Enteroviruses are a type of RNA-strained virus with more than 100 different genotypes. Infection can be asymptomatic, and, if any, symptoms can range from mild to severe. Some patients can develop neurological involvement, such as aseptic meningitis, encephalitis, or even cardiorespiratory failure. However, in children, the risk factors for developing severe neurological involvement are not well understood. The aim of this retrospective study was to analyze some characteristics associated with severe neurological involvement in children hospitalized for neurological disease after enterovirus infection.
Methodsretrospective observational study analyzing clinical, microbiological and radiological data of 174 children hospitalized from 2009 to 2019 in our hospital. Patients were classified according to the World Health Organization case definition for neurological complications in hand, foot and mouth disease.
ResultsOur findings showed that, in children between 6 months old and 2 years of age, the appearance of neurological symptoms within the first 12h from infection onset—especially if associated with skin rash—was a significant risk factor for severe neurological involvement. Detection of enterovirus in cerebrospinal fluid was more likely in patients with aseptic meningitis. By contrast, other biological samples (e.g., feces or nasopharyngeal fluids) were necessary to detect enterovirus in patients with encephalitis. The genotype most commonly associated with the most severe neurological conditions was EV-A71. E-30 was mostly associated with aseptic meningitis.
ConclusionsAwareness of the risk factors associated with worse neurological outcomes could help clinicians to better manage these patients to avoid unnecessary admissions and/or ancillary tests.
Los enterovirus son virus ARN con más de 100 genotipos diferentes. Las infecciones que producen pueden ser asintomáticas y, si producen síntomas, estos varían de leves a graves. Algunos pacientes pueden desarrollar afectación neurológica, como meningitis aséptica, encefalitis o incluso fallo cardiorrespiratorio por lesión del tronco encefálico. Sin embargo, en niños, los factores de riesgo para desarrollar clínica neurológica grave no son bien conocidos. El objetivo de este estudio es analizar las características asociadas a la afectación neurológica grave en niños hospitalizados con infección por enterovirus.
MétodosEstudio retrospectivo observacional que analiza datos clínicos, radiológicos y microbiológicos de 174 niños ingresados desde 2009 a 2019 en nuestro hospital. Los pacientes fueron clasificados según la definición de caso de la OMS para las complicaciones neurológicas de la enfermedad boca mano pie.
ResultadosNuestros resultados muestran que, en niños de 6 meses a 2 años de edad, la aparición de síntomas neurológicos en las primeras 12 horas del inicio de la infección, y especialmente si asocian exantema cutáneo, se asoció a un riesgo estadísticamente significativo para desarrollar formas neurológicas graves. La detección de enterovirus mediante PCR en líquido cefalorraquídeo fue más probable en los niños con meningitis. En cambio, se precisaron estudios en otras muestras biológicas (heces, moco nasofaríngeo) para detectar enterovirus en pacientes con encefalitis. El genotipo que se asoció más frecuentemente con las formas clínicas más graves fue EV-A71. E-30 es el que más frecuentemente se asoció a meningitis.
ConclusionesEl conocimiento de los factores de riesgo asociados con las formas más graves podría ayudar al manejo de estos niños, así como evitar pruebas o ingresos innecesarios.
Enteroviruses (EV) are single-strained RNA viruses that are endemic in most countries. Four different species of EV (A, B, C and D) are known to affect humans, with more than 100 different genotypes.1 These viruses are responsible for common viral infections in children, with most of them being mild or even asymptomatic (90%).2 However, in some cases, infection can lead to more serious conditions with neurological, cardiac, or hepatic involvement. Neurological involvement ranges from mild (e.g., meningitis) to severe (e.g., encephalitis or cardiopulmonary failure), with the latter conditions associated with high morbidity and mortality rates. Given the potential risks, early diagnosis of EV infections in children at high risk of developing the most severe forms is crucial.1,2 Most EV genotypes can cause neurological symptoms; however, some of them appear to have higher affinity for the central nervous system (CNS).1
EVs are detected in the cerebrospinal fluid (CSF) in 50–80% of patients with aseptic meningitis.3–5 The most common isolated genotype in the CSF in patients with meningitis is echovirus 30 (E-30), which has been associated with meningitis and meningoencephalitis outbreaks around the world.6 E-30 was isolated in more than 30% of children diagnosed with meningitis from 2002 to 2012 in East China.7
On the other side, enterovirus A71 (EV-A71) and enterovirus D68 (EV-D68) have been associated to the more severe forms of neurological disease, such as brainstem encephalitis or acute flaccid paralysis.8
Knowing the genotype of the EV could help in decision making; however, EV characterization is not performed in the emergency setting and clinicians should manage patients according to clinical symptoms and other ancillary tests. In some patients, first symptoms allow an early diagnose, but in other children unspecific symptoms are followed by a quick dramatic deterioration and death. Despite the potential severe consequences of EV infection, little is still known about the risk factors for this severe neurological involvement in children. In this context, the objective of the present retrospective study was to evaluate the clinical and ancillary characteristics of patients diagnosed of an EV infection from 2009 to 2019 (before and after the 2016 Spanish EV-A71 outbreak) in a tertiary hospital in Catalonia, in order to identify potential risk factors associated to the development of neurological involvement.
Materials and methodsStudy designThis was a retrospective, observational study of children diagnosed with EV-associated neurological disease at the Hospital Sant Pau in Barcelona (Catalonia), Spain. The study period was from January 2009 to July 2019.
Case definition was the presence of neurological symptoms and a confirmed EV from a biological sample (CSF, feces, nasopharyngeal fluids). Patients with febrile seizures without other neurological involvement were excluded.
In order to classify patients according to the clinical severity, we used the World Health Organization (WHO) case definitions in the clinical guidelines for the management of HFMD9: Aseptic meningitis was defined as a febrile illness with headache, vomiting and meningism associated with presence of more than 5–10 white cells per cubic millimeter (WC/m3) in CSF, and negative results on CSF bacterial culture. Brainstem encephalitis was defined as myoclonus, ataxia, nystagmus, oculomotor palsies, and bulbar palsy in various combinations, with or without abnormalities in neuroimaging. Encephalitis included patients with impaired consciousness, including lethargy, drowsiness or coma, or seizures or myoclonus. Encephalomyelitis was defined as an acute onset of hyporeflexic flaccid muscle weakness with myoclonus, ataxia, nystagmus, oculomotor palsies and bulbar palsy in various combinations. Acute flaccid paralysis included patients with an acute onset of flaccid muscle weakness and lack of reflexes. Finally, Autonomic Nervous System (ANS) dysregulation was defined as the presence of cold sweating, mottled skin, tachycardia, tachypnea, and hypertension.13
Besides, we classified patients in two groups: the milder form (acute meningitis) and the more severe forms (encephalitis, brainstem encephalitis, encephalomyelitis, acute flaccid paralysis and ANS dysregulation) in order to find specific risk factors for the more severe clinical forms.
Assuming younger patients will be the most severely affected, we classified patients in these age groups: less than 3 months old, 3–6 months, 6 months to 2 years old, 2–5 years and more than 5 years of age, to better study differences in children's first years of life.
To confirm EV infection, we performed two types of microbiological studies: To detect the presence of EV in CSF, we performed reverse transcription polymerase chain reaction (RT-PCR) assays (Cepheid®Xpert EV). To detect and isolate EV in nasopharyngeal and feces samples, cell cultures in different lines (MRC-5, A549, HEp2 and RD), were used, in accordance with WHO recommendations for the detection of poliomyelitis. All culture-isolated and all CSF-detected EVs were characterized. The patients’ demographic, clinical, blood tests, microbiological and radiological data were collected.
Statistical analysisDescriptive data are provided. Categorical variables are expressed as number of cases and percentages and quantitative variables as means with interquartile range (p25–p75). For categorical variables, Chi-square contingency tables were prepared. We used likelihood ratio as the inferential test because it can be used regardless of the number of cells. For quantitative variables we used the Student's t-test, without assuming equal variances. No multiple comparison correction was performed as this was an exploratory study to identify risk factors, which could then be used to facilitate future studies. The level of significance was set at 5% (α=0.05). All analyses were performed with the IBM-SPSS (v.26) statistical software package.
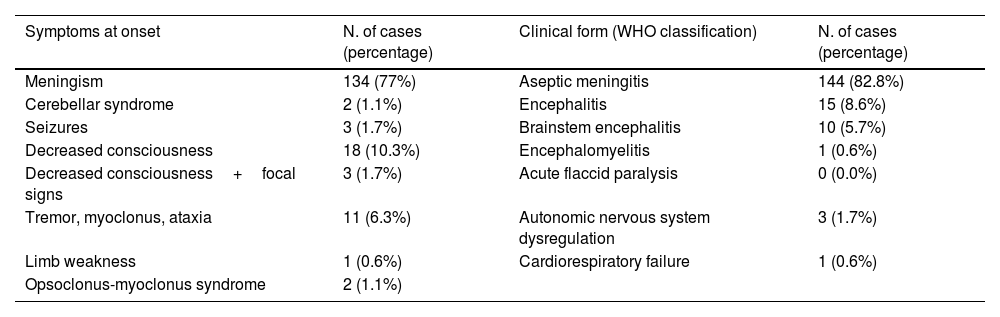
ResultsA total of 174 patients met the case definition during the study period. The mean patient age was 3.1 years (interquartile range: 0.2–5.1 years). Patients aged 2–5 years made up the largest age group (n=57). Most children were healthy prior to infection (>80%). Patients’ characteristics are shown in Table 1.
Patient characteristics.
| Characteristics | Number of patients | Percentage |
|---|---|---|
| Sex | ||
| Male | 119 | 68.4% |
| Female | 55 | 31.6% |
| Age | ||
| <3 months | 44 | 25.3% |
| 3–6 months | 7 | 4.0% |
| ≥6 months to <2 years | 18 | 10.3% |
| ≥2 to <5 years | 57 | 32.8% |
| ≥5 years | 48 | 27.6% |
| Time of first neurological sign after prodromal onset | ||
| 0–12h | 78 | 44.8% |
| 12–24h | 51 | 29.3% |
| 24–72h | 26 | 14.9% |
| 3–7 days | 19 | 10.9% |
| Fever | ||
| Yes | 148 | 85.1% |
| Skin rash | ||
| Yes | 13 | 7.5% |
| Hand foot and mouth disease | ||
| Yes | 4 | 2.3% |
| Herpangina | ||
| Yes | 8 | 4.6% |
| Respiratory symptoms | ||
| Yes | 25 | 14.4% |
| Meningism | ||
| Yes | 134 | 77.0% |
| Seizures | ||
| Yes | 10 | 5.7% |
| Apneas | ||
| Yes | 3 | 1.7% |
| Autonomic nervous system (ANS) dysregulation | ||
| Yes | 5 | 2.9% |
| Admission to Intensive Care Unit | ||
| Yes | 23 | 13.2% |
| Mechanical ventilation | ||
| Yes | 6 | 3.4% |
| Inotropic treatment | ||
| Yes | 4 | 2.3% |
| Rhombencephalitis (neuroimaging) | ||
| Yes | 9 | 5.2% |
| Myelitis (neuroimaging) | ||
| Yes | 6 | 3.4% |
| Pleocytosis | ||
| Yes | 155 | 89.1% |
| Traumatic lumbar puncture | 3 | 1.7% |
| No | 16 | 9.2% |
| Increased transaminases | ||
| Yes | 34 | 19.5% |
| Coagulopathy | ||
| Yes | 10 | 5.7% |
| Increased troponin level | ||
| Yes | 2 | 1.1% |
| Treatment with immunoglobulins | ||
| Yes | 9 | 5.2% |
| Treatment with glucocorticoids | ||
| Yes | 6 | 3.4% |
| Plasma exchange | ||
| Yes | 1 | 0.6% |
| Outcome | ||
| Death | 1 | 0.6% |
| Alive without sequelae | 173 | 99.4% |
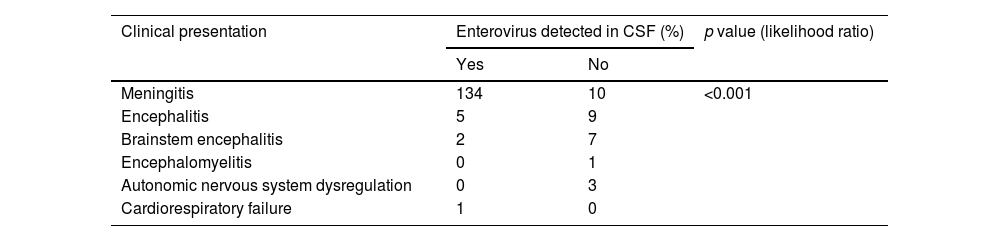
Eighty-five percent of patients presented with fever and 7.5% had a skin rash: 4 (2.3%) showed HFMD and 8 (4.6%) herpangina. Twenty-five patients (14.4%) had respiratory symptoms. In 44.8% of the children (n=78) the first neurological symptoms developed within the first 12hours of illness onset; in 10.9% of children (n=19), the symptoms appeared after three days of disease onset. The most common neurological symptom at onset was headache (n=134; 77%). Patients were then classified according to their clinical signs following the WHO case definition for neurological complications of HFMD (Table 2).
Clinical presentation and classification of patients according to WHO case definition.
| Symptoms at onset | N. of cases (percentage) | Clinical form (WHO classification) | N. of cases (percentage) |
|---|---|---|---|
| Meningism | 134 (77%) | Aseptic meningitis | 144 (82.8%) |
| Cerebellar syndrome | 2 (1.1%) | Encephalitis | 15 (8.6%) |
| Seizures | 3 (1.7%) | Brainstem encephalitis | 10 (5.7%) |
| Decreased consciousness | 18 (10.3%) | Encephalomyelitis | 1 (0.6%) |
| Decreased consciousness+focal signs | 3 (1.7%) | Acute flaccid paralysis | 0 (0.0%) |
| Tremor, myoclonus, ataxia | 11 (6.3%) | Autonomic nervous system dysregulation | 3 (1.7%) |
| Limb weakness | 1 (0.6%) | Cardiorespiratory failure | 1 (0.6%) |
| Opsoclonus-myoclonus syndrome | 2 (1.1%) |
Aseptic meningitis was the most common disease in patients younger than 6 months old, while encephalitis and brainstem encephalitis were the two most common conditions among patients in the 2–5-year age range.
Fifty-eight patients (33.3%), most of whom were diagnosed with aseptic meningitis, did not require hospitalization. The other 136 patients (77.7%) required hospitalization (mean duration: 4.5 days; IQR: 1–5 days). The most common reasons for admission were decreased consciousness, signs of neurological involvement, and age under 3 months. The mean duration of admission was longer in infants younger than 3 months of age (9.6 days; IQR: 3.7–15.4) with respect to other age groups. Twenty-three patients (13.2%) required admission to the pediatric intensive care unit (PICU) due to severe encephalopathy (n=19) or status epilepticus (n=4). Six children required mechanical ventilation and four of these patients received inotropic drugs.
Twenty-eight patients (16.1%) underwent magnetic resonance imaging (MRI), with abnormal findings in 9 cases: rhombencephalitis (9/9) and myelitis (6/9). In the other children, the MRI results were normal.
A total of 306 samples were obtained from CSF (n=172), nasopharyngeal fluids (n=72), and feces (n=62). EV was detected (RT-PCR) in the CSF of 142 children (81.6%), most of whom were diagnosed with aseptic meningitis (n=134). In patients with encephalitis, EVs were detected in CSF only in five cases (33%) and in those with brainstem encephalitis, only in two (20%). RT-PCR in CSF was negative in patients diagnosed with ANS dysregulation or encephalomyelitis; EV infection was later confirmed in these patients through cell cultures in nasopharyngeal fluids or feces (Table 3).
Enterovirus detection in cerebrospinal fluid (CSF) according to clinical presentation.
| Clinical presentation | Enterovirus detected in CSF (%) | p value (likelihood ratio) | |
|---|---|---|---|
| Yes | No | ||
| Meningitis | 134 | 10 | <0.001 |
| Encephalitis | 5 | 9 | |
| Brainstem encephalitis | 2 | 7 | |
| Encephalomyelitis | 0 | 1 | |
| Autonomic nervous system dysregulation | 0 | 3 | |
| Cardiorespiratory failure | 1 | 0 | |
Pleocytosis was detected in the CSF, with a mean value of 192.82 leukocytes/mm3 (IQR: 14–197), mainly neutrophils (44%; IQR: 18–71%) and lymphocytes (40%; IQR: 15–60%). CSF glucose and protein levels were normal in all cases.
In blood samples, 54% patients (n=94) showed leukocytosis (mean value: 12449×10E9/L [IQR: 9410–14770]) and 7% had neutrophilia. High transaminase levels were detected in 34 cases (19.5%).
Patients received symptomatic treatment and, when necessary, supportive treatment, consisting of intravenous immunoglobulin therapy (n=9; 5.2%), corticosteroids (n=6; 3.4%), and/or plasma exchange (n=1; 0.6%).
All but one of the patients survived. Three patients—diagnosed with brainstem encephalitis, encephalitis, and ANS dysregulation, respectively—showed mild action tremor on follow-up. In all three cases, these conditions resolved within a few months. None of the other patients showed neurological symptoms after resolution of the infection. The one death in this series involved a preterm newborn with multisystemic involvement due to echovirus 11 (E-11) infection.
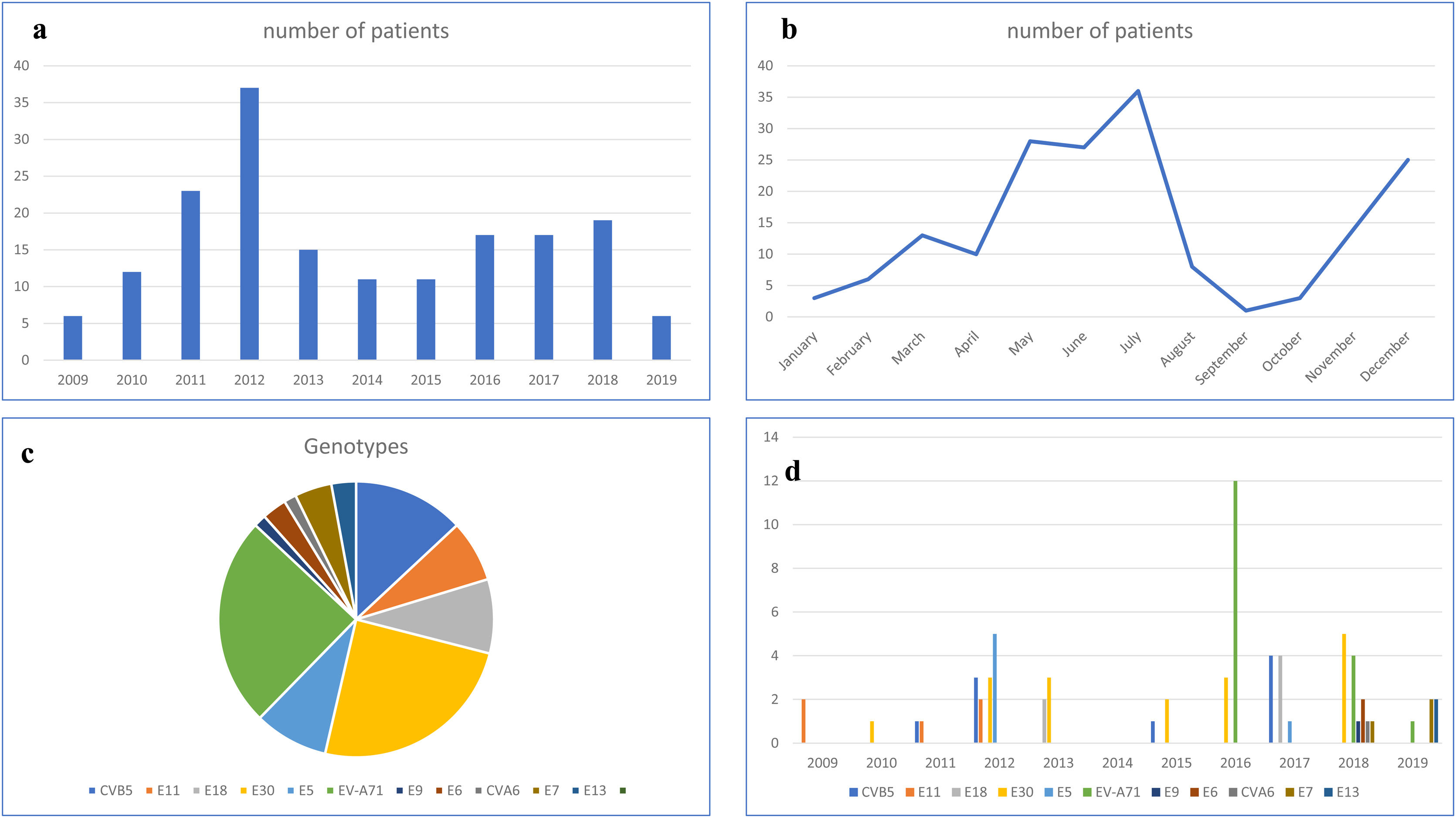
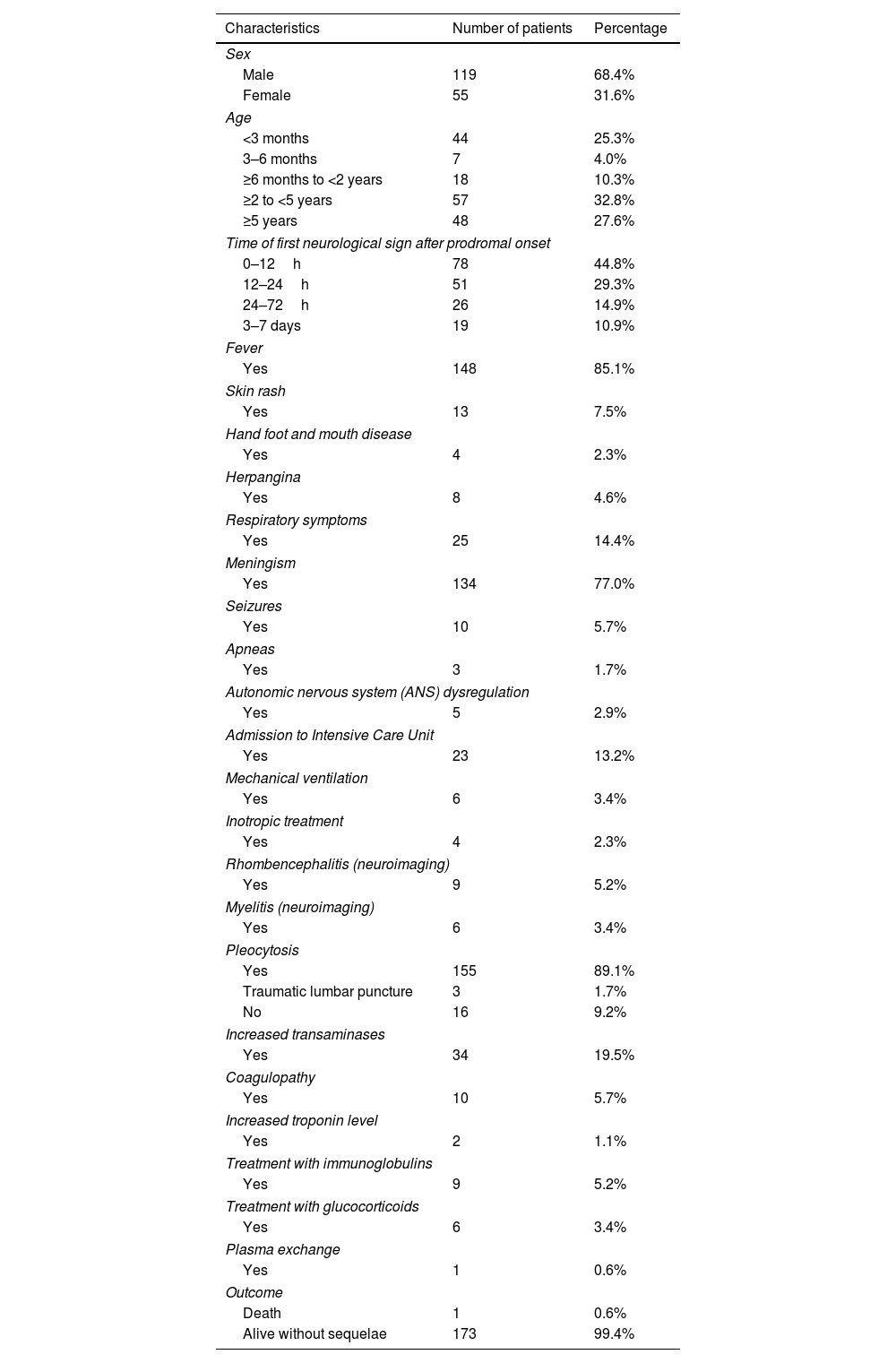
Chronological and temporal distribution of genotypesDuring the study period, a mean of 17 cases were diagnosed annually. The largest number of cases (n=37) was observed in 2012 (Fig. 1a).
In terms of temporal distribution in incidence rates over the year, there were two peaks in the May–July period and November–December period (Fig. 1b).
EV genotyping was obtained in only 69 patients (39.65%), because it could only be performed in children with a positive EV cell culture. Eleven different genotypes were identified. E-30 and EV-A71 (17 patients each, 50%), coxsackievirus B5 (CV-B5) (n=9) and echovirus 18 and 5 (E-18 and E-5; 6 patients each) were the most frequent detected genotypes (Fig. 1c).
EV-A71 was first observed in 2016 (12 cases), disappeared in 2017, and reappeared in 2018 and 2019, but with a much lower incidence. Echovirus 11 (E-11) was detected from 2009 to 2011, but then disappeared. In the last two years of the study period (2018 and 2019), five new genotypes appeared: echovirus 6, 7, 9 and 13 (E-6, E-7, E-9 and E-13) and coxsackievirus A6 (CV-A6). Only two genotypes—E-30 and CV-5—were detected in every year (Fig. 1d).
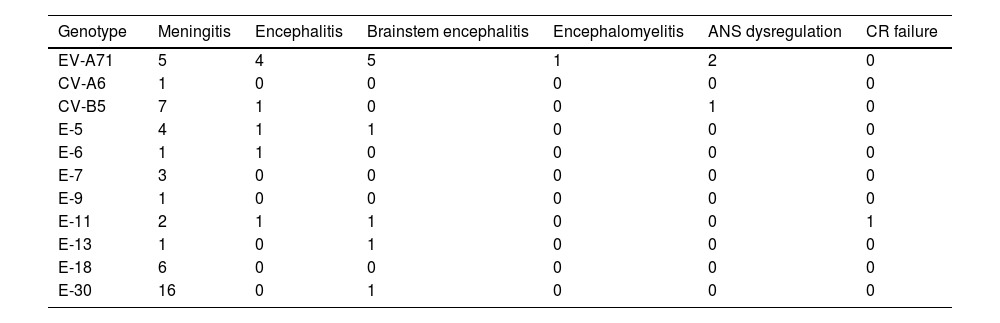
Clinical and analytical characteristics according to EV genotypeWhile all EV genotypes were associated with aseptic meningitis, the most commonly isolated were E-30, CV-B5 and E-18 (Table 4).
Clinical presentation according to genotype.
| Genotype | Meningitis | Encephalitis | Brainstem encephalitis | Encephalomyelitis | ANS dysregulation | CR failure |
|---|---|---|---|---|---|---|
| EV-A71 | 5 | 4 | 5 | 1 | 2 | 0 |
| CV-A6 | 1 | 0 | 0 | 0 | 0 | 0 |
| CV-B5 | 7 | 1 | 0 | 0 | 1 | 0 |
| E-5 | 4 | 1 | 1 | 0 | 0 | 0 |
| E-6 | 1 | 1 | 0 | 0 | 0 | 0 |
| E-7 | 3 | 0 | 0 | 0 | 0 | 0 |
| E-9 | 1 | 0 | 0 | 0 | 0 | 0 |
| E-11 | 2 | 1 | 1 | 0 | 0 | 1 |
| E-13 | 1 | 0 | 1 | 0 | 0 | 0 |
| E-18 | 6 | 0 | 0 | 0 | 0 | 0 |
| E-30 | 16 | 0 | 1 | 0 | 0 | 0 |
In patients with the more severe involvement (encephalitis, brainstem encephalitis, encephalomyelitis, or ANS dysregulation), the most common genotype was EV-A71 (Table 4). Seventeen patients (all aged from 6 months to 2 years) presented with EV-A71 infection; all of these patients (100%) developed fever at onset; 13 (76.4%) had skin lesions (4 were diagnosed of HFMD and 3 of herpangina); and 2 developed seizures. Ten children required admission to the PICU: of these, two required mechanical ventilation and one received inotropic treatment.
Only 3 children had a positive EV RT-PCR in the CSF; in the other 14 patients, the virus was detected in nasopharyngeal fluid or feces. Mean leukocyte counts were higher in patients with the more severe EV-A71 infection (Table 5).
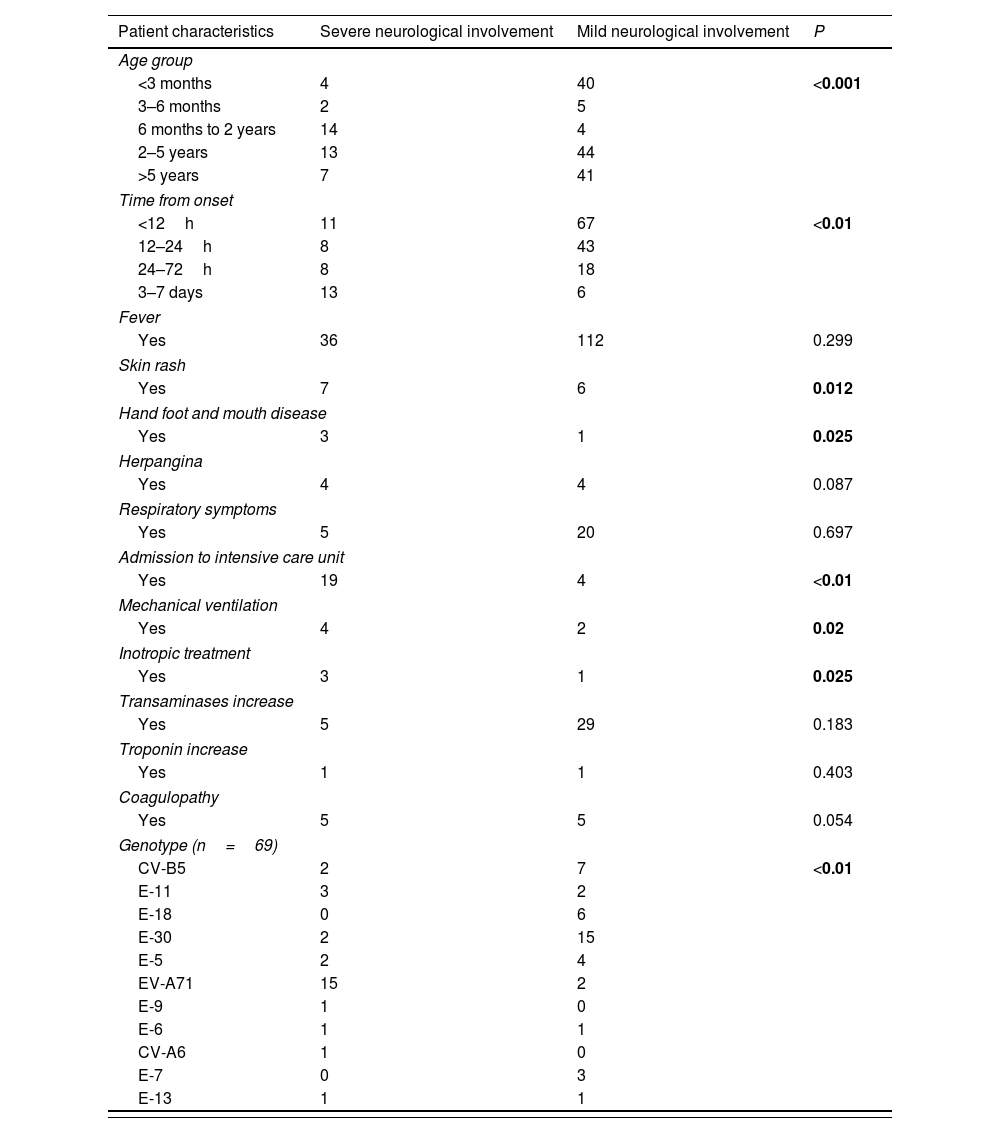
Risk factors for severe neurological involvement.
| Patient characteristics | Severe neurological involvement | Mild neurological involvement | P |
|---|---|---|---|
| Age group | |||
| <3 months | 4 | 40 | <0.001 |
| 3–6 months | 2 | 5 | |
| 6 months to 2 years | 14 | 4 | |
| 2–5 years | 13 | 44 | |
| >5 years | 7 | 41 | |
| Time from onset | |||
| <12h | 11 | 67 | <0.01 |
| 12–24h | 8 | 43 | |
| 24–72h | 8 | 18 | |
| 3–7 days | 13 | 6 | |
| Fever | |||
| Yes | 36 | 112 | 0.299 |
| Skin rash | |||
| Yes | 7 | 6 | 0.012 |
| Hand foot and mouth disease | |||
| Yes | 3 | 1 | 0.025 |
| Herpangina | |||
| Yes | 4 | 4 | 0.087 |
| Respiratory symptoms | |||
| Yes | 5 | 20 | 0.697 |
| Admission to intensive care unit | |||
| Yes | 19 | 4 | <0.01 |
| Mechanical ventilation | |||
| Yes | 4 | 2 | 0.02 |
| Inotropic treatment | |||
| Yes | 3 | 1 | 0.025 |
| Transaminases increase | |||
| Yes | 5 | 29 | 0.183 |
| Troponin increase | |||
| Yes | 1 | 1 | 0.403 |
| Coagulopathy | |||
| Yes | 5 | 5 | 0.054 |
| Genotype (n=69) | |||
| CV-B5 | 2 | 7 | <0.01 |
| E-11 | 3 | 2 | |
| E-18 | 0 | 6 | |
| E-30 | 2 | 15 | |
| E-5 | 2 | 4 | |
| EV-A71 | 15 | 2 | |
| E-9 | 1 | 0 | |
| E-6 | 1 | 1 | |
| CV-A6 | 1 | 0 | |
| E-7 | 0 | 3 | |
| E-13 | 1 | 1 | |
| Leukocytosis and pleocytosis according to neurological involvement | ||
|---|---|---|
| Pleocytosis (/mm3) | ||
| With neurological involvement | 177.68 | 0.784 |
| Without neurological involvement | 197.18 | |
| Lymphocytes in CSF (%) | ||
| With neurological involvement | 33.48 | 0.113 |
| Without neurological involvement | 42.93 | |
| Neutrophils in CSF (%) | ||
| With neurological involvement | 38.58 | 0.231 |
| Without neurological involvement | 46.17 | |
| Leukocytosis (/mm3) | ||
| With neurological involvement | 13,837 | 0.038 |
| Without neurological involvement | 12,035 | |
| Neutrophiles (/mm3) | ||
| With neurological involvement | 9605 | 0.394 |
| Without neurological involvement | 12,626 | |
| Lymphocytes (/mm3) | ||
| With neurological involvement | 5633 | 0.136 |
| Without neurological involvement | 3098 | |
| Leukocytosis and pleocytosis according to EV-A71 genotype or other genotypes | ||
|---|---|---|
| Pleocytosis (/mm3) | ||
| EV-A71 | 199.24 | 0.416 |
| Other EV | 288.30 | |
| Lymphocytes in CSF (%) | ||
| EV-A71 | 32.22 | 0.423 |
| Other EV | 39.24 | |
| Neutrophiles in CSF (%) | ||
| EV-A71 | 39.95 | 0.676 |
| Other EV | 43.88 | |
| Leukocytosis (/mm3) | ||
| EV-A71 | 14,820 | 0.088 |
| Other EV | 12,125 | |
Severe neurological involvement: brainstem encephalitis, encephalitis, encephalomyelitis, ANS dysfunction, acute flaccid paralysis. Mild neurological involvement: acute meningitis.
In this series, the incidence of the E-30 and EV-A71 genotypes were equally common (Table 5). E-30 was more common in children older than 2 years of age. No skin lesions or respiratory symptoms were observed in them, and they all had positive EV RT-PCR in CSF. Pleocytosis was significantly higher in these patients (374cells/camp) compared to children with other genotypes.
The most commonly detected genotype in children under 6 months of age was CV-B5, which was associated to respiratory symptoms in half of them, but not to skin involvement. The mean duration of admission was longer in patients with CV-B5 infection (19 days; IQR: 0.4–37.6 days) respect other EV, probably due to their younger age.
In order to find out risk factors for the most severe neurological disease, univariate analysis was performed. It showed that age between 6 months old and 2 years of age (p<0.001); time from onset to first neurological signs (p<0.001); presence of skin lesions (p=0.012); HFMD (p=0.025); and EV-A71 genotype (p<0.001) were associated to the development of the most severe neurological disease (Table 5).
DiscussionThe main aim of this study was to analyze clinical characteristics associated to severe neurological involvement in children with EV infections. Our main findings were that the children with the highest risk of developing severe neurological involvement associated to EV infection were those 6 months to 2 years of age who develop symptoms in the first 12h from illness onset, especially if unspecific skin rash or HFMD are present.
EV infection is common in children. However, given that some EV infections can have serious consequences, early diagnosis is essential, which is why it is important to determine the clinical signs and genotypes most commonly associated with severe disease. This would allow clinicians to offer patients better treatment while also avoid unnecessary tests and preventing hospitalization.
In Europe, only a few studies have been performed before the year 2015 to evaluate the prevalence of different genotypes in patients with EV infections. A report from Italy based on wastewater samples showed that CV-B5 and E-6 were the predominant genotypes in the 2006–2010 period, and coxsackievirus B4 (CV-B4) after 2010.10 In 2008, a multicenter study in Spain evaluated the EV genotypes in CNS infections, finding that the most prevalent EV genotypes were E-4, E-30, E-9, and E-6.11 Later on, one European study evaluated biological samples obtained in 2015–2017, finding that the most common genotypes changed over time, and showing that the most common EV in 2015 was E-6, while EV-D68 and EV-A71 were more prevalent in 2016, and E-30 and E-18 in 2017.12 It is important to note, however, that the data from that large series included all patients diagnosed with an EV infection, regardless of the clinical presentation. In our series, by contrast, we included only EV infections with neurological involvement, which is why some genotypes may be underrepresented in our series. The most common genotypes in our series by year were E-30 (2015), EV-A71 (2016), and E-18 and CV-B5 (2017).
In contrast, in Asia, due to HFMD outbreaks with neurological involvement, more studies are found from 2010. Between 2010 and 2016, the EV-A71 and coxsackievirus A16 (CV-A16) were the two most common genotypes associated with HFMD in Shanghai, China.13 Although that study reported a clear association between EV-A71 and worse clinical outcomes, the authors did not specify the type of neurological involvement, which makes it difficult to compare those findings to our results. Another Chinese study evaluated patients with HFMD in Sichuan between 2011 and 2017,14 finding that the most prevalent genotype changed over time, from the EV-A71 genotype in the early years to the CV-A16 genotype in the latter years. Until 2013, these were the most common isolated genotypes, but from this year to nowadays, there was an increase of other EV genotypes infection. However, those authors did not describe the type or extent of neurological involvement in patients.
In our series, we divided the patients into five age groups in order to evaluate genotypes and clinical presentation by age. In infants less than six months of age, the most common genotype was CV-B5, which was mainly associated with aseptic meningitis. In patients aged 6 months to 2 years, the most common genotype was EV-A71, which was associated with all types of neurological disease, but not with cardiorespiratory failure. Finally, in children up to 2 years of age, the most common neurological disease was aseptic meningitis and the most common genotype in this age group was E-30. Unlike EV-A71, neither CV-B5 nor E-30 were associated with skin rash or HFMD prior to onset of the neurological symptoms. CV-B5 was associated with respiratory symptoms in 50% of patients.
CV-B5 has been reported as a frequent agent causing CNS involvement in infants and neonates.15,16 Although much less frequent, it can cause HFMD and similar neurological involvement as EV-A71, such as brainstem encephalitis or ANS dysregulation: in a Chinese study in 2018, of 1844 children admitted to hospital with complicated HFMD, in only 8 patients CV-B5 was isolated.17 In our series, all patients but 2 were classified as acute meningitis, and the other 2 were diagnosed of encephalitis and ANS dysregulation, respectively. All our patients were 6 months old or younger.
EV-A71 has been associated with hand, foot, and mouth disease (HFMD) outbreaks in Asia, leading to neurological disease in some of the affected children, and later on, in Europe.12,18,19 In this infection, after a prodromal period with headache, malaise, weakness, and irritability, some children may develop ataxia, myoclonus, or lower cranial nerve palsy, suggesting cerebellar and/or brain stem involvement. It can also be followed by cardiopulmonary failure due to a cytokine storm, that can lead to death or severe sequelae.8 Treatment will depend on the specific neurological disease, and may include immunoglobulins, corticosteroids, and/or plasma exchange in more serious forms.20 On contrast, EVD-68 has been associated to respiratory disease followed by neurological involvement in few patients.21 It may present as encephalitis,22 but most frequently, acute flaccid paralysis.23–25 This EV directly invade the anterior horn gray matter and causes spinal motor neuron injury, probably via retrograde axonal transport of peripheral nerves after a respiratory tract infection.26 The mechanism for neuroinvasion in other EV genotypes is not well understood, but probably implies hematological dissemination from gastrointestinal or respiratory tissues.27
In an outbreak that occurred in Spain (mostly in the region of Catalonia) in 2016, approximately 100 cases of encephalomyelitis and brain stem encephalitis associated with EV-A71 infection were reported.28–30 This genotype was previously detected in other European countries (Germany and France),31 and later (2018) in the United States, although these outbreaks were milder than that in Spain.23 In our series, after the 2016 outbreak, EVA71 was seen in 2018 and 2019, in fewer patients.
Finally, E-30 has been also associated to encephalitis and, more often, to acute meningitis in world-wide outbreaks. It mostly affects children aged 5 years old or more. Main neurological findings in children infected by this EV are headache and meningism, and other neurological symptoms, as cranial nerve palsies, tremor or ataxia are rarely seen.32,33 In our series, this genotype was associated to acute meningitis in the eldest group of children and, only one patient (5.9%) presented with brainstem encephalitis.
Previous studies have shown that EV-A71, unlike other genotypes, is rarely isolated in CSF (in 0–5% of cases).28,34 In our series, this genotype was only detected in a few patients with aseptic meningitis, which is why if an EV infection is suspected in these patients, other biological samples, such as nasopharyngeal fluid or feces, should be sampled to detect the presence of enterovirus. We obtained a positive RT-PCR result in 69% of respiratory samples and in 62.9% of fecal samples. Some studies have found that detection rates are higher in fecal samples (up to 95% in an Australian study) than in nasopharyngeal samples (16% in a European study and 85% in the aforementioned Australian study).12,35
Clearly, if we could identify the patients most likely to develop severe neurological impairment this would help to optimize treatment. To this end, we classified our patients according to the WHO case definitions for neurological disease established in 2011 (Table 2), which permits a management strategy based on symptom severity.36–40 For example, in our series, patients with aseptic meningitis were discharged after a few hours of monitoring in the emergency room; by contrast, patients showing decreased consciousness, and especially those with symptoms suggestive of brainstem encephalitis, were admitted to the ICU for closer monitoring to watch for ANS dysregulation or cardiorespiratory failure.
Once patients were classified according to these case definitions, we evaluated the clinical, radiological, and analytical data to identify the risk factors associated with the most severe neurological involvement. Several variables—age between 6 months and 2 years, time from infection onset, presence of skin rash, HFMD, and the EV-A71 genotype—were significantly associated with it. Other studies have identified other risk factors, including the presence of leukocytosis at admission, age, male sex, and certain genetic risk factors, but only in the context of EV-A71 infection.28 A 2014 metanalysis found that fever higher than 37.5°C lasting more than three days, age, lethargy, hyperglycemia, vomiting, neutrophilia, and the EV-A71 genotype were associated with severe neurological impairment related to HFMD.41
Another important finding of this study is the high prevalence of aseptic meningitis among infants younger than 3 months of age. This is probably due to the fact that lumbar puncture is almost compulsory in infants under 1 month of age presenting with fever without a focus of infection, since it is nearly impossible to detect meningism at this age, and therefore some mildly symptomatic patients can be better diagnosed in this age group than in eldest children.42–44
Study strengths and limitationsThis study has some limitations. Firstly, it is a retrospective study and therefore needs further confirmation with future prospective studies. We included only patients with a microbiological confirmation of EV infection, so patients with a EV infection but with false negative results, or patients in whom no test were performed because of milder symptoms, may have been excluded. Finally, in the genotype study, we could only include 39% of the children, because we could not obtain a positive EV cell culture in the rest of the patients. All results regarding genotype only include this patients’ group. However, in spite of its limitations, we believe that the findings warrant additional research to more clearly define the risk factors associated with enterovirus infections in pediatric populations. Ideally, future studies should include a larger sample size with a multicentric design to confirm the findings of the present study.
ConclusionsIn this retrospective series, several risk factors were associated with neurological involvement in pediatric patients with EV infection. In children between 6 months to 2 years of age who develop neurological signs—associated with a non-specific skin rash or HFMD—within the first 12h from onset, a broad, early microbiological study should be considered. A lumbar puncture should be performed to check for the presence of an EV infection using RT-PCR. Other biological samples (feces or respiratory fluids) should also be collected for EV detection and genotyping. In cases with symptoms suggestive of brainstem encephalitis, cardiorespiratory monitoring is recommended to check for signs of severe neurological involvement such as lower cranial nerve palsy, arrhythmia, or respiratory distress. CSF should be evaluated in infants under 3 months of age who present fever without focus to check for aseptic meningitis, even if clinical signs of meningism are not present.
Author's contributionFC and ETV design the study and draw the initial manuscript. FC, LA and EC collected patients’ demographic and clinical data. MdC, NR collected patients’ microbiological data and reviewed final manuscript. ETV and EM reviewed final manuscript.
Ethics approvalThe present study was approved by the Ethics Board of Hospital Sant Pau.
DeclarationsInformed consent was obtained from the parents of all patients included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
FundingNo funding received for this study.
Conflict of interestsThe authors declare no competing financial interests in relation to this work.