Pseudomonas aeruginosa neurosurgical meningitis is a rare entity, usually related with intraventricular catheters and associated with high mortality rates. We describe the clinical characteristics, treatment and outcomes of a series of neurosurgical meningitis caused by P. aeruginosa along 1990–2016.
MethodsDescriptive, retrospective study of all postsurgical meningitis due to P. aeruginosa related to intraventricular catheters in Hospital Universitario Central de Asturias, between 1990 and 2016. Clinical features, therapeutic approaches and prognostic factors were analyzed statistically. A binary logistic regression analysis was performed to determine the factors influencing the infection mortality.
Result51 episodes from 51 different patients with CSF culture positive for P. aeruginosa were reviewed. Seventeen patients (33.3%) died as a direct consequence of the infection. Univariate analysis showed that mortality was higher in the group of patients treated with ceftazidime (12 vs. 15, p=0.068, OR 3.040 [0.877–10.544]) and lower in patients whom had received intrathecal therapy (2 vs. 13, p=0.050, OR 4.64 [0.80–34.93]), without differences observed between those patients treated with aminoglycosides or with colistin. Any patient treated with colistin died (0 vs. 6, p=0.067, OR: not defined). In the multivariate analysis mortality was only significant higher for patients without catheter withdrawal (p=0.014) and lower for those patients who received intrathecal therapy (p=0.05) or adequate empirical treatment (p=0.006).
ConclusionsThe mortality of P. aeruginosa meningitis is high especially in infections without catheter withdrawal and in patients for whom the intrathecal route of administration was not used. Catheter withdrawal was an independent factor of good outcome in our series.
La meningitis nosocomial por Pseudomonas aeruginosa es una entidad poco frecuente, generalmente relacionada con catéteres intraventriculares y asociada con altas tasas de mortalidad. Se describen las características clínicas, con especial hincapié en su tratamiento, de una serie de meningitis neuroquirúrgicas por P. aeruginosa entre 1990 y 2016.
MétodosEstudio descriptivo, retrospectivo, de todas las meningitis posquirúrgicas por P. aeruginosa relacionadas con catéteres intraventriculares en el Hospital Universitario Central de Asturias entre 1990 y 2016, con especial hincapié en los enfoques terapéuticos y factores pronósticos. Los factores asociados con mortalidad se analizaron mediante regresión logística binaria.
ResultadosSe revisaron 51 episodios de 51 pacientes diferentes con cultivos de LCR positivos para P. aeruginosa. Diecisiete pacientes (33,3%) murieron como consecuencia directa de la infección. La mortalidad fue mayor en el grupo de pacientes tratados con ceftazidima (12 vs. 15, p=0,068, OR 3,040 [0,877-10,544]) y menor en los pacientes que habían recibido terapia intratecal (2 vs. 13, p=0,050, OR 4,64 [0,80-34,93]), sin diferencias en estos últimos entre los tratados con aminoglucósidos o con colistina. Ningún paciente tratado con colistina falleció (0 vs. 6, p=0,067, OR no definida). El análisis multivariable únicamente confirmó la asociación con la ausencia de terapia intratecal (p=0,05) o tratamiento empírico adecuado (p=0,006).
ConclusionesLa mortalidad de la meningitis por P. aeruginosa es elevada, especialmente en pacientes en quienes no se utilizó la vía de administración intratecal. La retirada del catéter fue un factor independiente de buena evolución en nuestra serie.
Nosocomial bacterial meningitis commonly results from invasive procedures or complicated head trauma. The responsible etiological agent can be both Gram-negative and Gram-positive bacteria and depends on different features such as the pathogenesis and timing of the infection after a predisposing event.1 Nosocomial bacterial meningitis has an incidence rate up to 20% if catheters are placed for drainage.1
Pseudomonas aeruginosa neurosurgical meningitis is a rare entity, usually related with intraventricular catheters (IVC), and associated with high mortality rates and increased resource utilization and costs (including length of stay and antibiotic use/duration).2 The treatment of P.aeruginosa meningitis pose a severe challenge due to the scarce number of drugs available for its treatment and also for the poor diffusion of most of the antimicrobial agents into the central nervous system through the blood brain barrier.2–6 Traditionally, some drugs such as cephalosporins, quinolones, carbapenems, colistin or aminoglycosides, the two-latter used also intrathecally, have been used for the treatment of meningitis caused by P. aeruginosa.3–7 However, in recent years, the emerging and spread of multidrug-resistant (MDR) strains, especially those resistant to carbapenems, have really complicated the treatment of P. aeruginosa infections.8
The aim of the present study is to describe the clinical features and outcomes of a series of patients with nosocomial neurosurgical meningitis caused by P. aeruginosa treated with different therapeutic approaches and to identify the different prognostic factors related with these rare infections. Also, the scarce literature related to this rare entity has been thoroughly reviewed.
Material and methodsClinical and microbiological dataA retrospective study was performed including all adult patients diagnosed of nosocomial postsurgical meningitis due to P. aeruginosa related to IVC between 1990 and 2016. The study was carried out at the Hospital Universitario Central de Asturias (HUCA), a tertiary university hospital with a neurosurgery ward in northern Spain. Patients were identified and included in the study after reviewing the cerebrospinal fluid (CSF) cultures registered in the Clinical Microbiology Laboratory.
The medical chart of each patient was reviewed, and the epidemiological and clinical data was collected including information such as: underlying diseases, mean time of stay before surgery, reason for surgery, time elapsed since the intervention, presence of mixed cultures, adequacy of the empiric treatment establishment, and biochemical CSF features. Previous antibiotic treatment was defined as treatment for at least 48h during the 10 days before the meningitis diagnosis.
All patients received antibiotic prophylaxis for prevention of surgical site infection with intravenous (iv) cefazolin (1g every 8h for three doses). Nosocomial meningitis was considered according to current CDC definitions.9 CSF infection must have met at least one of the following two criteria: (i) presence of an organism isolated from CSF culture and fever; (ii) temperature of 38.8°C in the absence of another recognized origin; and one of more of the following; (i) increased white cells (10cells/mm3 with 50% polymorphonuclear leucocytes); (ii) increased proteins (45mg/dL); (iii) hypoglucorraquia (lower 40mg/dL or less than 50% of the glycaemia). A positive CSF culture or Gram stain with normal values of glucose, proteins and cell count in absence of clinical manifestations was considered as a contamination and discarded for the study.
Samples of CSF were obtained through an IVC if present and by lumbar puncture if not. Incubation, microbiological evaluation and bacterial subcultures were performed, as recommended by the Infectious Diseases Society of America (IDSA).10,11 A mixed bacterial infection was considered when two or more microorganisms were isolated from a CSF culture. Bacterial identification was performed by the Microscan System (MicroScan; Beckman Coulter, CA, USA), which was also applied for determination of the minimal inhibitory concentrations (MIC) to a panel of 18 antimicrobials. Etest strips (bioMérieux, Marcy-l’Étoile, France) were used for determining the MICs of colistin in those isolates resistant to this drug by the Microscan. The different P. aeruginosa isolates were considered resistant or susceptible to the different antimicrobials tested according to the CLSI guidelines available in the respective year of the bacteria isolation.
Evolution and treatmentAn empirical antimicrobial therapy was considered as adequate if it included at least one effective antibiotic according to antimicrobial susceptibility testing. After receiving the antibiogram results, the treatments included the following parenterally administered treatment schedules: meropenem 2g/8h, ceftazidime 3g/8h, amikacin 500mg/8h or sodium colismethate 5mg/kg/day administered in three doses in patients with normal renal function. In some cases the treatment was also administered intrathecally: colistin (10mg/12h), gentamycin or tobramycin (both at 10mg/24h) or amikacin (20mg/24h). Most patients received dexamethasone (up to 4mg/8h) for at least five days after surgery.
Infection cure was considered when two successive cultures were negative and clinical signs of infection (fever and/or meningismus) were absent. To assess the patient's survival, all of them were followed-up until they died in the hospital or were discharged. Treatment failure was defined as death attributable to meningitis or relapse within 30 days of infection. Death was considered not attributable to meningitis if the patient had negative CSF culture results before death, absence of inflammatory parameters, clinical signs of meningitis, and when a cause other than meningitis was found to be more probable according to the responsible physician. Relapse was defined as an isolation of the same microorganism from CSF in the next 30 days after treatment.
Statistical analysisContinuous values were expressed as means and standard deviation [SD] and compared using Student's t-test or the Mann–Whitney U-test. Categorical values were expressed as absolute and relative frequencies and were compared using Fisher's exact test or χ2 test. A p value less than 0.05 was considered as statistically significant. A binary logistic regression analysis using a stepwise (Wald) approach was performed to determine the factors influencing the infection mortality.
ResultsDuring the period of the study 51 CSF cultures of 51 different patients positive for P. aeruginosa were reviewed. Fifty-eight percent of patients were male and the mean age was 5018 years. All patients had undergone surgical procedures and also carried an IVC. The time elapsed between surgery and the onset of infection was 2120 days (limits 3-112 days). The mean of permanence of IVC before meningitis diagnosis were of 2214 days.
The most frequent underlying diseases were neoplasm and hemorrhage (33.3% both) followed by head trauma (27.5%) and hydrocephalus (6%). From a clinical point of view, fever was the most frequent symptom (100%), followed by headache (43%) and low level of consciousness (39%). Signs of meningeal irritation were detected in only 12 patients (23%). The CSF features were as follows: white cell count 6964 [33,569]cells/mm3, protein 321 [314]mg/dL, and glucose 49 [37]mg/dL.
Ten patients (19.6%) were diagnosed with polymicrobial meningitis. The other accompanying microorganisms recovered were Staphylococcus aureus (5 cases), Acinetobacter baumannii (3 cases) and Enterococcus faecalis (2 cases).
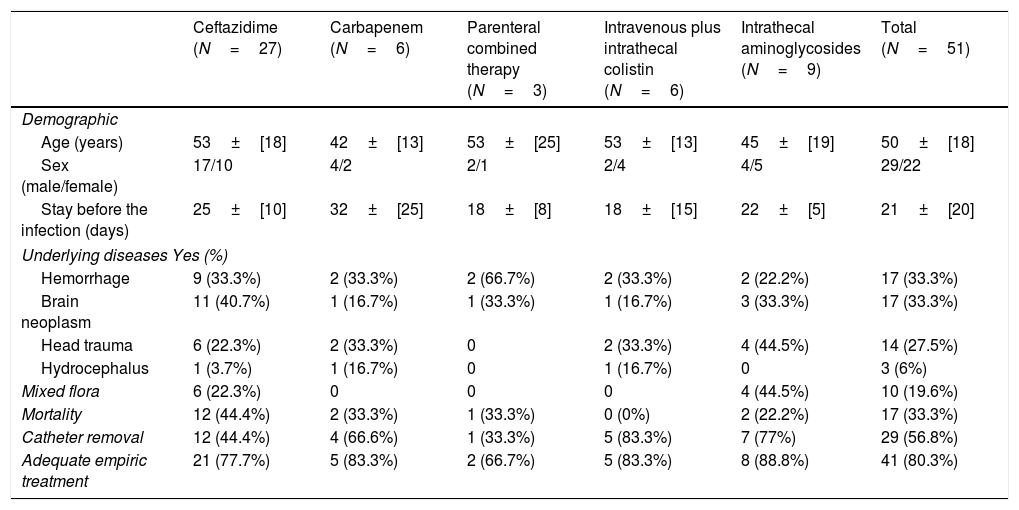
Regarding treatment, 33 patients received intravenous monotherapy with antipseudomonal cephalosporins (27 patients) or carbapenems (six patients). Other patients received combined therapy with ceftazidime and aminoglycoside (three cases). Fifteen patients received both intravenous and intrathecal therapy with cephalosporin or cabapenems plus intrathecal aminoglycosides (nine cases), or intravenous plus intrathecal combined therapy with colistin (six cases). The median duration of antimicrobial therapy was 19 days (range 2-29 days). In patients with combined therapy (intravenous and intrathecal) both treatments were maintained during the same time (mean 17 days; range 2-23). Furthermore, in 29 patients the antimicrobial therapy was combined with removal of the IVC. In 19.7% of the cases the empirical treatment established was considered inadequate. Treatment features are summarized in Table 1.
Clinical and demographic features of patients with postneurosurgical meningitis by P. aeruginosa divided in groups treated with different approaches.
| Ceftazidime (N=27) | Carbapenem (N=6) | Parenteral combined therapy (N=3) | Intravenous plus intrathecal colistin (N=6) | Intrathecal aminoglycosides (N=9) | Total (N=51) | |
|---|---|---|---|---|---|---|
| Demographic | ||||||
| Age (years) | 53±[18] | 42±[13] | 53±[25] | 53±[13] | 45±[19] | 50±[18] |
| Sex (male/female) | 17/10 | 4/2 | 2/1 | 2/4 | 4/5 | 29/22 |
| Stay before the infection (days) | 25±[10] | 32±[25] | 18±[8] | 18±[15] | 22±[5] | 21±[20] |
| Underlying diseases Yes (%) | ||||||
| Hemorrhage | 9 (33.3%) | 2 (33.3%) | 2 (66.7%) | 2 (33.3%) | 2 (22.2%) | 17 (33.3%) |
| Brain neoplasm | 11 (40.7%) | 1 (16.7%) | 1 (33.3%) | 1 (16.7%) | 3 (33.3%) | 17 (33.3%) |
| Head trauma | 6 (22.3%) | 2 (33.3%) | 0 | 2 (33.3%) | 4 (44.5%) | 14 (27.5%) |
| Hydrocephalus | 1 (3.7%) | 1 (16.7%) | 0 | 1 (16.7%) | 0 | 3 (6%) |
| Mixed flora | 6 (22.3%) | 0 | 0 | 0 | 4 (44.5%) | 10 (19.6%) |
| Mortality | 12 (44.4%) | 2 (33.3%) | 1 (33.3%) | 0 (0%) | 2 (22.2%) | 17 (33.3%) |
| Catheter removal | 12 (44.4%) | 4 (66.6%) | 1 (33.3%) | 5 (83.3%) | 7 (77%) | 29 (56.8%) |
| Adequate empiric treatment | 21 (77.7%) | 5 (83.3%) | 2 (66.7%) | 5 (83.3%) | 8 (88.8%) | 41 (80.3%) |
There were not statistically significant differences in age, sex, underlying diseases, presence of mixed culture and catheter removal between the different treatments (Table 1). Only three patients had a relapse. Seventeen patients (33.3%) died as a direct consequence of the infection. They had been treated with ceftazidime (12 cases, 70.5%), carbapenems (2 cases, 11.7%), carbapenem plus intrathecal aminoglycosides (2 cases 11.7%) or combined intravenous therapy (1 case, 5.8%).
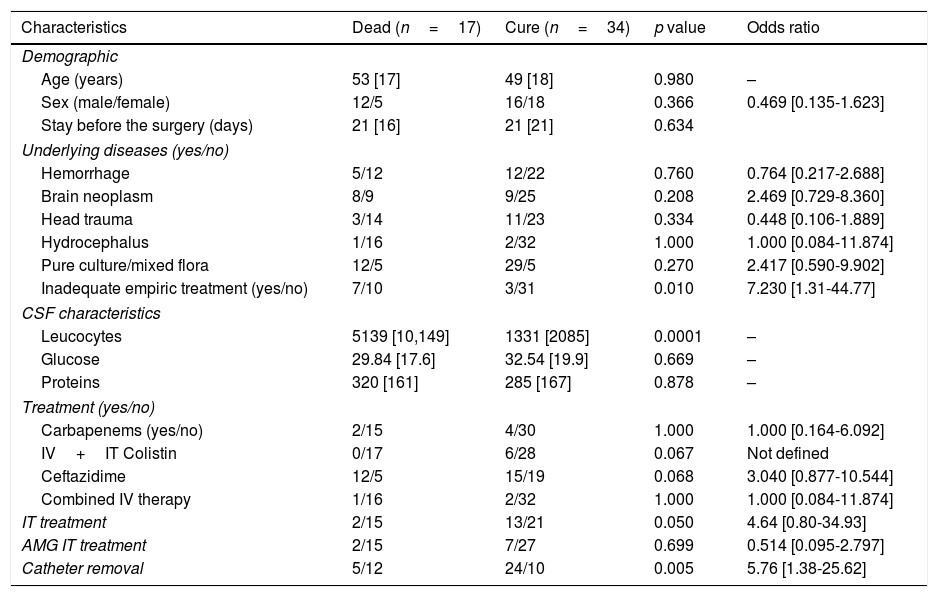
In the univariate analysis mortality was higher in the group of patients treated with ceftazidime (12 vs. 15, p=0.068, OR: 3.040 [0.877-10.544]) and also in patients under inadequate empirical treatment (7 vs. 3 p=0.010, OR: 7.230 [1.31-44.77]. Moreover, mortality was lower in patients whom had received intrathecal therapy (2 vs. 13, p=0.050, OR: 4.64 [0.80-34.93], without differences observed between those patients treated with aminoglycosides and those treated with colistin. Finally, no patients treated with colistin died (0 vs. 6 p=0.067, OR: not defined) (Table 2). In the multivariate analysis mortality was only significantly higher in patients without catheter withdrawal (p=0.014) and statistically significant lower in patients who received intrathecal therapy (p=0.05) or adequate empirical treatment (p=0.006).
Univariate analysis of factors associated with death among patients with P. aeruginosa meningitis.
| Characteristics | Dead (n=17) | Cure (n=34) | p value | Odds ratio |
|---|---|---|---|---|
| Demographic | ||||
| Age (years) | 53 [17] | 49 [18] | 0.980 | – |
| Sex (male/female) | 12/5 | 16/18 | 0.366 | 0.469 [0.135-1.623] |
| Stay before the surgery (days) | 21 [16] | 21 [21] | 0.634 | |
| Underlying diseases (yes/no) | ||||
| Hemorrhage | 5/12 | 12/22 | 0.760 | 0.764 [0.217-2.688] |
| Brain neoplasm | 8/9 | 9/25 | 0.208 | 2.469 [0.729-8.360] |
| Head trauma | 3/14 | 11/23 | 0.334 | 0.448 [0.106-1.889] |
| Hydrocephalus | 1/16 | 2/32 | 1.000 | 1.000 [0.084-11.874] |
| Pure culture/mixed flora | 12/5 | 29/5 | 0.270 | 2.417 [0.590-9.902] |
| Inadequate empiric treatment (yes/no) | 7/10 | 3/31 | 0.010 | 7.230 [1.31-44.77] |
| CSF characteristics | ||||
| Leucocytes | 5139 [10,149] | 1331 [2085] | 0.0001 | – |
| Glucose | 29.84 [17.6] | 32.54 [19.9] | 0.669 | – |
| Proteins | 320 [161] | 285 [167] | 0.878 | – |
| Treatment (yes/no) | ||||
| Carbapenems (yes/no) | 2/15 | 4/30 | 1.000 | 1.000 [0.164-6.092] |
| IV+IT Colistin | 0/17 | 6/28 | 0.067 | Not defined |
| Ceftazidime | 12/5 | 15/19 | 0.068 | 3.040 [0.877-10.544] |
| Combined IV therapy | 1/16 | 2/32 | 1.000 | 1.000 [0.084-11.874] |
| IT treatment | 2/15 | 13/21 | 0.050 | 4.64 [0.80-34.93] |
| AMG IT treatment | 2/15 | 7/27 | 0.699 | 0.514 [0.095-2.797] |
| Catheter removal | 5/12 | 24/10 | 0.005 | 5.76 [1.38-25.62] |
CSF: cerebrospinal fluid; IV: intravenous; IT: intrathecal; AMG: aminoglycoside.
P. aeruginosa meningitis is uncommon and is frequently associated with CSF fistulas or invasive procedures derived from neurosurgical interventions. As in most of nosocomial meningitis, the presence of IVC has been shown to be an important risk factor for the onset of infection by this microorganism.1,12
The lack of clinical expression of meningitis caused by P. aeruginosa can cause it to be masked thus delaying its diagnosis. As previously described in similar studies, the most frequent clinical manifestation in our patient series was fever which was present in all patients studied, followed by other symptoms such as headache or low level of consciousness.13 Interestingly, only 23% out of the patients presented with meningeal irritation signs. The absence of clinical expression may be particularly relevant in critical patients subjected to mechanical ventilation, for whom the presence of clinical signs go unnoticed because of their sedation status. This emphasizes the importance of a prompt and an accurate diagnosis taking control microbiological cultures to each patient with fever of unknown origin carrying an IVC or recently submitted to neurosurgical interventions, in order to establish an early diagnosis.
In addition to diagnosis, an important complexity of the P. aeruginosa meningitis is its therapeutic approach. The emerging and spread of MDR strains, resistant to drugs traditionally used against P. aeruginosa infections, complicates the antimicrobial therapy and is associated with higher morbi-mortality rates.8,14 Ceftazidime has been so far the treatment of choice for infections caused by P. aeruginosa susceptible strains.15 However, mortality in our series was higher in patients treated with ceftazidime, although this did not reach statistical significance. Other antipseudomonal cephalosporins such as cefepime, has been used to treat P. aeruginosa meningitis. Huang et al., compared the clinical efficacy of cefepime administered in prolonged perfusion versus intermittent perfusion for the treatment of 68 patients with nosocomial meningitis, seven of them caused by P. aeruginosa.16 They found a higher bactericidal activity and a length of stay reduction in patients treated with prolonged perfusions. Regarding carbapenems, meropenem is the only drug of the family approved for the treatment of meningitis, due to its less common neurological adverse effects. It is known to be a secure alternative for the treatment of susceptible strains,17,18 however patients treated with this drug presented a mortality rate close to 12% in our series. The establishment of an optimal meropenem dosage for treating meningitis has been the object of numerous studies.3,17 For instance, Lu et al. studied a series of 82 patients receiving meropenem 2g every 8h, 1g every 8h, or 1g every 6h for at least 3 days, concluding that the best curing results were obtained when the 2g were administered every 8h in extended perfusion (4h).17 Other studies had displayed that prolonged 4h meropenem perfusion is associated with clinical improvements of infection, microbiological eradication and less mortality than conventional dosage.18 With respect to other carbapenems, doripenem is more active that meropenem against P. aeruginosa and is capable to cross the blood-brain barrier in the absence of inflammation.19 However, there are only a few reports of doripenem usage to treat meningitis caused by P. aeruginosa leading to a good clinical evolution, but in combination with rifampicin or colistin.20
The emerging of MDR microorganisms have lead to an increase of the intrathecally treatment of meningitis, especially using aminoglycoside plus β-lactamic or colistin intrathecal plus colistin iv combinations.5,6,13,14,21–31 The crossing of the colistin through the blood brain barrier is limited, even in the presence of meningeal inflammation, so the combination of both routes of administration seems mandatory. Nevertheless, the correct dosage is unclear with recommendations ranging from 2.61 to 20mg/day.5,6,24–28 Although the 20mg/day administered in our study is a higher dose than the 10mg/day recommended by the IDSA,15 not local adverse effects were observed in our study in patients receiving this regimen.
Taken all data together, the use of intrathecal route of administration was a factor favoring good evolution in our study according to the multivariable analysis, without differences observed between the patients treated with aminoglycosides versus colistin. To highlight, no patients treated with colistin died. These results are similar to those obtained by Tangden et al., who found the highest survival rates in patients with neurosurgical Gram-negative bacilli ventriculitis treated with aminoglycosides administered intrathecally,23 although only four meningitis by P. aeruginosa were included in their study. Contrary, Shah et al., found a 3-fold increases mortality in children suffering Gram-negative bacilli meningitis treated with intraventricular aminoglycosides compared to those treated with intravenous monotherapy.31 However, the results obtained from last study are not extrapolable to ours, in which most of patients were adults in many cases without other therapy regimens.
The attributable mortality to P. aeruginosa meningitis was high in our study (33%), especially in patients without catheter withdrawal and for which the intrathecal route of administration was not used. This agrees with other studies in which mortalities of P. aeruginosa meningitis range between 20 and 80%.2,14 However, it contrasts with the study of Pai et al., in which not deaths attributable to P. aeruginosa ventriculitis occurred during the study, although a high diversity of the treatments were administered.13 Catheter withdrawal, was an independent factor of good outcome in our series, what supports the IDSA recommendations.15
As far as we know, our work is the broader described series of nosocomial post-surgical meningitis by P. aeruginosa and it shed some light in issues such its therapeutic approach. However, it presents limitations such as it is an observational retrospective study, not randomized, and in which the antimicrobial dosage was not constant since it was prescribed according to different year guidelines. For instance, any patient was treated with loading dose of colistin, as recommended by the currently available guidelines.
While recognizing the limitations of our study, we want to highlight that nosocomial post-surgical meningitis by P. aeruginosa is an entity associated with high morbi-mortality, especially in those patients without intrathecal treatment and without catheter withdrawal. More studies are required to contribute to improve management of patients with this uncommon infection.
FundingThis research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interestsThe authors declare no conflict of interests.
An abstract based on this paper was presented at the ECCMID 2017 on 22 April, Vienna.