Ceftolozane/tazobactam is a cephalosporin indicated for the treatment of intra-abdominal and urinary tract infections caused by Enterobacteriaceae, non-fermenting Gram-negative bacilli (except Stenotrophomonas maltophilia or Acinetobacter spp.) and Pseudomonas aeruginosa (PAE). The most prevalent adverse effects (1–10%) are thrombocytosis, hypokalaemia, hypotension, insomnia, anxiety, headache, dizziness, rash, pyrexia, infusion site reactions, elevation of transaminases and gastrointestinal disorders.1
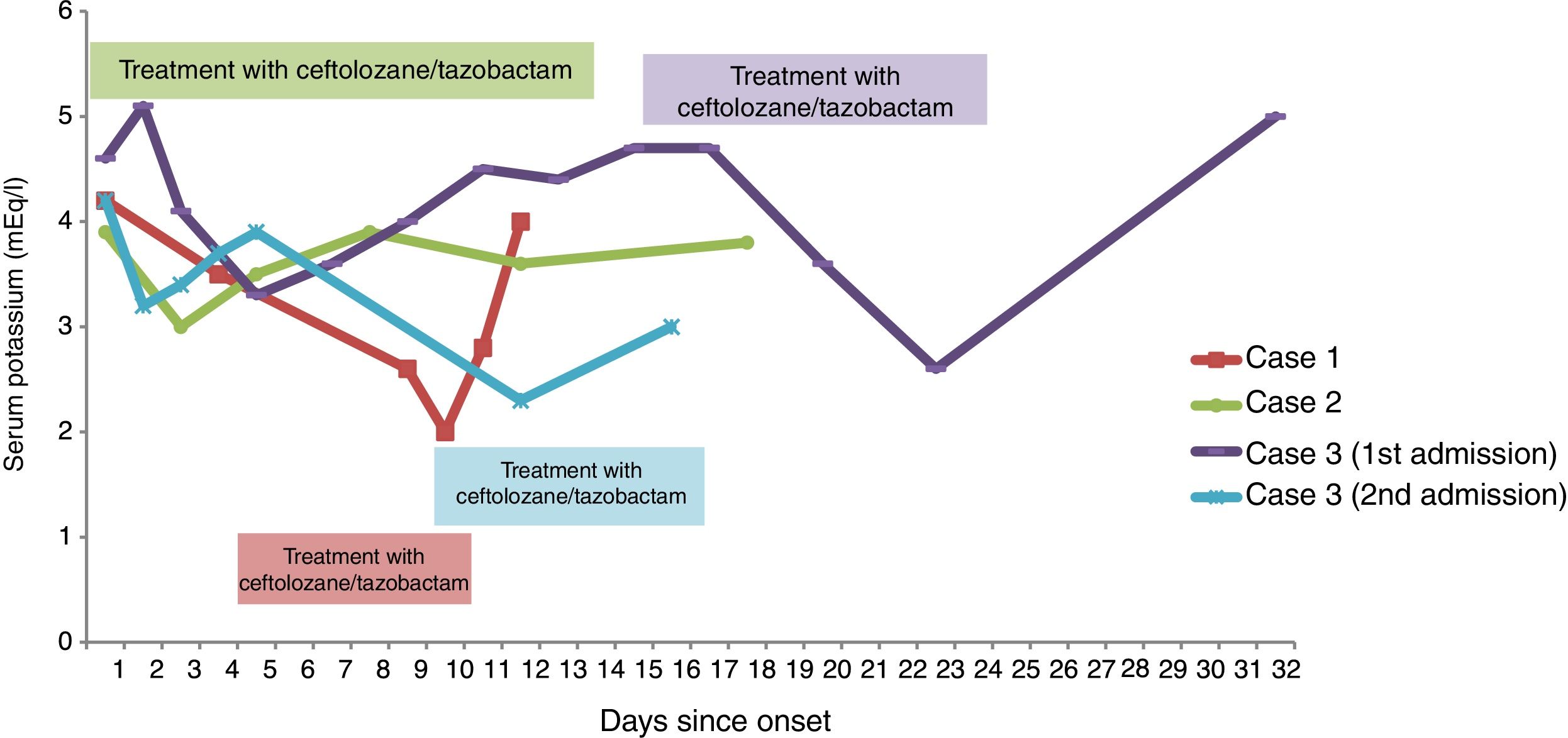
ResultsCase 1This was a 75-year-old woman who was admitted with septic shock secondary to obstructive cholangitis. She spent 26 days in hospital and had a percutaneous transparietal-hepatic biliary drain inserted. She was readmitted 24h after discharge with pyrexia and increased drain output. She was started on treatment with omeprazole, phytonadione, paracetamol, pancreatic enzymes, ciprofloxacin, linezolid and fluconazole. An extremely drug-resistant (XDR) PAE sensitive to ceftolozane/tazobactam, colistin and amikacin was isolated in the patient's bile fluid culture. On day +5, the antimicrobial treatment was adjusted according to cultures: amikacin, ceftolozane/tazobactam, linezolid and fluconazole. At the start of the treatment, her serum potassium (SP) was 3.5mEq/l.
On day +9, she developed moderate hypokalaemia (K=2.6mEq/l) and treatment was started with oral potassium (25mEq/12h). On day +10, her SP was 2mEq/l and the supplements were increased (25mEq/8h orally and intravenous potassium chloride 80mEq/day). During this period there were no changes to the patient's pharmaceutical therapy, she was tolerating diet and the biliary drain had been removed (day +4). An adverse event (AE) related to ceftolozane/tazobactam was suspected and it was changed to colistin. On day +12 her SP was 4mEq/l.
Case 2This was a 68-year-old man admitted for a purulent abscess in a graft on the right lower leg which required debridement and antibiotic therapy to cover the bacteria isolated in the latest cultures: ceftolozane/tazobactam (XDR PAE in rectal swab) and amikacin, vancomycin and metronidazole for Acinetobacter baumannii, methicillin-resistant Staphylococcus aureus and Bacteroides fragilis respectively. He was also on treatment with omeprazole, ondansetron, enoxaparin, paracetamol, dexketoprofen and lorazepam. His SP on admission was 3.9mEq/l and by 12h later, he was tolerating diet. After 32h of treatment with ceftolozane/tazobactam, the patient was found to have mild hypokalaemia (K=3mEq/l) and treatment was started with oral potassium (25mEq/12h). He was kept on the same antibiotics for 13 days and the hypokalaemia resolved 48h after starting the supplement.
Case 3This was a 69-year-old woman diagnosed with bladder perforation due to radiation cystitis who required radical cystectomy. After six days, she was started on treatment with colistin and amikacin because of an XDR PAE in urine culture. On day +16, she was found to have renal deterioration (GFR-CKD-EPI 28ml/min) and the colistin was changed to ceftolozane/tazobactam after verifying its sensitivity.
The prescribed treatment when starting on ceftolozane/tazobactam was: omeprazole, ondansetron, enoxaparin, dexpanthenol, paracetamol, metamizole and dexketoprofen. At the beginning of the treatment with ceftolozane/tazobactam, the patient's SP was 4.7mEq/l and at seven days she developed moderate hypokalaemia (K=2.6mEq/l). The antibiotics were discontinued after 14 days of treatment and she was discharged.
Two weeks later, she was readmitted with pyrexia and low back pain and treatment was restarted with colistin and amikacin, changing the colistin to ceftolozane/tazobactam due to nephrotoxicity on day +10. On day +12, the patient was again found to have moderate hypokalaemia (K=2.3mEq/l) and was started on 25mEq/12h of oral potassium. On day +16 the treatment was completed with her potassium level at 3mEq/l.
In both admissions her potassium returned to normal a few days after finishing the treatment.
The changes in the potassium levels of all three cases are shown in Fig. 1.
DiscussionWe have found in our clinical practice that the incidence of hypokalaemia caused by treatment with ceftolozane/tazobactam may be higher than described in clinical trials (<3%).2,3 In our hospital, three of the 10 patients treated with ceftolozane developed hypokalaemia.
For the other drugs prescribed, hypokalaemia is only described in the summary of product characteristics for linezolid (1–10%) and fluconazole (0.01–0.1%), both prescribed in the first patient. That patient was on treatment with linezolid and fluconazole for almost four weeks without developing hypokalaemia.
Applying the Naranjo algorithm, levels of imputability for PROBABLE were obtained for the first and second cases and DEFINITE for the third. All three cases were reported to the Spanish Pharmacovigilance System.
Based on our experience, we consider it strongly advisable to monitor SP in patients treated with ceftolozane/tazobactam.
Please cite this article as: Toro Blanch C, Gratacós Santanach L, Díez Vallejo C, Sacrest Güell R. Probable hipocalemia secundaria al tratamiento con ceftolozano/tazobactam: a propósito de 3 casos. Enferm Infecc Microbiol Clin. 2019;37:483–484.