by 2012 |
| NCT00843531 | II | Not randomized, single arm | Everolimus 5mg/24h+erlotinib 100mg/24h | 44 | Well or moderately differentiated, progressing neuroendocrine tumors | RR | OS. PFS Tolerability | Results expected by 2013 |
| NCT01374451 | II | Randomized, two arms | Everolimus 10mg/24h+pasireotide LAR 60mg/28d IM | 150 | Well or moderately differentiated, progressing neuroendocrine tumors | PFS | RR. OS. Tolerability | |
| NCT00576680 | I/II | Not randomized, single arm | Everolimus 10mg/24h+temozolamide 150mg/24h | 12 | Progressing, metastatic pancreatic tumors | RR | PFS, Tolerability | Results expected by 2012 |
| NCT01229943 | II | Randomized, 2 arms | Everolimus 10mg/24h+octreotide LAR 30mg IM/28days versus everolimus 10mg/24h+octreotide LAR 30mg IM/28days+BVZ 5mg/kg 2 wks | 138 | Progressing, metastatic pancreatic tumors | PFS | OS. RR. Tolerability | Results expected by 2012 |
| NCT00607113 | II | Randomized, 3 arms | Everolimus 10mg/24h versus everolimus 10mg/24h+BVZ 15mg/kg 3 wks versus BVZ 15mg/kg 3 wks | 41 | Well or moderately differentiated, progressing neuroendocrine tumors | Tumor perfusion changes measured by CT scan | Results expected by 2012 | |
| NCT01115803 | I | Not randomized, single arm | LY2584702, dose titrated+everolimus 5–10mg/24h | 45 | Progressing, metastatic pancreatic and intestinal tumors | Tolerability | PFS. RR. Pharmacokinetics | Phase 1b, two arm. Neuroendocrine tumors accepted in everolimus arm only |
| Studies with inhibitors of insulin-like growth factor receptor type (IGFR-1) | ||||||||
| NCT01204476 | I | Not randomized, single arm | Everolimus 10mg/24h, octreotide LAR 30mg IM/28days+cixutumab | 27 | Well or moderately differentiated neuroendocrine tumors progressing without standard treatment | Tolerability | Pharmacokinetics | |
| Rothenberg et al.51 | I | Not randomized, single arm | AMG 479 20mg/kg 2 wks | 55 (5 neuroendocrine tumors) | Metastatic or locally advanced, progressing carcinoid tumors | PFS: 39 wks RR: 25% | Tolerability | Phase 1 in solid advance tumors, including 5 endocrine tumors |
AMG 479: monoclonal antibody against IGFR-1; cixutumab: monoclonal antibody against IGFR-1; IM: intramuscular; LY2584702: AKT inhibitor; DCR: disease control rate; RR: response rate; PFT: progression-free time.
Temsirolimus (CCI-779) is an intravenous drug that binds to immunophilin FKBP-12 and creates a complex that inhibits the activity of protein kinase mTOR, causing cell cycle arrest in the G1 phase. A single phase II study analyzed the effect of this drug in patients with advanced GEP-NETs. The study enrolled 37 patients who were treated with temsirolimus 25mg/week. The main study endpoint was radiographic response using RECIST criteria, and the study was considered negative because only two partial responses were achieved (5.6%).22 As it occurs in most studies with targeted therapies in solid tumors, there were few radiographic tumor responses, although most patients (54%) showed tumor size reductions ranging from 1% to 29%, which were considered stabilizations based on RECIST criteria. This study included a pharmacodynamic study of matched tumor biopsies in 13 patients, which showed decreased phosphorylation of mTOR products such as S6 and an increased pAKT expression reflecting adequate target inhibition by temsirolimus. In addition, elevated baseline levels of pS6 and pmTOR correlated to a better response, and high pAKT levels with an improved PFS.
Although the initial development of temsirolimus in GEP-NETs was discontinued after what were considered negative results of this phase II study, research on this product in NETs has now been restarted based on the good results reported with the other mTOR inhibitor, everolimus, which showed an activity similar to temsirolimus in its early phase II development phases.
EverolimusEverolimus (RAD001) is an oral derivative of rapamycin which has shown a potent inhibitory activity of mTOR in tumor cell lines and lymphocytes. Because of this, the drug was initially developed as an immunosuppressant.
The first evidence of activity in GEP-NETs was found in a phase II study enrolling 60 patients with GEP-NETs in two consecutive cohorts and using two different doses of everolimus (5 and 10mg). The first cohort of 30 patients received everolimus 10mg/day and octreotide LAR 30mg IM every 28 days. The second cohort received everolimus as a monotherapy. The study showed promising activity, with response rates of 17% in NETs in the small bowel and 27% in NETs of a pancreatic origin. Median PFS (mPFS) times were 63 and 50 weeks, respectively.23 Everolimus showed a higher activity in the 10mg/day cohort, confirming pharmacodynamic data of the prior phase I study with everolimus in solid tumors.24 The toxicity profile was acceptable, and the most common grade 3–4 side effects were aphthous stomatitis, fatigue, diarrhea, hyperglycemia, and hypophosphatemia.
Based on the interesting results achieved in the initial study, RADIANT studies for the development of everolimus in GEP-NETs were designed. RADIANT-1 was an international phase II study to confirm the initial study of two strata of patients with NETs of pancreatic origin. The first stratum consisted of 115 patients treated with everolimus 10mg/day, while the second stratum consisted of 45 patients who received a combination of everolimus 10mg/day and octreotide LAR 30mg IM every 28 days.25 The results seen were significantly poorer as compared to the initial study, with overall response rates of 7.8% and 4.4% in the first and second strata, respectively. In the same study, the use of chromogranin A levels as a biomarker of drug response was considered, and decreased chromogranin A levels were found to be associated with the benefit of everolimus (mPFS 13.3 vs 7.5 months). Finally, the hypothesis of a synergistic effect between mTOR inhibitors and somatostatin analogues was tested, and better PFS data were seen in patients included in the combination cohort (12.9 vs 9.3 months).
The results of the two regulatory studies of everolimus in NETs, RADIANT-2 and 3, have recently been reported. These are two phase III international, double-blind, placebo-controlled studies in patients with clinically functional extrapancreatic NETs and pancreatic NETs, respectively. RADIANT-2 assessed the efficacy and safety of the combination of everolimus 10mg/day or placebo with octreotide LAR 30mg every 28 days in 429 patients with advanced carcinoid tumors and history of associated hormonal symptoms. Although the study failed to achieve the primary endpoint based on the centralized radiographic review, the combination of everolimus and octreotide LAR showed a significant increase of 5.1 months in mPFS as compared to placebo (HR=0.77; 95% CI, 0.59–1.00; p=0.026). After adjusting for imbalances between the treatment arms and inconsistencies between central and local radiographic evaluation, the results showed that the combination of everolimus and octreotide LAR significantly decreased the risk of disease progression by 40% (HR=0.60; 95% CI, 0.44–0.84; p=0.0014).26 The overall results of the RADIANT-2 study reflect the activity of the combination of everolimus and octreotide, but the US and European regulatory authorities (FDA and EMA) have not approved the use of everolimus extrapancreatic NETs because the primary endpoint of the study was not achieved. For this reason, an ongoing phase III study, RADIANT-4, will assess the activity of everolimus monotherapy compared to placebo in patients with advanced NETs of intestinal and pulmonary origin (www.clinicaltrials.gov).
The RADIANT-3 study recruited 410 patients with advanced NETs of pancreatic origin who were randomized to receive everolimus 10mg/day or placebo, together with the best support therapy, which allowed the use of somatostatin analogues.27 Central remission showed a significant increase in PFS after treatment with everolimus. mPFS more than doubled, increasing from 4.6 to 11 months (HR=0.35; 95% CI, 0.27–0.45; p<0.0001), and the primary study endpoint was achieved. At 18 months, PFS in the everolimus arm was 34%, and a patient subgroup especially benefiting from long-term treatment was identified. The toxicity profile was favorable. PFS increases were seen in all patient subgroups irrespective of prior treatments received, ECOG PS (Eastern Cooperative Oncology Group Performance Status), age, tumor burden, time since diagnosis, tumor grade, or combined treatment with somatostatin analogues. The response rate using RECIST criteria was relatively low (5%), although 64.4% of patients experienced a reduction ranging from 1% and 29% in the size of their target lesions. The increased benefit of everolimus was therefore due to its minor response and stable disease rates. No differences were seen in overall survival because 73% of patients in the placebo arm were crossed over to the everolimus arm. The toxicity profile was as expected for everolimus, including stomatitis, anemia, and hyperglycemia as the most common grade 3 or 4 adverse effects, although all these occurred in less than 10% of patients. The results of the RADIANT-3 study made possible the approval, both by the EMA and FDA, of everolimus 10mg/day for the treatment of patients with well and moderately differentiated advanced NETs of pancreatic origin.
The efficacy of everolimus in GEP-NETs is not limited to its antiproliferative effect, but also includes a potential effect on hormone release and carbohydrate metabolism. There have been reports of series cases where the administration of everolimus was able to control the clinical picture related to hormone release in GEP-NETs, such as hypoglycemia in insulinoma or carcinoid syndrome in intestinal NETs.28,29 The molecular rationale behind these effects has not been elucidated yet, but a deeper understanding of the metabolic consequences of mTOR inhibition is expected in the near future.
The current development of everolimus is focused on NETs of extrapancreatic origin and on the combination of mTOR inhibition with inhibition of other therapeutic targets so as to increase synergism and prevent resistance mechanisms (Table 2).
Future avenues for researchThe introduction into clinical practice of two new drugs for the treatment of patients with GEP-NETs has facilitated advances in the complex management of this group of patients. However, the number of questions raised continues to be higher than the number of questions answered, even after the results of the largest studies ever completed in patients with NETs. One of the main problems in the management of patients with NETs is their classification. Ever since Williams and Sandler proposed their embryological classification system nearly 50 years ago, various classification systems based on tumor grade, histological characteristics, hormone secretion, tumor stage, or site of origin have been used. This variety has generated even more confusion, impairing the interpretation of clinical trial results, as occurred in RADIANT-2. The standardization of criteria is still lacking, and three classifications may be used (AJCC TNM, ENETS TNM, and WHO). Improved understanding of the molecular mechanisms involved in NET development and progression should allow for the devising of classifications with greater prognostic value in the future. In this regard, recent gene expression profiling studies have related the decreased expression of tumor suppressor genes of the PI3K–AKT–mTOR pathway, such as PTEN and TSC2, to a poorer prognosis, and microRNA (miR-21) expression to the occurrence of liver metastases and a higher tumor grade.30–32 Other molecular changes such as the relevance of HIF1α, the p53 pathway, changes in menin expression or AKT levels have been related to the behavior of GEP-NETs.33,34 The results of gene expression profiling studies and recent advances in deep sequencing will soon make possible not only the devising of prognostic classifications with a molecular basis, but also the selection of those patients who are most likely to respond to targeted therapies.35
An additional major disadvantage for testing targeted drugs in GEP-NETs is the lack of a variety of animal models that allow for reliable and reproducible preclinical development in humans. The most widely used model, RIP-TAG, has allowed for the development of sunitinib in this patient population, so reflecting the value of xenograft as an angiogenesis model. Its relevance for the study of other metabolic pathways has yet to be shown, although recent studies with mTOR inhibitors and anti-EGFR have led to a reversal of resistance to the inhibition of the PI3K–AKT–mTOR pathway being hypothesized.36
Future treatment of GEP-NETs will be based on molecular typing of the different tumors in order to facilitate the design of clinical trials directed to patient subgroups that share similar molecular characteristics and are more likely to benefit from treatment. Combinations of targeted therapies that allow for the aborting of the resistance mechanisms of inhibition of a single metabolic pathway and combinations with standard cytotoxics or hormone therapies are currently being developed and will change the paradigm of management of these patients in the near future (Tables 1 and 2; www.clinicaltrials.gov). In addition, the development of new targeted drugs should run parallel with a potent program of predictive biomarkers of response so as to increase the efficiency of such treatments.37
ConclusionsThe rapid progression of therapies directed to specific targets is changing the management of cancer patients. The situation is similar in the field of GEP-NETs, where the two new therapies approved in the past year have still to be integrated into the complex management required by these patients. These advances have been made possible by an international effort encompassing all the specialties involved in the treatment of these patients and facilitating the enrollment of patients in studies that will change daily clinical practice. In the current social and economic situation, this kind of multidisciplinary approach which allows for the integration of all the therapeutic options available is essential for good clinical practice and for optimizing the scarce resources invested in the research of what are still considered to be orphan or uncommon diseases.
Conflicts of interestThe authors state that they have no conflicts of interest.
Please cite this article as: Capdevila J, et al. Tumores neuroendocrinos: la era de las terapias dirigidas. Endocrinol Nutr. 2012;59(7):438–51.