The aim of the study was to analyze the clinical and microbiological characteristics of adult patients with cerebrospinal fluid (CSF) drainage-related ventriculitis.
MethodsRetrospective study from January 2010 to June 2019 performed in the Complexo Hospitalario Universitario de Vigo (Spain). Cases of CSF drainage-related ventriculitis in patients ≥18-year-old were gathered. Clinical characteristics of patients, type of drainage devices, management and microbiological isolates were analyzed.
ResultsNinety-one episodes of CSF drainage-related ventriculitis were identified. The most frequent organisms isolated were Gram-positive cocci (65%), mainly Staphylococcus epidermidis (48%). Multidrug-resistant microorganisms were detected in 21 episodes (23%). In multivariate analysis, the independent factors related with multidrug-resistant ventriculitis were the length of hospital stay >14 days (HR 6.7; 95%CI 1.75–25.86, p=0.006) and previous antimicrobial therapy (HR 5.58; 95%CI 1.44–21.65, p=0.013).
ConclusionsOur study shows a large number of drainage-related ventriculitis episodes caused by multidrug-resistant organisms and reinforce the importance of a judicious use of antibiotics.
El objetivo del estudio fue analizar las características clínicas y microbiológicas de pacientes adultos con ventriculitis asociada a dispositivos de drenaje de líquido cefalorraquídeo (VaD-LCR).
MétodosEstudio retrospectivo entre enero de 2010 y junio de 2019 realizado en el Complexo Hospitalario Universitario de Vigo (España). Se recogieron los casos de VaD-LCR y se analizaron las características clínicas, los tipos de drenaje, el tratamiento y los aislamientos microbiológicos.
ResultadosSe identificaron 99 episodios de VaD-LCR. Los microorganismos más frecuentemente aislados fueron los cocos grampositivos (65%), principalmente Staphylococcus epidermidis (48%). Se detectaron microorganismos multirresistentes en 21 episodios (23%). En el análisis multivariante, los factores asociados con VaD-LCR por cepas multirresistentes fueron la estancia hospitalaria >14días (HR: 6,7; IC95%: 1,75-25,86; p=0,006) y el uso previo de antibióticos (HR: 5,58; IC95%: 1,44-21,65; p=0,013).
ConclusionesEn nuestro estudio la tasa de VaD-LCR causada por bacterias multirresistentes fue elevada. Estos datos refuerzan la importancia del uso adecuado de antibióticos.
Cerebrospinal fluid (CSF) drainage devices are used for multiple clinical conditions.1 Infection is the major complication of these devices, and is associated with high morbidity and mortality.2,3 The reported incidence of shunt infection is 4%–17%.2,3 In regard to external ventricular drainages (EVD), a pooled incidence of 10.6–11.4 per 1000 catheter-days was observed in a large metanalysis.3
Symptoms of infection are very heterogeneous and depend on the type of device and pathogen virulence.2,4 The most frequently isolated microorganisms are Gram-positive pathogens, especially Staphylococcusepidermidis.1–5 However, in recent years there has been an increase in infections due to multidrug-resistant Gram-negative bacilli6,7 and Candida spp.2,8
Although conservative management may be appropriate for selected patients,5 complete device removal in combination with targeted antibiotic therapy have been associated with improved clinical outcome,2,4,9 and thus it is the current recommendation.1
Most of the published series on infection of CSF derivation devices collected data in pediatric patients, whereas available data in adults is scarce.4,9–12 The objective of this study was to analyze the characteristics of CSF derivation device infections, management and clinical evolution in adult patients.
MethodsThe Complexo Hospitalario Universitario de Vigo (Spain) is a tertiary hospital with 1250 beds and is the reference center for Neurosurgery serving an area of more than 700,000 inhabitants. All positive CSF cultures obtained between January 2010 and June 2019 were retrospectively reviewed. We included all patients aged ≥18 years with any type of CSF drainage infection.
Case definition: CSF drainage device infection was considered for all patients with positive CSF cultures and at least one of the following symptoms: fever in the absence of another source of infection; neurological manifestations of unclear etiology (headache, lethargy, nausea/vomiting); drainage malfunction (hydrocephalus, ventricular size increase with respect to previous studies, periependimary edema); purulent exudate or inflammatory signs on the tunneled path; turbidity of the CSF in the external drainage carriers or abdominal discomfort in patients with ventriculoperitoneal shunt.
Positive cultures were considered to be a contamination if there was a CSF positive culture without symptoms. Those with more than one CSF positive culture with the same microorganism, without signs or symptoms suggestive of infection, were considered colonized. Both contaminated and colonized cultures were excluded from the analysis.
Clinical cure was defined as the disappearance of symptoms at the end of treatment; relapse, as infection due to the same microorganism, after resolution of the symptoms of the previous episode; and reinfection was considered in patients infected with a different microorganism, after the resolution of a previous episode of infection. Patients that died during the acute episode of CSF drainage device infection and without other causes of death were classified as attributable mortality.
The CSF sample was obtained under aseptic conditions from the distal end of the device, by puncture of the reservoir or by lumbar puncture. The CSF was inoculated on chocolate agar, blood agar and thioglycolate broth and incubated at 36°C in 5% CO2. Gram staining was performed. For identification and susceptibility testing VITEK 2 system (bioMérieux) was used. Minimum inhibitory concentrations of linezolid and vancomycin were analyzed by gradient-diffusion, using Etest® strips (bioMérieux). Antimicrobial breakpoints were defined following the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria.13 Epidemiological and clinical characteristics, microbiological isolates, management and clinical evolution were analyzed.
The study was approved by the local ethics committee. Informed consent was not required.
Statistical analysis was performed using the statistical package SPSS 22.0. The normality of the continuous variables was checked by the Kolmogorov–Smirnov test. Continuous variables were compared by means of Mann–Whitney U, and were described as median and interquartile range (IQR), as appropriate. The χ2 and the Fisher exact test were used to compare categorical variables, as appropriate. A multivariate analysis of the risk factors associated with multidrug-resistant infections was carried out using a logistic regression model. Variables with a p-value <0.2 in the univariate analysis were included in the model. Statistical significance was set at p-value <0.05.
ResultsIn our center, 844 CSF drainage devices were placed during the study period; of these, 631 were external drainages and 213 shunts. Ninety-one episodes of CSF drainage device infection were identified in 74 patients: 70 episodes involved external drainages (2 lumbar and 68 ventricular drainages) and 21 episodes occurred in shunts (18 ventriculo-peritoneal and 3 ventriculo-atrial). The majority of patients were male (57%), with a median age of 64 years (IQR, 49–72 years). External device-related infection rate was 6.67 per 1000 catheter-days. Most patients (n=61) had a single episode of ventriculitis; 9 patients had two episodes and four patients had three episodes.
Analysis of infection according to drainage typeThe characteristics of external drainages and shunt infections are compared in Table 1. Median duration of systemic antimicrobial therapy was 16 days, IQR 11–21 days. Fifteen patients received intrathecal therapy for a median of 10 days, IQR 6–20 days.
Clinical characteristics of episodes of CSF drainage-related infections.
| Clinical characteristics | CSF external drainages (n=70) | CSF shunts (n=21) | p |
|---|---|---|---|
| Median age, years (IQR) | 64 (49–72) | 66 (32–75) | 0.830 |
| Sex, male/total patients (%) | 32/58 (55) | 10/16 (63) | 0.777 |
| Time since implantation, median days (IQR) | 12 (9–18) | 60 (34–325) | <0.001 |
| Clinical manifestations, n (%) | |||
| • Device malfunction | 5 (7) | 11 (42) | <0.001 |
| • Fever | 62 (89) | 17 (81) | 0.462 |
| • Neurological disorders | 31 (44) | 9 (43) | 1 |
| • Abdominal symptoms | 0 | 5 (24) | <0.01 |
| Drainage removal, n (%) | 63 (90) | 21 (100) | 0.195 |
| Temporary external ventricular drainage, n (%) | 41 (59) | 16 (76) | 0.322 |
| Time to device withdrawal, median days (IQR) | 2 (1–6) | 1 (0–8) | 0.300 |
| Outcome, n (%) | |||
| • Clinical cure | 51 (73) | 17 (81) | 0.691 |
| • Reinfection | 7 (10) | 3 (14) | 0.746 |
| • Relapse | 6 (9) | 2 (10) | 0.853 |
| • Death at 90 days | 16/58 (28) | 2/16 (13) | 0.327 |
| • Attributable death | 5/16 (31) | 0 | 1 |
Clinical cure was achieved in 73% of patients with external drainage infections and 81% of patients with shunts. Crude mortality at 90 days was 28% in patients with external devices and 13% in those with shunts. In patients with external drainage infection, the attributable mortality was 31%. There was no observed shunt infection related mortality.
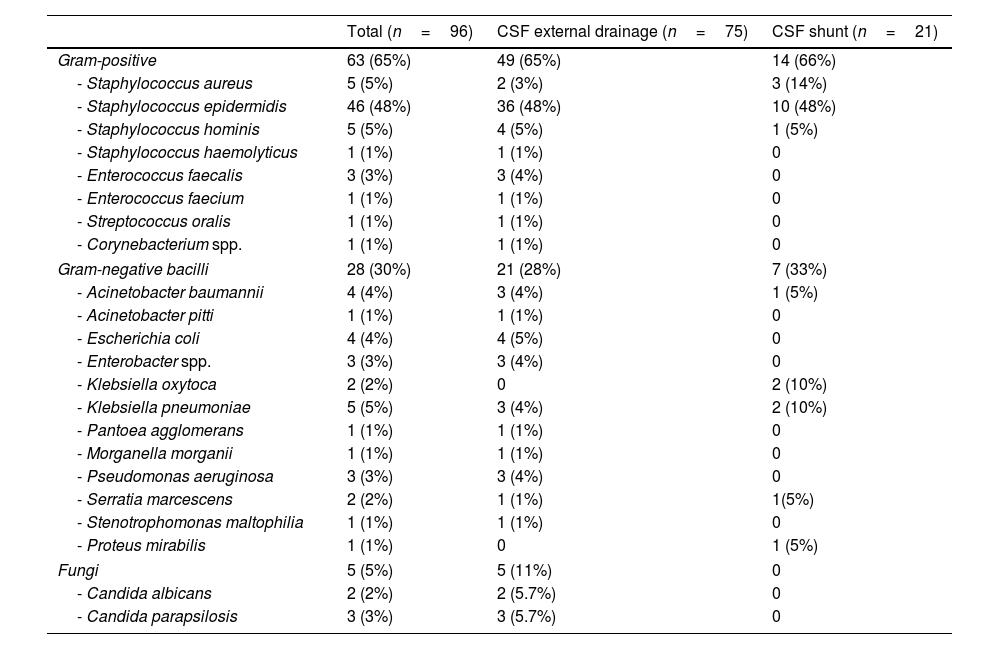
Microbiological isolatesMicrobiological isolates are detailed in Table 2. Twenty-one episodes (23%) of device-related infections due to multidrug-resistant microorganisms were detected. The most frequent was linezolid-resistant S. epidermidis (12 episodes). In addition, we observed 1 episode of meticilin-resistant Staphylococcus aureus, 3 of extended-spectrum beta-lactamase-producing organisms (2 Escherichia coli and 1 Klebsiella pneumoniae), 2 of multidrug-resistant Acinetobacter baumanii, 2 of carbapenem-resistant P. aeruginosa, and 1 due to OXA-48-like carbapenemase-producing K. pneumoniae.
Microbiological isolates obtained in the episodes of device-associated infection.
| Total (n=96) | CSF external drainage (n=75) | CSF shunt (n=21) | |
|---|---|---|---|
| Gram-positive | 63 (65%) | 49 (65%) | 14 (66%) |
| - Staphylococcus aureus | 5 (5%) | 2 (3%) | 3 (14%) |
| - Staphylococcus epidermidis | 46 (48%) | 36 (48%) | 10 (48%) |
| - Staphylococcus hominis | 5 (5%) | 4 (5%) | 1 (5%) |
| - Staphylococcus haemolyticus | 1 (1%) | 1 (1%) | 0 |
| - Enterococcus faecalis | 3 (3%) | 3 (4%) | 0 |
| - Enterococcus faecium | 1 (1%) | 1 (1%) | 0 |
| - Streptococcus oralis | 1 (1%) | 1 (1%) | 0 |
| - Corynebacterium spp. | 1 (1%) | 1 (1%) | 0 |
| Gram-negative bacilli | 28 (30%) | 21 (28%) | 7 (33%) |
| - Acinetobacter baumannii | 4 (4%) | 3 (4%) | 1 (5%) |
| - Acinetobacter pitti | 1 (1%) | 1 (1%) | 0 |
| - Escherichia coli | 4 (4%) | 4 (5%) | 0 |
| - Enterobacter spp. | 3 (3%) | 3 (4%) | 0 |
| - Klebsiella oxytoca | 2 (2%) | 0 | 2 (10%) |
| - Klebsiella pneumoniae | 5 (5%) | 3 (4%) | 2 (10%) |
| - Pantoea agglomerans | 1 (1%) | 1 (1%) | 0 |
| - Morganella morganii | 1 (1%) | 1 (1%) | 0 |
| - Pseudomonas aeruginosa | 3 (3%) | 3 (4%) | 0 |
| - Serratia marcescens | 2 (2%) | 1 (1%) | 1(5%) |
| - Stenotrophomonas maltophilia | 1 (1%) | 1 (1%) | 0 |
| - Proteus mirabilis | 1 (1%) | 0 | 1 (5%) |
| Fungi | 5 (5%) | 5 (11%) | 0 |
| - Candida albicans | 2 (2%) | 2 (5.7%) | 0 |
| - Candida parapsilosis | 3 (3%) | 3 (5.7%) | 0 |
The factors related with multidrug-resistant microorganisms were previous ventriculitis (OR 3.7, 95%CI 1.86–7.30, p=0.001), previous antimicrobial therapy (OR 4.5, 95%CI 1.42–14.28, p=0.003) and length of hospital stay >14 days (OR 5.3; 95%CI 1.63–16.13, p=0.001). We did not observe an association between multidrug-resistant strains and mortality (OR 1.3, 95%CI 0.34–5, p=1). In multivariate analysis, the independent factors related to multidrug-resistant ventriculitis were the length of hospital stay>14 days (HR 6.7; 95%CI 1.75–25.86, p=0.006) and previous antimicrobial therapy (HR 5.58; 95%CI 1.44–21.65, p=0.013).
DiscussionOur study represents, to our knowledge, the largest series of CSF drainage-related ventriculitis in adults. In our series, we found a high prevalence of multidrug-resistant organisms, not only carbapenem-resistant Gram-negative bacilli, but also linezolid-resistant S. epidermidis.
The recent worldwide emergence of linezolid-resistant staphylococci raises significant challenges for the treatment of infections caused by these microorganisms.14,15 Mutations at the ribosomal target site (23S rRNA) is the main mechanism of resistance to linezolid.14 In addition, staphylococci become resistant to linezolid by acquisition of a transferable gene (cfr) whose product is responsible for a specific post-transcriptional modification in the target site.14
In our study, length of hospital stay, and previous antimicrobial treatment were independent factors associated with multidrug-resistant ventriculitis. Both factors have been related with the emergence of antimicrobial resistant microorganisms.6
We observed a higher mortality in patients with external devices (28%), as compared to those carrying shunts (13%). Data was similar to recent published studies.10 On the other hand, shunt-related mortality is usually very low.4,11 Differences in mortality could also be related to other factors, such us, higher severity of the underlying condition, and a lower rate of and a delayed device removal.
Furthermore, we could not demonstrate an association between mortality and isolation of multidrug-resistant strains. Previous studies had found higher mortality in patients with ventriculitis due to Pseudomonasaeruginosa.6 However, in another study with high prevalence of multidrug-resistance Gram-negative bacilli, the mortality was very low (7.5%).7
Our study has some limitations. It is a retrospective study conducted in a single center. In addition, no molecular analysis were performed in order to investigate the linezolid resistance mechanisms. Despite these limitations, this study reports one of the largest series on CSF drainage device-related ventriculitis.
In conclusion, multidrug-resistant microorganisms are responsible of high numbers of CSF drainage-related infections in our study. Previous use of antibiotics and length of hospital stay were associated with infection by multidrug-resistant strains. Our data reinforce the importance of a judicious use of antibiotics.
FundingNo.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors wish to thank to Manuel Crespo, Cesareo Conde and Adrián Sousa their support to carry out this work.