Introduction
This document was contemplated as a practical guideline for determining the level of care, etiologic tests, treatment and the follow-up of immunocompetent adult patients with community-acquired pneumonia (CAP), including both those treated at home and those hospitalized. The recommendations contained herein are not intended to be an extensive review of CAP or an adaptation of the most important guidelines for this disease1-3, to which we direct all readers interested in studying CAP in depth. These guidelines are based on scientific evidence. To refine them, we followed the standards of quality proposed by the Infectious Diseases Society of America4.
Epidemiology of CAP
General morbidity and mortality
Community-acquired pneumonia is a frequent cause of morbidity and mortality within the general population, with an incidence of 2-10 cases per 1000 inhabitants/year, among which 20% to 35% require hospitalization. In immunocompetent patients, CAP-related mortality ranges from 1% to 36.5%, and is commonly cited at around 5%. This considerable mortality range is mainly determined by the form of clinical presentation of the pneumonia, its etiology, and the characteristics of the individual patient. The rate is lower than 1% in outpatients, 2% to 30% in hospitalized patients, and about 30% (20%-54%) in patients who require admission to an intensive care unit.
Risk factors and etiology of CAP
The risk factors for CAP are numerous and well recognized, having been identified in several studies. The incidence of pneumonia increases in patients over 50 years old and is highest in those over 70. Other factors determining a higher incidence of pneumonia include influenza A virus epidemic, alcoholism, asthma, and nursing home residency.
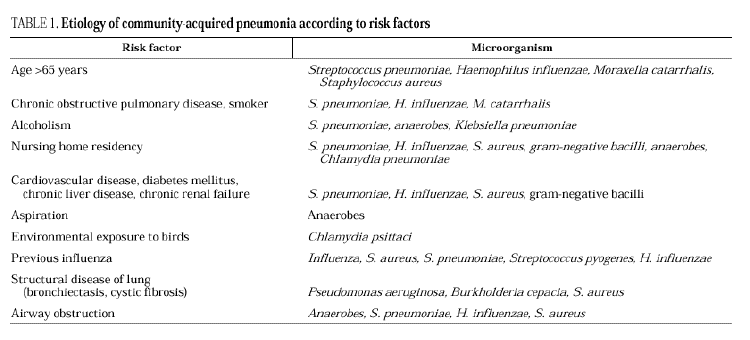
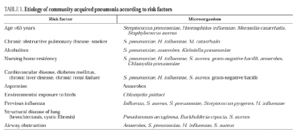
Streptococcus pneumoniae is the most common cause of CAP, at a rate of 9.6% to 48.8% of cases with an etiologic diagnosis. Mycoplasma pneumoniae is one of the primary causes of pneumonia in young patients, mainly those under 20 years old. Chlamydia pneumoniae can affect young patients and adults with underlying diseases, causing 5%-15% of CAP episodes. Chlamydia psittaci and Coxiella burnetii are uncommon causes of CAP, as are viral influenza and respiratory syncytial virus, which can cause pneumonia in adults during the cold months. Legionella pneumophila accounts for 2%-6% of CAP in the majority of series of hospitalized patients. Haemophilus influenzae is an infrequent cause of pneumonia in adults, affecting mainly elderly people and patients with underlying diseases, such as chronic airway obstruction and tobacco use. Moraxella catarrhalis mainly affects patients with underlying bronchopulmonary disease, such as chronic airway obstruction. Staphylococcus aureus is also an uncommon causal agent, accounting for 1.7% of 2145 cases of pneumonia,5 although it is more frequent in patients with severe CAP. Aerobic gram-negative bacilli, such as Klebsiella spp. (1.2% of 2458 cases of CAP) and Escherichia coli (0.8%) are infrequent causes of CAP5. In severe cases, the frequency of enterobacteria is higher, causing 11.8% of the episodes. Pseudomonas aeruginosa is a quite infrequent causal agent, accounting for 0.5% of cases without criteria of severity and with an etiologic diagnosis, and 3.8% of severe cases5.
The frequency of CAP involving anaerobic microorganisms is unknown, though it has been estimated at about 10%. The most common predisposing factor is the aspiration of oropharyngeal secretions during episodes of altered mental status or other circumstances favoring this event, such as dysphagia, intestinal obstruction, periodontal disease, tonsillectomy or dental extractions.
In series of patients with severe community pneumonia, the most frequent agents are S. pneumoniae, followed by Legionella spp. and H. influenzae6 and other gram-negative bacilli, and aspiration pneumonia, whereas in series of cases treated on an outpatient basis, pneumococcus, Chlamydia spp. and M. pneumoniae predominate. The relationships among the various etiologic agents and predisposing factors are summarized in Table 1.
Principal clinical syndromes
Clinical and radiological findings can be very similar in cases of CAP with different etiologies, but attempts at determining the etiology of pneumonia based on the epidemiological and clinical characteristics and complementary findings are worthwhile. Three main types of pneumonia can be established: pneumonia suggestive of pneumococcal etiology, pneumonia suggestive of atypical pathogens, and pneumonia without a clinically suspected pathogen or group of pathogens. A fourth group would include suspected aspiration pneumonia. This division has been criticized by several authors, but it can be useful from the practical point of view, since it allows a theoretical approximation of the etiology of pneumonia and the treatment to follow.
Pneumonia suggestive of pneumococcal etiology
This form is characterized by an acute syndrome of sudden onset with high fever, chills, productive cough with purulent or rusty expectoration, pain having pleural characteristics and, eventually, labial herpes. Signs of pulmonary consolidation (crackles, abnormal breath sounds) may be found on physical exploration and leukocytosis with neutrophilia is usually present. The x-ray often shows a lobar alveolar infiltrate with a bronchogram, affecting several lobes in severe cases. The presence of three of these criteria suggest pneumococcal pneumonia7. Visualization of gram-positive cocci on Gram stain of a representative, purulent sputum sample (>25 polymorphonuclear cells and >10 epithelial cells per field) has diagnostic value.
CAP suggestive of atypical microorganisms
This clinical form is differentiated from the previous one by a subacute course involving fever without chills and few respiratory symptoms, mainly consisting of non-productive cough. Initially, patients often refer to upper respiratory tract symptoms. Extrapulmonary symptoms predominate, mainly consisting of headache, general malaise, diarrhea and vomiting. The physical examination characteristically evidences a clinical-radiological disassociation. Chest x-rays usually show a multilobar interstitial pattern, frequently affecting the lower lobes and sometimes, an alveolar lobar pattern. The principal causative agents in this group of CAP are M. pneumoniae and C. Pneumoniae and less frequently, C. psittaci, C. burnetii and viruses.
Aspiration pneumonia
Aspiration pneumonia usually has a subacute course, though acute or a chronic pneumonia is sometimes seen. Most patients with pneumonitis present fever and cough with significant purulent expectoration, which is putrid in 5% of the cases. Pneumococcal pneumonia might be suspected when the illness begins suddenly; the duration of symptoms is usually longer, however, and an episode of aspiration is often documented. Without treatment, the process evolves to tissue necrosis and abscess formation. Cavitations may be found on chest plain films, depending on the phase of progression of the pneumonia.
Antimicrobial susceptibility of bacteria causing CAP
The most recent data on the patterns of resistance of S. pneumoniae in Spain come from a multicenter study including 1684 strains isolated between 1998 and 1999 in patients with exacerbations of chronic obstructive pulmonary disease and CAP8. The strains were collected from 17 health care areas in 12 regions of the country. It was found that 21.7% of strains were resistant to penicillin (MIC ≥ 2 µg/mL); MIC90 was 2 mg/L and the highest MIC was 4 µg/mL (6.5% of strains). Resistance to amoxicillin and amoxicillin/clavulanate was 5.1% for both treatments; MIC90 was 2 µg/mL and the highest MIC was 16 µg/mL (1.2% and 1.1% of strains, respectively).8 The rate of cefuroxime resistance was 31.4%; MIC90 was 8 mg/L and the highest MIC was 16 mg/L (2% of strains). Cefotaxime resistance was documented at 6.8%; MIC90 was 1 mg/L and highest MIC was 8 mg/L (0.1% of strains).
Nevertheless, penicillin reaches high concentrations in the lower respiratory tract, so it can still be used to treat respiratory infections, including pneumonia, caused by pneumococcal strains with MICs ≤ 2 µg/mL.9 In the case of the betalactams, the time during which plasma concentrations remain above the MIC is the pharmacodynamic parameter that predicts therapeutic outcome. Thus, 500 mg of oral amoxicillin or amoxicillin/clavulanate every 8 hours results in plasma levels above the MIC of S. pneumoniae (MIC=2 µg/mlL), during 41% of the between-dose interval, which has been found to be appropriate for therapeutic effectiveness in animal models and in clinical trials.10
Pneumococcal resistance rates for erythromycin, clarithromycin and azithromycin are 34.7% to 34.9%, with a MIC90 ≥ 64 µg/mL in 23.9-25.5% of the strains.8 This high level of resistance precludes the use of macrolides in cases in which pneumococcal etiology is suspected. Telithromycin shows good antimicrobial activity for pneumococci, with a MIC90 of 0.016-0.06 µg/mL for macrolide-susceptible strains and 0.125-0.5 µg/mL for macrolide non-susceptible strains11-13.
The MIC90 values of levofloxacin and moxifloxacin for pneumococcus are 1 µg/mL14 and 0.12-0.25 µg/mL15 respectively, regardless of the susceptibility to penicillin and erythromycin. Cases of treatment failure with levofloxacin have been described in relation to the isolation of non-susceptible S. pneumoniae strains. In some of these cases, resistance was acquired during pneumonia treatment with levofloxacin16. Mutations of the gyrase and topoisomerase regions, responsible for this resistance, occur more commonly with the use of less potent quinolones (ciprofloxacin and levofloxacin) than with moxifloxacin17.
Considering the high proportion of β-lactamase-producing strains of H. influenzae (29%-55%) and M. catarrhalis (82%)18-20, penicillin should always be associated with a betalactamese inhibitor, such as amoxicillin/clavulanate, in these cases.21 Second- and third-generation cephalosporins are also active against these two pathogens19,20,22.
Erythromycin is only active against 3-15% of H. Influenzae strains22. Azithromycin is four times more active (MIC90 0.5-4 µg/mL) against this microorganism22,23, whereas clarithromycin is less active (MIC90 16 µg/mL)22. The MIC90 of macrolides for M. catarrhalis is 0.06-0.5 µg/mL24. The MIC90 of telithromycin for H. influenzae and M. catarrhalis is 2 µg/mL and 0.12 µg/mL, respectively24-27. Levofloxacin and moxifloxacin show good activity against H. influenzae and M. catarrhalis14,28,29.
Telithromycin, levofloxacin and moxifloxacin are active against atypical pathogens, including L. Pneumophila14,30-32. Anaerobic bacteria have to be taken into account in aspiration pneumonia; amoxicillin/clavulanate and moxifloxacin show good activity against these pathogens32,33.
Prognosis and level of care of patients with CAP
Determining where a patient with CAP will be treated may be the most important decision the physician will have to make over the course of the disease. This choice will determine the exams requested, the antimicrobial therapy administered and the cost of managing the disease.
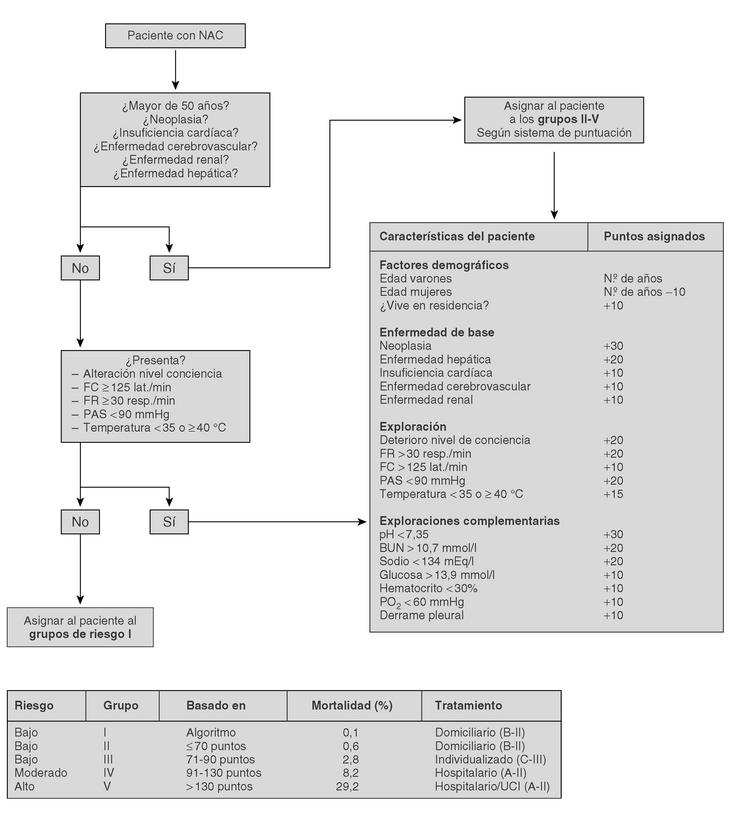
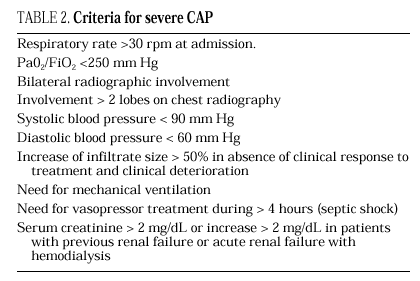
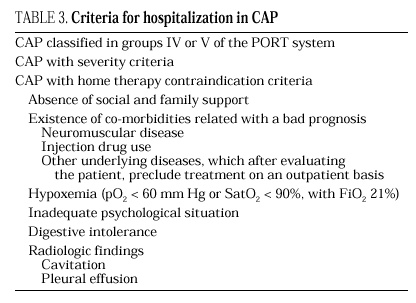
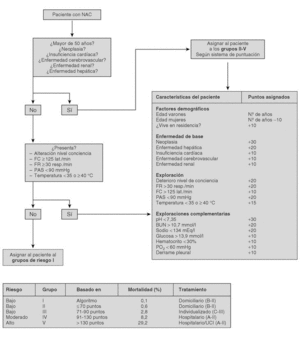
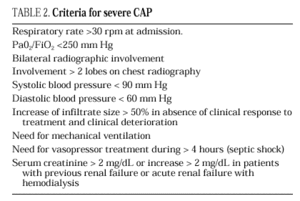
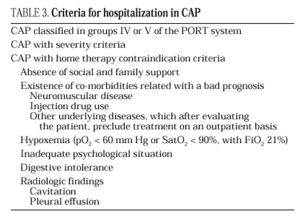
The current standard for evaluating mortality risk in patients with CAP is the Pneumonia Patient Outcome Research Team (PORT) predictive rule developed by Fine et al34 The PORT prognostic system divides patients with CAP into five groups, each with a different risk of mortality, by a process involving two steps (Figure 1). All patients with criteria for severe pneumonia3,35 require hospitalization (Table 2), but the decision to admit a patient with CAP must be made on an individual basis, taking into account social, clinical, and psychological factors (Table 3). Another important aspect in the management of CAP is selection of patients who require intensive care. Briefly, a patient will require admission to the ICU if he needs close observation and ventilatory and/or hemodynamic support.
Figure 1. Prognostic Predictive Model CAP (Fine M. et al. N Engl J Med 1997).
Additional tests according to the level of care
The additional examinations requested depend on the severity of CAP and on the level of care required.
Laboratory tests
Trials that identify the "routine" laboratory tests needed for the management of a patient with CAP are lacking. In patients classified in group I of the PORT system, routine laboratory tests are not considered necessary (C-III).
In CAP patients outside of group I, some additional studies must be performed for their classification by the PORT system (Figure 1); these include, a hemogram, serum electrolyte analysis, and renal function and oxygen saturation testing2,36,37 (A-II). In patients with chronic obstructive lung disease, an arterial blood gasometry study, instead of oxygen saturation, is needed to investigate hypercapnia (B-III).
These determinations should be performed in the emergency area in order to evaluate the severity of pneumonia and the need for hospital admission. There is no evidence of the usefulness of these studies in the routine evaluation of patients without clinical or radiological data indicating severity. Such cases can be managed on an outpatient basis, regardless of where they were first evaluated (B-II).
Microbiology tests
Routine microbiological study is not recommended for outpatients. The following techniques are performed in hospitalized patients:
1. Gram stain and sputum culture. In spite of its limitations in sensitivity and specificity, sputum study is a noninvasive method that is relatively inexpensive and easy to perform and that can provide useful information for the choice of initial treatment. For these reasons, this study is recommended in all hospitalized patients (C-III), preferably before antimicrobial administration.
Other tests, such as Pneumocystis jiroveci or Mycobacterium tuberculosis stains, are required in cases in which clinical or epidemiological data suggest these etiologies.
2. Blood cultures. The performance of blood cultures within 24 hours of admission is associated with a significant reduction in 30-day mortality38. Therefore, this procedure is recommended in all patients hospitalized with CAP (A-I). If it is possible, the procedure should be carried out before beginning antimicrobial treatment.
3. Serologic tests. Serologic tests have an epide miological value in the study of the etiology of CAP. However, since positive results are not obtained until several weeks after the onset of the symptoms, these tests are not recommended for initial patient management (C-III).
4. Legionella pneumophila urinary antigen. This test is recommended in all CAP cases requiring hospital admission and in patients that fail to respond to initial treatment with betalactams (A-II). In CAP outpatients, the test is done when there is a suspected Legionella outbreak.
5. Thoracentesis. Thoracentesis must be performed in all patients with pleural effusion measuring >1 cm on a lateral decubitus radiograph39 or when it is considered significant in imaging techniques.
6. Invasive methods. We do not recommend carrying out invasive methods (fiberbronchoscopy or transthoracic pulmonary aspiration) in the routine management of patients with CAP (B-II). These procedures should be reserved for cases with a fulminant course and those that do not respond to treatment.
Antimicrobial treatment of CAP
General Considerations
Empirical treatment
Treatment for CAP is empirical in most cases and should be selected according to the knowledge obtained in clinical trials, and in microbiological, pharmacokinetic/ pharmacodynamic, and experimental studies. Empirical treatment should conform to a series of general principles that will help us to obtain the best outcome for the individual patient while minimizing the development of bacterial resistance40-46:
Antimicrobial agents should only be used for bacterial infections
Diagnostic tests and other measures should be used to reduce the prescription
Antimicrobial treatment should achieve bacterial eradication
The pharmacokinetic/pharmacodynamic characteristics of the antimicrobial should be optimum to completely eradicate the bacteria.
The local resistance data to antimicrobial agents should be considered when deciding upon an agent.
The least expensive antimicrobial agent should be used when the above-mentioned characteristics are equal.
Recommendations for antimicrobial treatment of CAP in adult patients treated at home
After analyzing all the variables mentioned previously and considering the results of numerous controlled clinical trials in which the effectiveness of older antibiotics is shown to be equivalent to that of new agents, the recommendations for outpatient CAP treatment in adults may be structured as follows7,40,41,44,45,47-50:
1. Pneumonia suggestive of pneumococcal etiology in young patients without underlying diseases:
Oral amoxicillin 1 g tid, for 10 days (A-I).
In cases of allergy to betalactams or therapeutic failure: oral moxifloxacin (400 mg q24h) or oral levofloxacin (500 mg q24h) for 7 to 10 days.
2. Pneumonia suggestive of pneumococcal etiology in patients over 65 years old or those or with chronic underlying diseases. In these cases, H. influenzae should be suspected in addition to pneumococcus, particularly in patients with chronic bronchitis:
Oral amoxicillin/clavulanate 875 mg/125 mg tid for 10 days (A-I).
In cases of allergy to betalactams or therapeutic failure: oral moxifloxacin (400 mg q24h) or oral levofloxacin (500 mg q24h) for 7 to 10 days.
3. Pneumonia suggestive of atypical pathogens. Any macrolide can be recommended, using the most appropriate according to the characteristics of the patient (gastrointestinal tolerance, drug interactions, etc.) (A-II):
Erythromycin (500 mg/qid), clarithromycin (250 mg/bid), roxithromycin (150 mg/bid), or azithromycin (500 mg/q24h), all by oral route for 14 days except azithromycin (3 days).
If the epidemiologic context suggests Coxiella burnetii or Chlamydia spp., the treatment of choice would be oral doxycycline, 100 mg/bid, for 14 days.
4. When there is no etiological suspicion, an agent active against pneumococcus should be used. In this case the antimicrobials recommended include:
Amoxicillin (1 g tid) plus a macrolide, both orally, 10 days.
Moxifloxacin (400 mg q24h) or levofloxacin (400 mg q24h), both orally, for 7 to 10 days.
Telithromycin, 800 mg q24h, for 7 to 10 days.
Recomendations for antimicrobial treatment in hospitalized adult patients with CAP
Antimicrobial treatment must be initiated in the first 8 hours following diagnosis, since a delay in its administration is related to increased 30-day mortality and longer hospital stay (A-II)51,52.
The length of the treatment will depend on the patient's response. In general, pneumonia caused by pyogenic bacteria can be treated for 7-10 days, whereas pneumonia caused by M. pneumoniae, C. pneumoniae and L. pneumophila should be treated from 10 to 14 days. In necrotizing pneumonia, it is advisable to continue treatment for at least 3 weeks (C-III):
1. Pneumonia without criteria of severity. The most probable etiology for these cases is S. pneumoniae, H. influenzae, S. aureus, or enteric gram-negative bacilli:
Intravenous amoxicillin/clavulanate (1000 mg/200 mg tid) or intramuscular or intravenous ceftriaxone (1 g q24h) (A-I) until the patient is afebrile. At this time the patient can be treated orally with amoxicillin/clavulanate (875/125 mg tid) to complete 10 days (A-II).53,54 Betalactams with a wider antimicrobial spectrum (piperacillin/tazobactam, cefepime, carbapenems) should only be used in suspected P. aeruginosa infection (structural airway anomalies, bronchiectasis, cystic fibrosis).
In cases of intolerance or allergy to β-lactams: oral moxifloxacin (400 mg q24h), or oral or intravenous levofloxacin (500 mg q24h) can be used (A-I)55. Considering their high bioavailability56,57, quinolones can be administered orally when there is no gastrointestinal intolerance or hemodynamic instability (B-II)58,59.
Based on retrospective studies in which a reduction in mortality has been proven60, some guidelines1,3 recommend the use of macrolides in combination with β-lactams in all hospitalized patients (B-II). In our opinion, the retrospective character of these studies, the fact that the etiology of the cases was sometimes not included, the possible existence of selection bias, and the absence of data regarding the administration route of the drug and the length of treatment preclude the systematic use of this recommendation in non-severe pneumonia.
2. Pneumonia with criteria of severity. Treatment must be active against S. pneumoniae, L. pneumophila, H. influenzae, S. aureus and enteric Gram-negative bacilli6,61:
Ceftriaxone (1 g q24h) plus levofloxacin (500 mg q24h), both intravenously. An alternative would be to use an intravenous macrolide instead of the fluoroquinolone (B-III).
In cases of risk of infection by P. aeruginosa (structural airway anomalies, bronchiectasis, cystic fibrosis or previous antimicrobial treatment), treatment should consist of cefepime (2 g tid) plus ciprofloxacin (400 mg bid), both intravenously (B-II).
In case of betalactams allergy: intravenous levofloxacin (500 mg bid) (C-III), adding aztreonam (2 g tid) plus amikacin (15 mg/kg q24h), both intravenously, when P. Aeruginosa is suspected.
3. Pneumonia with aspiration criteria:
Intravenous amoxicillin/clavulanate (2000 mg/200 mg tid) plus intravenous levofloxacin when there are pneumonia criteria of severity (500 mg q24h), (B-III) 62.
Follow-up of patients with CAP
Follow-up of outpatients. Favorable response to treatment, considered to be the absence of high fever and stabilization of the symptoms and clinical signs, as well as any adverse effects, will be assessed 48-72 hours after starting antimicrobial therapy44,63,64. When the response is not favorable, the patient should be sent to his referral hospital to be evaluated the same day. A clinical control at the end of treatment is required to assess the clinical cure and adherence to treatment (B-III). If there is a favorable response, a chest x-ray should be repeated within 30 days. In cases without complete radiological resolution at this time, another x-ray should be repeated a month later. If the anomalous findings persist, additional studies will be needed to identify other possible airway pathologies.
Follow-up of patients who need initial hospitalization. In patients with favorable clinical evolution, an x-ray will be performed upon discharge or at the end of treatment and at 30 days. Resolution of the infiltrate may be slower than in non-severe CAP, in which case the control will be repeated periodically until resolution is complete (B-III). Abnormal laboratory parameters will be repeated according to the clinical evolution of the patient. In most cases the course is satisfactory in 3 to 7 days48.
When evolution is not satisfactory, (persistent fever, respiratory failure, hemodynamic instability) the patient must be reevaluated clinically, new radiological techniques (chest radiography and/or computerized tomography) should be applied, and after assessing the available microbiological results, fiberbronchoscopy and/or transthoracic pulmonary aspiration should be considered. In these cases, the new antimicrobial treatment should be individualized.
Change from parenteral to oral treatment and criteria for discharge. Patients who are initially treated intravenously do not need to continue with this route over the entire treatment. Once clinical stability is obtained, worsening of the illness is not common65,66. The symptoms and clinical signs indicating clinical stability include66 temperature ≤ 37ºC, systolic blood pressure ≥90 mm Hg, pulse ≤ 100 bpm, respiratory rate ≤24 rpm, oxygen saturation ≥ 90%, oral intake ability and recovery of the level of consciousness. At this time, the change to oral treatment shows an efficacy comparable to the continuation of parenteral treatment, and the patient can be discharged65.