Aortic endograft infection is an infrequent but life-threatening complication after endovascular abdominal aortic repair (EVAR). There is no consensus on management of endograft infection and little evidence has been published in our country. Endograft explantation is considered the “gold standar” treatment whereas percutaneous or surgical perigraft and sac drainage associated to antibiotics should be considered and alternative therapy.
MethodsWe carried out a retrospective and descriptive review of abdominal aortic endograft infections at our tertiary center (Hospital Universitario Cruces) during last ten years (2010–2019).
ResultsWe describe the clinical and microbiological characteristics of 10 EVAR infections, their management and outcomes. The incidence of graft infection after EVAR was 3%. The mean time to the clinical presentation of infection was 16.9 months (median 4.5 months). The microbiological diagnosis was reached in 100% of cases (predominance of gram-positive species). The overall mortality rate was 50% (although the survival rate was 100% after surgical drainage of the sac).
ConclusionPerigraft or aneurysm sac aspiration culture show their diagnostic utility as microbiological diagnosis was reached in all cases despite of blood cultures being only positive in 50% of the samples. Surgical drainage and endograft preservation combined with antibiotherapy show remarkable results. The high heterogeneity in our case series makes difficult to offer general recommendations, thus far, a tailored approach to treatment is suggested.
La infección de endoprótesis de aorta abdominal (EVAR) es una entidad infrecuente, pero con una elevada mortalidad. Son escasas las publicaciones al respecto en nuestro país y no hay un consenso definitivo acerca de su manejo. Se acepta como gold standard la cirugía de explante, planteándose como alternativas la antibioterapia asociada a drenaje o limpieza del saco.
MétodosRevisión retrospectiva descriptiva de las endoprótesis aórticas tipo EVAR infectadas en nuestro centro terciario (Hospital Universitario de Cruces) en la última década (2010–2019).
ResultadosDescribimos las características clínicas y microbiológicas de 10 infecciones de EVAR, así como su manejo y resultados obtenidos. La incidencia de infección de EVAR fue del 3%. Tiempo medio hasta la presentación clínica de la infección 16,9 meses (mediana de 4,5 meses). Se logró el diagnóstico microbiológico en el 100% de los casos (predominio de cocos Gram positivos). La mortalidad global fue del 50% (sin embargo, la supervivencia fue del 100% tras limpieza quirúrgica del saco).
ConclusionesEl cultivo de muestras de colecciones periprotésicas y saco aneurismático muestra una gran rentabilidad, llegando al diagnóstico microbiológico en todos los casos, a pesar de ser negativos los hemocultivos en un 50%. Destacan los buenos resultados obtenidos con la limpieza quirúrgica y preservación del dispositivo, asociada a antibioterapia. Sin embargo, en nuestra serie de casos se aprecia una importante heterogeneidad, lo que dificulta elaborar recomendaciones de manejo y obliga a individualizar el tratamiento.
Endovascular Aneurysm Repair (EVAR) is currently regarded as the preferred technique for the treatment of abdominal aortic aneurysms (AAA).1,2 Given the lower initial mortality of EVAR compared to open surgery (1.2%–1.6% vs. 4.2%–5.2%3,4) and its lower perioperative morbidity, approximately 80% of repairs are currently performed using this technique.1–4
EVAR is not without complications of several kinds (endoleaks, haematomas, limb ischaemia, postimplantation syndrome, etc.). One of the most feared complications is infection of the aortic endograft, which is rare but potentially fatal. Its incidence ranges from 0.2%–5%, according to the different published series, although overall mortality is close to 50%.5
The European Society for Vascular Surgery (ESVS) recently published clinical guidelines for the management of vascular graft and endograft infections. The authors recommend the surgical explantation of the graft with in situ reconstruction as the treatment of choice. Conservative management (without explantation) with percutaneous or surgical drainage is regarded as an alternative in high surgical risk patients.6
Few Spanish publications are available on the optimal treatment of EVAR-associated infections and they are limited to small case series. The largest series was published by Fernández Prendes et al.5 with 7 cases treated at the Hospital Universitario Central de Asturias [Asturias Central University Hospital].7,8 Our objective is to describe the series of cases diagnosed with aortic endograft infection in recent years at our centre, as well as to publish our therapeutic strategy based on our experience and a literature review to stimulate the generation of information about this difficult-to-manage infectious complication. It would be interesting to be able to join efforts to create consensuses about diagnosis and treatment to improve these patients' prognosis.
MethodsA retrospective review of the EVAR database was carried out in the Angiology and Vascular Surgery Department of the Hospital Universitario Cruces from January 2010 to December 2019, both elective and emergency. Cases complicated by endograft infection were selected.
Aortic endograft infection was defined by clinical criteria, compatible diagnostic imaging (on CT or PET) and microbiological isolation in blood samples, preoperative percutaneous aspirate from periprosthetic collection or explanted graft material or other intraoperative samples.9 A consensus was reached on diagnosis by the Angiology and Vascular Surgery, Internal Medicine and Infectious Diseases multidisciplinary group.
An Excel-format database was designed and an anonymised review was carried out with a follow-up of medical records until December 2019. The descriptive analysis was carried out using the SPSS Statistics 19® software.
ResultsDemographic characteristics and comorbiditiesDuring the 10-year period (2010–2019) included in the study, 10 aortic endograft infections were identified from a total of 329 procedures, with a cumulative incidence of 3%. All the cases were male, with a mean age of 71.10 years. Attention should be drawn to the large size of the aneurysms, which had an average diameter of 7.56 cm. Smoking and arterial hypertension were the most common comorbidities (70%). Dyslipidaemia (DLP) (50%) and ischaemic heart disease (30%) were next in prevalence. The remaining comorbidities (kidney failure, diabetes, immunosuppression and cancer) occurred in <20% of the cases. All the patients received preoperative antibiotic prophylaxis with a single intravenous dose of cefazolin 2 g, in accordance with the hospital's protocol.
Risk factors and period until infectionOnly 2 of the cases presented postoperative infections of a different origin that triggered implant infection: one case of bacteraemia and one case of intra-abdominal infection. From initial surgery to endograft infection (interval period), the patients had a mean of 1.9 hospital admissions and 27.6 days in hospital. In the interval period, 30% underwent major surgery, another 30% invasive intravascular procedures and 60% invasive extravascular procedures.
Clinical presentation, diagnosis and management (Table 1)The mean time from EVAR to clinical presentation of infection was 16.9 months, with a median of 4.5 months. Five (5) of the 10 cases were defined as early infections (in the first 4 months post-implant). Fever was present in 70% of patients at diagnosis, abdominal or lower back pain in 60%, and 40% were a combination of both. In the laboratory tests, all of the patients presented an increase in CRP, with a mean 175 mg/l at diagnosis. Other data, such as leukocytosis (only present in 30%), were less significant.
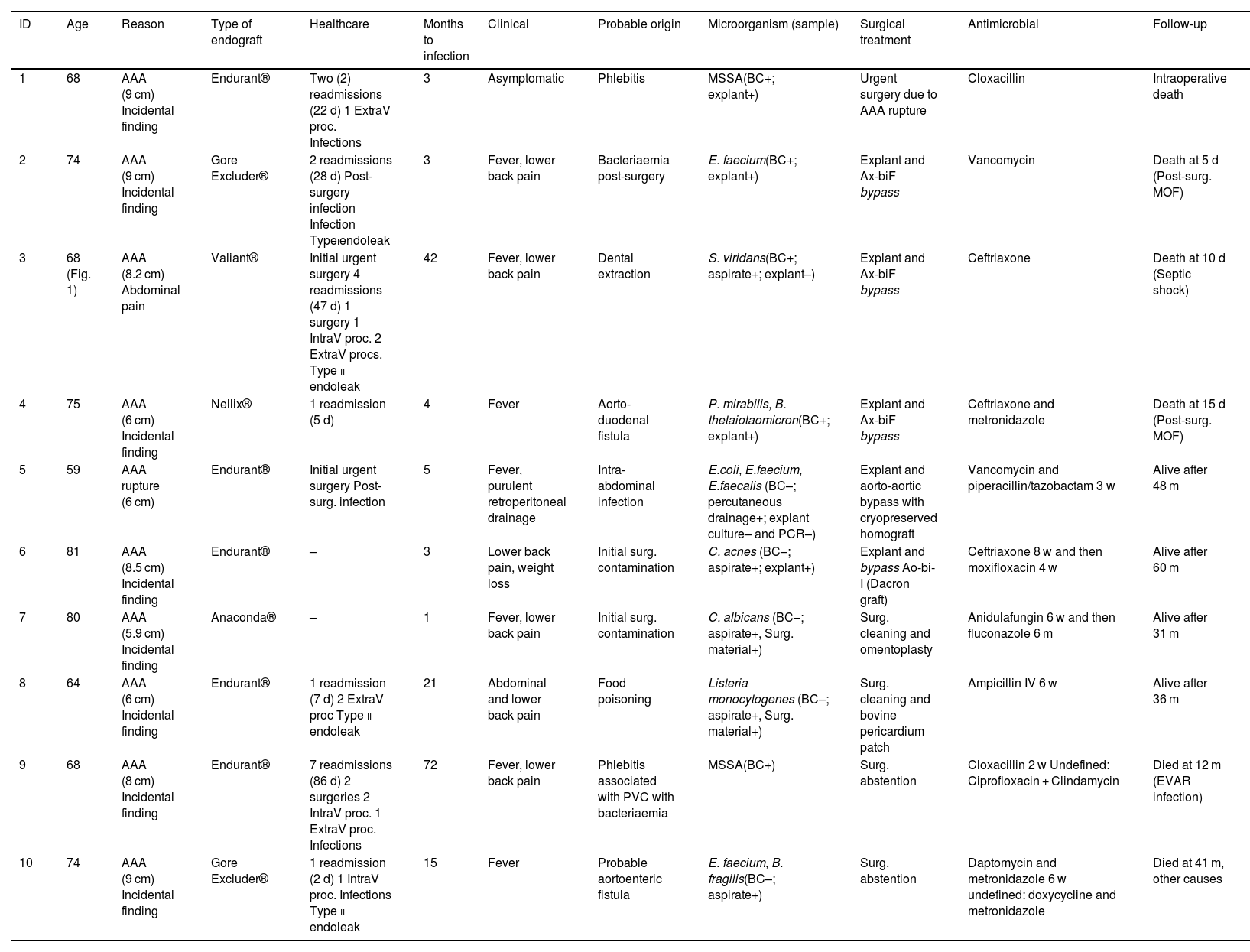
Clinical characteristics, diagnostic method, treatment and prognosis.
| ID | Age | Reason | Type of endograft | Healthcare | Months to infection | Clinical | Probable origin | Microorganism (sample) | Surgical treatment | Antimicrobial | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 68 | AAA (9 cm) Incidental finding | Endurant® | Two (2) readmissions (22 d) 1 ExtraV proc. Infections | 3 | Asymptomatic | Phlebitis | MSSA(BC+; explant+) | Urgent surgery due to AAA rupture | Cloxacillin | Intraoperative death |
| 2 | 74 | AAA (9 cm) Incidental finding | Gore Excluder® | 2 readmissions (28 d) Post-surgery infection Infection Typeiendoleak | 3 | Fever, lower back pain | Bacteriaemia post-surgery | E. faecium(BC+; explant+) | Explant and Ax-biF bypass | Vancomycin | Death at 5 d (Post-surg. MOF) |
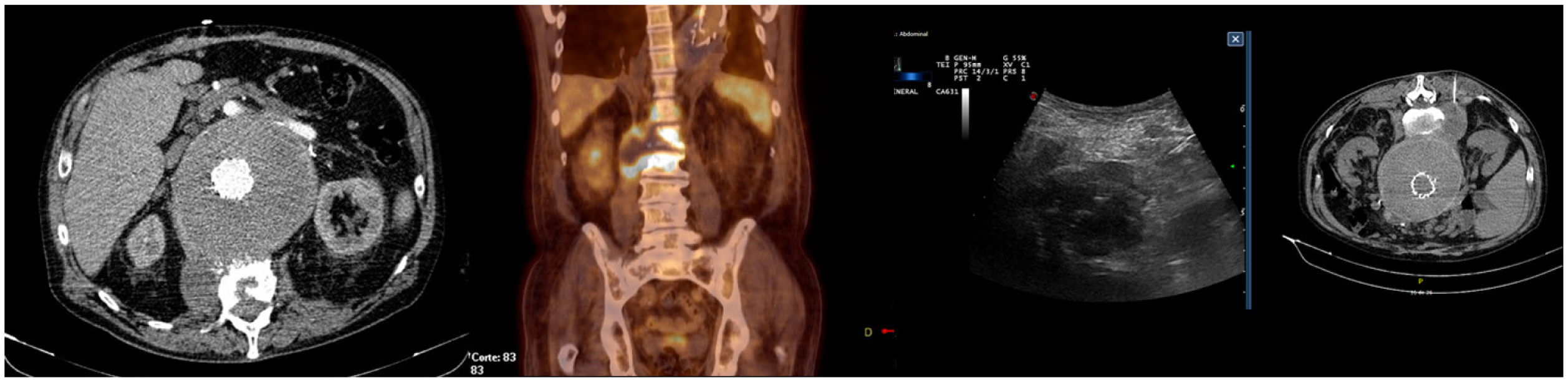
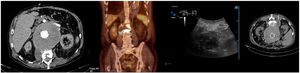
| 3 | 68 (Fig. 1) | AAA (8.2 cm) Abdominal pain | Valiant® | Initial urgent surgery 4 readmissions (47 d) 1 surgery 1 IntraV proc. 2 ExtraV procs. Type ii endoleak | 42 | Fever, lower back pain | Dental extraction | S. viridans(BC+; aspirate+; explant–) | Explant and Ax-biF bypass | Ceftriaxone | Death at 10 d (Septic shock) |
| 4 | 75 | AAA (6 cm) Incidental finding | Nellix® | 1 readmission (5 d) | 4 | Fever | Aorto-duodenal fistula | P. mirabilis, B. thetaiotaomicron(BC+; explant+) | Explant and Ax-biF bypass | Ceftriaxone and metronidazole | Death at 15 d (Post-surg. MOF) |
| 5 | 59 | AAA rupture (6 cm) | Endurant® | Initial urgent surgery Post-surg. infection | 5 | Fever, purulent retroperitoneal drainage | Intra-abdominal infection | E.coli, E.faecium, E.faecalis (BC–; percutaneous drainage+; explant culture– and PCR–) | Explant and aorto-aortic bypass with cryopreserved homograft | Vancomycin and piperacillin/tazobactam 3 w | Alive after 48 m |
| 6 | 81 | AAA (8.5 cm) Incidental finding | Endurant® | – | 3 | Lower back pain, weight loss | Initial surg. contamination | C. acnes (BC–; aspirate+; explant+) | Explant and bypass Ao-bi-I (Dacron graft) | Ceftriaxone 8 w and then moxifloxacin 4 w | Alive after 60 m |
| 7 | 80 | AAA (5.9 cm) Incidental finding | Anaconda® | – | 1 | Fever, lower back pain | Initial surg. contamination | C. albicans (BC–; aspirate+, Surg. material+) | Surg. cleaning and omentoplasty | Anidulafungin 6 w and then fluconazole 6 m | Alive after 31 m |
| 8 | 64 | AAA (6 cm) Incidental finding | Endurant® | 1 readmission (7 d) 2 ExtraV proc Type ii endoleak | 21 | Abdominal and lower back pain | Food poisoning | Listeria monocytogenes (BC–; aspirate+, Surg. material+) | Surg. cleaning and bovine pericardium patch | Ampicillin IV 6 w | Alive after 36 m |
| 9 | 68 | AAA (8 cm) Incidental finding | Endurant® | 7 readmissions (86 d) 2 surgeries 2 IntraV proc. 1 ExtraV proc. Infections | 72 | Fever, lower back pain | Phlebitis associated with PVC with bacteriaemia | MSSA(BC+) | Surg. abstention | Cloxacillin 2 w Undefined: Ciprofloxacin + Clindamycin | Died at 12 m (EVAR infection) |
| 10 | 74 | AAA (9 cm) Incidental finding | Gore Excluder® | 1 readmission (2 d) 1 IntraV proc. Infections Type ii endoleak | 15 | Fever | Probable aortoenteric fistula | E. faecium, B. fragilis(BC–; aspirate+) | Surg. abstention | Daptomycin and metronidazole 6 w undefined: doxycycline and metronidazole | Died at 41 m, other causes |
AAA, abdominal aortic aneurysm; AGE, acute gastroenteritis; Ao-bi-I, aorto-bi-iliac; aspirate, percutaneous aspirate; Ax-biF, axillo-bifemoral; BC, blood cultures; d, days; ExtraV/IntraV, Extra/intravascular; MOF, multiple organ failure; ID, identifier; IV, intravenous; m, months; PCR, PCR of the 16S ribosomal RNA gene; Pip-Tazo, piperacillin-tazobactam; PVC, peripheral venous catheter; MSSA, methicillin-susceptible S. aureus; w, weeks.
A presurgical microbiological aetiological diagnosis was obtained in all cases (10/10). The blood cultures taken at the time of the suspected EVAR infection were positive in 50% of cases, and in the remaining 50% the diagnosis was made through ultrasound-guided aspiration of the infected aneurysmal sac before surgery. Regarding the microorganisms involved, gram-positives were present in 80% of the samples, gram-negative enterobacteriaceae in 20% and Candida sp. in 10%; 30% were polymicrobial infections. Regarding radiological diagnosis, CT was diagnostic in 90% of cases. PET-CT was performed in 6 of the patients in our series and was pathological in 5 of the 6 cases.
Surgery was performed in 8 of the 10 patients. In 6 patients the decision was taken to explant the infected endograft (ID 1–6). One patient underwent emergency surgery due to rupture of the aneurysmal sac associated with the endograft infection and died during surgery. Explantation was performed successfully in the other 5 patients. Among these patients, early intrahospital mortality (<30 days post-surgery) was 60% (3/5), with the patients dying in the resuscitation unit at 5, 10 and 15 days from multiple organ failure. The other 2 patients (40%) are still alive. Surgical treatment of the focus was performed in 2 patients (ID 7.8), with preservation of the implant: surgical opening and cleaning of the aneurysmal sac was performed followed by coverage of the endograft with a bovine pericardial patch and omentoplasty. In the last 2 patients (ID 9,10), the decision to administer conservative treatment with antibiotic therapy was taken in view of the comorbidities and high surgical risk. In these last four cases, in which the endograft was not explanted, early intrahospital mortality was 0% and overall long-term survival was 75% (100% in patients with surgical cleaning and 50% in those with medical treatment). Overall mortality was 50%.
DiscussionPost-EVAR endograft infection has a variable incidence of between 0.2% and 5%. The two meta-analyses published by Li et al.10 and by Argyriou et al.2 show a downward trend with an incidence of 0.2% and 0.6%, respectively. The incidence in small case series is higher, as in the only case series recently published in our country by the Fernández Prendes et al.5 group, who describe an incidence of 1.48%. Our case series shows a slightly higher incidence (3%), which is within expectations and could be accounted for by the high rate of risk factors to which our cases were exposed in the interval period (from EVAR to presentation of the infection), where the requirement for new hospital admissions and other invasive procedures should be highlighted, as well as the inclusion of emergency EVARs.
EVAR infections are classified as early when they occur in the first 4 months after implantation, when the infection occurs mainly due to contamination during the initial surgery. In these cases, more virulent microorganisms are usually involved and give rise to more acute and severe symptoms. During this period, the neointima is forming and endothelialisation of the device occurs, whereby the risk of infection, in the case of haematogenous seeding, is also higher. Tardive infections, beyond the fourth month, are mainly secondary to the haematogenous seeding of microorganisms in invasive procedures or bacteraemia from other foci. Occasionally, aortoenteric fistulae may occur due to growth of the aneurysmal sac and sac wall infection.6,9,11–14 Tardive infections are particularly relevant in the main series and meta-analyses, with a mean time of 25 months from implantation to the presentation of infection.2,15–17 In our series, 50% of EVAR infections were early, although contamination in the initial surgery was only considered to be a pathogenic mechanism in 20% of them. The most common origin of all infections was bacteraemia of another origin (50%), and 20% were associated with aortoenteric fistula. Im sumamry, in any case of bacteraemia in patients with aortic endografts, antibiotic treatment should be aggressive, and a diagnostic effort should be made to rule out graft infection.
The risk factors that have been identified for EVAR infection could be grouped as follows2,5,6,17:
- -
Comorbidities.
- -
Surgical factors: emergency EVAR, fever before EVAR, procedure in the radiology room and hypogastric artery embolisation.
- -
Postoperative factors: postoperative infections and bacteraemia (<30 days), invasive vascular and non-vascular procedures.
- -
Others: type II endoleak, aortoenteric fistula.
It is worth reflecting upon antibiotic (ATB) prophylaxis in invasive dental procedures that require manipulation of the gingival or periapical region of the tooth or perforation of the oral mucosa, as some authors suggest, due to extrapolation of the risk of infective endocarditis, although in the case of vascular implants there is controversy in the literature.1,6 In one of our patients, the endograft infection was due to a dental extraction without prophylaxis with secondary bacteraemia by Streptococcus viridans, hence we could consider patients with EVAR as high-risk for infection due to haematogenous seeding of an odontogenic origin.
The clinical presentation of aortic endograft infection is often insidious, hampering and delaying diagnosis. The most common clinical manifestations are fever, general malaise and/or abdominal pain, which according to studies are present in 60%–80% of cases. On other occasions, it may present as a general syndrome, with gastrointestinal bleeding (due to aortoenteric fistula) or even remain asymptomatic in 5%–10% of cases, with the diagnosis made through imaging controls at successive scheduled check-ups.2,5,15,18,19 Cases with clinical presentation of contiguous spondylodiscitis have also been described (our experience with patient ID 3),11,20–22 lower limb ischaemia or septic embolisms. Our series demonstrates proportions similar to those described in the literature.
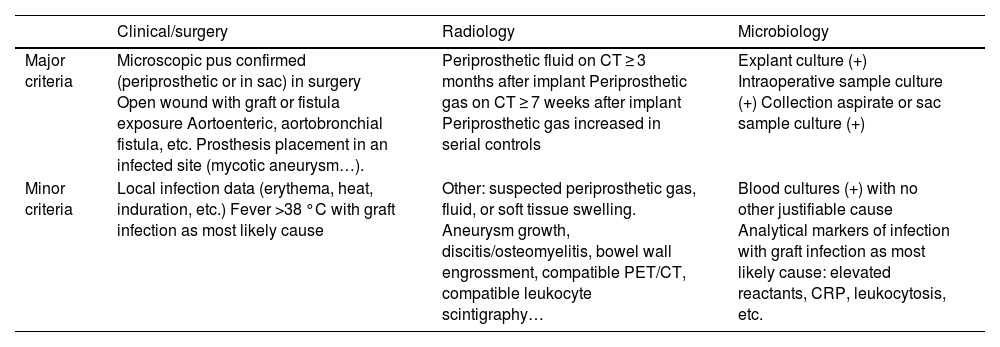
Therefore, a combination of clinical, radiological and microbiological criteria is required for diagnosis. In 2016, the Management of Aortic Graft Infection Collaboration (MAGIC) criteria were published in an attempt to simplify and facilitate diagnostic criteria that will help to identify vascular graft infections early.6,9 According to these criteria, a diagnosis is considered made when one major criterion from one category and another criterion (major or minor) from any other category are met (Table 2).
MAGIC Criteria.
| Clinical/surgery | Radiology | Microbiology | |
|---|---|---|---|
| Major criteria | Microscopic pus confirmed (periprosthetic or in sac) in surgery Open wound with graft or fistula exposure Aortoenteric, aortobronchial fistula, etc. Prosthesis placement in an infected site (mycotic aneurysm…). | Periprosthetic fluid on CT ≥ 3 months after implant Periprosthetic gas on CT ≥ 7 weeks after implant Periprosthetic gas increased in serial controls | Explant culture (+) Intraoperative sample culture (+) Collection aspirate or sac sample culture (+) |
| Minor criteria | Local infection data (erythema, heat, induration, etc.) Fever >38 °C with graft infection as most likely cause | Other: suspected periprosthetic gas, fluid, or soft tissue swelling. Aneurysm growth, discitis/osteomyelitis, bowel wall engrossment, compatible PET/CT, compatible leukocyte scintigraphy… | Blood cultures (+) with no other justifiable cause Analytical markers of infection with graft infection as most likely cause: elevated reactants, CRP, leukocytosis, etc. |
Adapted from Lyons et al.9
In the imaging tests, CT is a fast and relatively cheap technique. In very early and chronic or low virulence infections, sensitivity decreases drastically.12,23 Several studies have recently sought to validate PET-CT, obtaining an overall sensitivity and specificity close to and even higher than the reference technique, which was CT.11,12,23–26 The new ESVS guidelines recommend CT as the first-line diagnostic technique and PET/CT as an additional technique in cases with inconclusive CT (Fig. 1).6
Microbiological diagnosis is not easy. Approximately one third of blood cultures tend to be negative.10,15,27 Therefore, whenever possible, an aspirate of the periaortic collections or of the aneurysmal sac will be obtained to increase diagnostic yield, and of course a microbiological study of the graft material is mandatory in the event of explantation, as well as of intraoperative periprosthetic tissues (aortic wall, thrombus or periprosthetic fluid), either by traditional culture methods or molecular biology methods.6,13 Understanding the flora involved in aortic endograft infections facilitates the choice of empiric antibiotic therapy, although it is highly variable and requires a broad-spectrum antibiotic therapy regimen. Gram-positive cocci are involved in up to half of the cases: S. aureus 19.2%–53% (one in five is usually MRSA); Coagulase-negative staphylococci (CoNS) in 3.3%–15%, Streptococcus sp. in 15% and enterococci in 3.3%–7.7%. Gram-negative bacilli are isolated in approximately 30%: predominantly E. coli with 7.7%–23,1%, whereas the prevalence of P. aeruginosa is approximately 4%. Finally, fungal infections may be found in 10%, and obligate anaerobes (Bacteroides sp., Cutibacterium sp.) in 5%. Anaerobic infections usually occur in the context of polymicrobial infections which, in the case of enteric flora (enterobacteriaceae, enterococci, Bacteroides sp., etc.) should lead us to suspect the presence of aortoenteric fistula.2,10,12,16,28
The treatment of choice is explantation of the infected graft. Early postoperative mortality (<30 days) is variable in the different case series, between 5.5% and 30%.2,15,18,19 The new ESVS guidelines recommend explantation with in situ reconstruction with autologous vein graft as the treatment of choice, although they acknowledge that each in situ or extra-anatomic technique has their advantages and disadvantages and they do not recommend in situ reconstruction with graft material in highly contaminated areas.6,12 The largest review of infected EVAR cases treated with graft maintenance, conducted by Moulakakis et al.,27 reflects an overall mortality of 44.8% (40% in cases treated with surgical cleaning and 50% in those treated with antibiotic therapy alone). Recently, Shukuzawa et al.17 published a series of 15 EVAR infections, 80% of which were treated preserving the infected implant (the procedure of choice in their centre) with an early mortality of 16.7%. Patients who are unlikely to survive surgical explantation due to age and comorbidity may benefit from a semi-conservative approach with partial removal of the graft, intraoperative cleaning, or conservative/palliative medical treatment.6 In summary, for choice of treatment, a multidisciplinary and individualised approach that takes patient characteristics and the centre's experience into account is necessary.
Studies addressing antimicrobial therapy are scarce. In most series, broad-spectrum antibiotic therapy is initiated, followed by directed therapy in the event of microbiological isolation, which is prolonged for up to six weeks or more, or indefinitely in the event of suboptimal treatment or high risk of recurrence (virulent germs, in situ reconstruction with extensive infection or other circumstances that do not guarantee the resolution of the clinical symptoms).12,28
The Groupe de Recherche sur les Infections de Prothèses vasculaires published the first consensus document for the antibiotic management of vascular graft infections in 2015.28 More recently, an Italian working group published an update on the multidisciplinary management of abdominal aortic graft infections.29 These are recommendations with a low grade of evidence - B-III/C-III, with evidence often extrapolated from the management of infective endocarditis or osteoarticular graft infections.
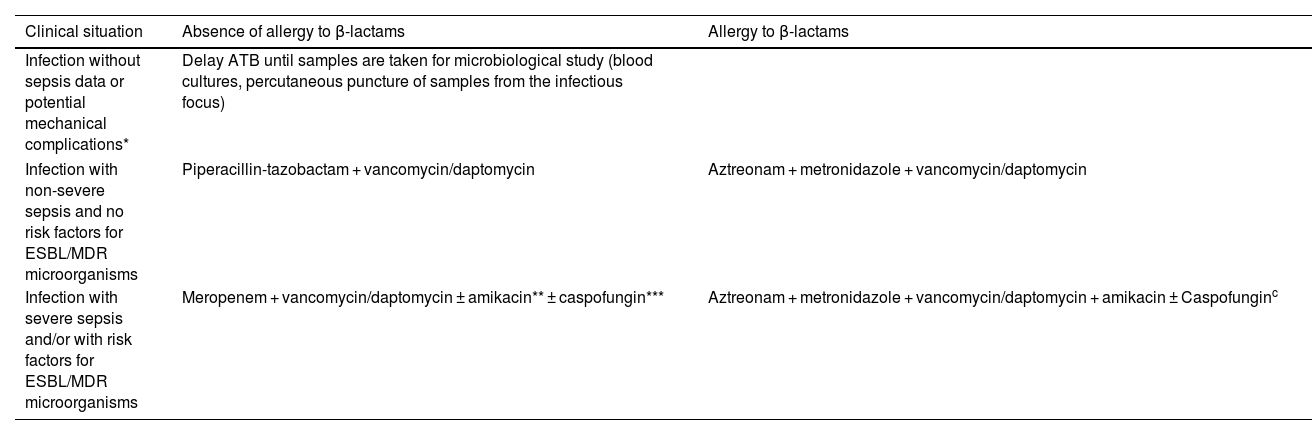
Tables 3 and 4 describe our hospital's empiric and directed antimicrobial therapeutic strategy in aortic endograft infections based on the years of experience accumulated, the resistance pattern of the microorganisms in our health area and periodic literature updates.
Empiric antibiotic treatment.
| Clinical situation | Absence of allergy to β-lactams | Allergy to β-lactams |
|---|---|---|
| Infection without sepsis data or potential mechanical complications* | Delay ATB until samples are taken for microbiological study (blood cultures, percutaneous puncture of samples from the infectious focus) | |
| Infection with non-severe sepsis and no risk factors for ESBL/MDR microorganisms | Piperacillin-tazobactam + vancomycin/daptomycin | Aztreonam + metronidazole + vancomycin/daptomycin |
| Infection with severe sepsis and/or with risk factors for ESBL/MDR microorganisms | Meropenem + vancomycin/daptomycin ± amikacin** ± caspofungin*** | Aztreonam + metronidazole + vancomycin/daptomycin + amikacin ± Caspofunginc |
ATB, antibiotic; ESBL, extended spectrum β-lactamases; MDR, multi-drug resistant.
Directed antibiotic treatment (conditional on susceptibility according to antibiogram).
| Microorganism | Initial treatment | Sequential treatment | |
|---|---|---|---|
| No allergies | Allergy to β-lactams | ||
| MSSAMSCoNSMRSAMRCoNS | Cloxacillin + rifampicin* | Vancomycin/daptomycin + rifampicin*Daptomycin/vancomycin + rifampicin* | LinezolidQuinolone + rifampicinQuinolone + cotrimoxazoleDoxycycline + rifampicinCotrimoxazole + rifampicin |
| Streptococcus sp. | Ceftriaxone | Vancomycin | Cefixime/levofloxacin |
| Enterococcus sp. | Sensitive to penicillin: Ampicillin ± gentamicin | Penicillin-resistant or allergic to β-lactams:Vancomycin/daptomycin | Amoxicillin Clavulanic acid/LevofloxacinPenicillin-resistant: linezolid |
| Enterobacteriaceae | Ceftriaxone/ceftazidime/cefepimeESBL: meropenem | Aztreonam/quinolone + aminoglycoside | Cefixime/ciprofloxacin/cotrimoxazoleESBL: ertapenem** |
| Pseudomonas sp. | Ceftazidime/piperacillin tazobactam | Aztreonam/quinolone + aminoglycoside | Quinolone |
| Anaerobes*** | Bacteroides sp.: MetronidazoleC. acnes: ceftriaxone | MetronidazoleCefixime/moxifloxacin | |
| Candida sp. | Echinocandin | Fluconazole | |
MSSA/MRSA, Methicillin-susceptible/resistant S. aureus; MSCoNS/MRCoNS: Methicillin-susceptible/resistant coagulase-negative S.
Once we obtain the microbiological results, we can modify the initial antibiotic regimen towards directed therapy depending on the microorganism's sensitivity and adapted to the severity of the clinical symptoms. An effort will be made to administer bactericidal antibiotics that reach high plasma levels until the patient's clinical stability is confirmed. In our hospital, in cases in which a definitive surgical procedure has been performed with explantation of the endograft, the cleaning of any collections from the initial focus and placement of an extra-anatomic bypass, we give intravenous antibiotic therapy for 2 weeks after surgery and for 4–6 weeks in case of in situ reconstruction. However, when the decision is taken to maintain the implant after surgical cleaning and cover it with autologous tissue or patch, we prolong the antibiotic treatment until 12 or 16 weeks have been completed after a clinical assessment and PET/CT imaging to verify evolution. In the case of yeast infection alone (Candida albicans) treated by this method, we prolong the antifungal treatment for up to six months. On occasions on which surgery cannot be performed on the infected aortic endograft, antibiotic treatment will be continued, with the strategy based on the chronic suppression of the infection depending on the sensitivity of the microorganism and patient tolerance. We must emphasise the possibility and the benefit to these patients of receiving directed intravenous treatments in an at-home intravenous antibiotic therapy regimen, similar to patients with infective endocarditis under the supervision of the home hospitalisation service, thus avoiding long hospitalisation periods. This method allows us to prolong the intravenous treatment, particularly when it is difficult to ensure adequate levels of some antibiotics in the vascular tissue and on the non-explanted graft when they are administered orally. In this sense, the appropriate time for sequential IV-to-oral antibiotic therapy is decided based on clinical evolution, evolution by CT and the available options for effective oral therapy for the causative microorganism and its tolerance.
We may conclude that post-EVAR graft infection is a rare entity but that it has high morbidity and mortality. For microbiological aetiology, in addition to blood cultures, ultrasound-/CT-guided culture of the aneurysmal sac sample should be attempted whenever possible, since it improves diagnostic performance. Initial empiric antibiotic therapy should be broad-spectrum with coverage for gram-positive cocci (including MRSA), enterobacteriaceae (individuating the risk of enterobacteriaceae with resistance mechanisms) and anaerobes, and the possible involvement of yeasts should also be considered in the case of aortoenteric fistula. In all patients with an acceptable surgical risk, the treatment of choice is explantation of the endograft with in situ or extra-anatomic reconstruction, although surgical cleaning while preserving the implant has proven to be a reasonable alternative. Unfortunately, hitherto there has been no consensus on scientific evidence-based clinical guidelines to recommend optimal antibiotic treatment in terms of efficacy and duration.
FundingNo funding was received for this study.
Conflicts of interestThe authors declare that they have no conflicts of interest.