A group of experts from the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC) and the Spanish Society of Medical Oncology (SEOM) have reviewed in this paper the main aspects to be considered in the evaluation of patients with solid cancer and infectious diseases. They have established a series of recommendations on the prevention of the most prevalent infections in these patients, the use of vaccines, the control measures of vascular catheter infection and prevention of infections before certain surgical procedures. Also the criteria for management of febrile neutropenia and the use of colony-stimulating factors were revised. Finally they provide a series of recommendations for the treatment of cancer patients with severe infection. The document is completed with a series of measures for the control of hospital infection.
Un grupo de expertos de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) y de la Sociedad Española de Oncología Médica (SEOM) han revisado en este documento los principales aspectos que deben considerarse en la evaluación de los pacientes con cáncer sólido y complicaciones infecciosas. Para ello se han establecido unas recomendaciones sobre la profilaxis de las infecciones más prevalentes en estos pacientes, el uso de vacunas, las medidas de control de la infección por catéteres vasculares y la prevención de la infección ante determinadas maniobras quirúrgicas. A continuación, se han revisado los criterios de manejo de la neutropenia febril y del uso de factores estimulantes de colonias, para terminar dando una serie de pautas sobre el tratamiento del paciente oncológico con infección grave. El documento se completa con una serie de medidas para el control de la infección hospitalaria.
Over the last two decades, notable advances have been made as regards the treatment of cancer patients. Undoubtedly, one of the most noteworthy has been the reduction in morbidity and mortality due to infectious complications, as a result of the progress achieved in preventing and treating these infections, as well as a decrease in the duration of neutropenia thanks to the use of haematopoietic growth factors.
Despite these advances, infectious complications continue to be one of the main causes of death in cancer patients. These patients are at a greater risk of certain infections being reactivated and also have an increased risk of suffering nosocomial infections as a result of surgery, the use of venous or urinary catheters and other devices, as well as the procedures that they undergo. The emergence of multidrug-resistant microorganisms in recent years has made an antibiotic approach difficult in these patients. Moreover, the increasingly frequent use of new monoclonal antibodies and biological therapies has increased the risk of these patients suffering a number of severe infections.
Although there are various clinical guidelines intended for haematological patients, there are few that specifically address those with a solid tumour. As such, experts from the Spanish Society of Infectious Diseases (SEIMC) and the Spanish Society of Medical Oncology (SEOM) have decided to produce this document, which reviews existing information on the topic and makes a series of recommendations based on the best available evidence, for use by oncologists and infectious diseases specialists in daily clinical practice.
Initial cancer patient assessmentThe initial assessment of the cancer patient seeks to detect active or latent infections that are at risk of reactivation in those with a solid cancer who are due to receive a potentially immunosuppressive treatment.
The clinical assessment should include: (1) previous history of infectious diseases that may have remained latent and be reactivated in the event of immunosuppression; (2) complete epidemiological history, including contact with patients suffering from an infectious disease, as well as with other immunocompromised patients; (3) patient origin and stays in or trips to foreign countries with endemic diseases that could be potentially reactivated; and (4) history of any drug reactions to antibiotics. In women, a gynaecological examination should also be advised, along with human papillomavirus (HPV) screening.
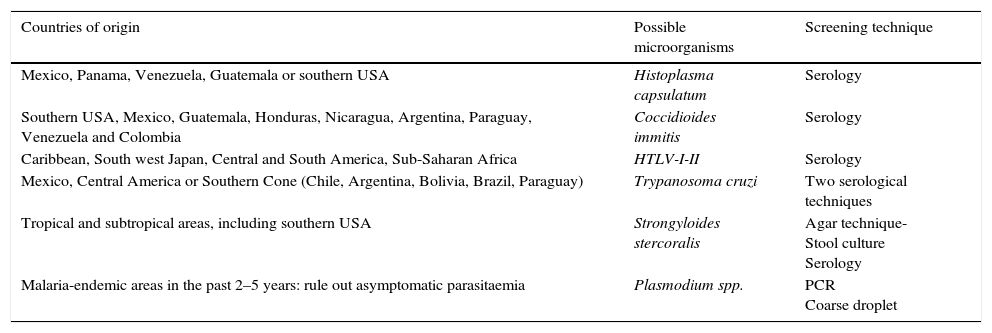
The initial microbiological assessment is intended to screen for the main chronic or latent infections that may be reactivated in the event of the patient's immunosuppression, and will depend on the type of chemotherapy treatment received as well as the specific immunosuppression risk in each cancer patient. In indicated cases, according to the treatment and immunosuppression risk, serological testing for the following viruses would be useful: (1) hepatitis A, B and C (HAV, HBV and HCV); (2) varicella zoster virus (VZV); and (3) human immunodeficiency virus (HIV). Moreover, the existence of latent tuberculosis (TB) should be ruled out by means of a Mantoux test and/or an interferon gamma release assay (IGRA) in all patients with a suspected history of the disease, those in contact with affected patients or in high-risk populations such as institutionalised patients. In patients from certain geographical areas, a number of regional diseases should be taken into account (Table 1).
Regional or imported diseases according to geographical area of origin.
| Countries of origin | Possible microorganisms | Screening technique |
|---|---|---|
| Mexico, Panama, Venezuela, Guatemala or southern USA | Histoplasma capsulatum | Serology |
| Southern USA, Mexico, Guatemala, Honduras, Nicaragua, Argentina, Paraguay, Venezuela and Colombia | Coccidioides immitis | Serology |
| Caribbean, South west Japan, Central and South America, Sub-Saharan Africa | HTLV-I-II | Serology |
| Mexico, Central America or Southern Cone (Chile, Argentina, Bolivia, Brazil, Paraguay) | Trypanosoma cruzi | Two serological techniques |
| Tropical and subtropical areas, including southern USA | Strongyloides stercoralis | Agar technique- Stool culture Serology |
| Malaria-endemic areas in the past 2–5 years: rule out asymptomatic parasitaemia | Plasmodium spp. | PCR Coarse droplet |
HTLV-I-II, human T-lymphotropic virus, types I and II; PCR, polymerase chain reaction.
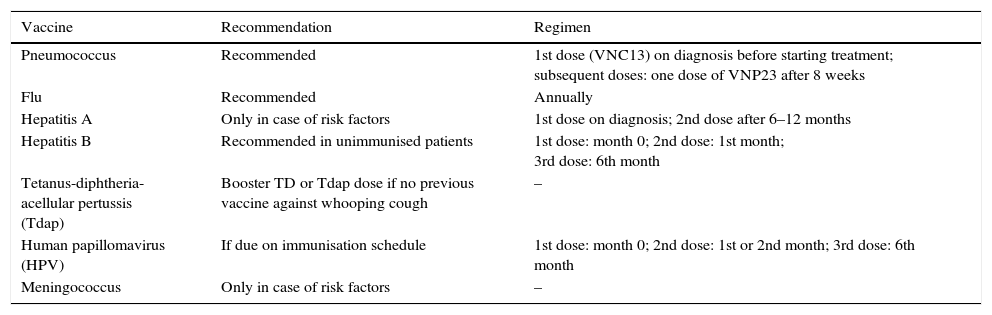
The vaccines indicated in solid cancer patients are described in Table 2 and should be inactivated vaccines.1 Vaccines with live attenuated microorganisms such as rotavirus, MMR (measles, mumps and rubella) and varicella are contraindicated during chemotherapy.2
Recommended vaccines in adults with solid tumours.
| Vaccine | Recommendation | Regimen |
|---|---|---|
| Pneumococcus | Recommended | 1st dose (VNC13) on diagnosis before starting treatment; subsequent doses: one dose of VNP23 after 8 weeks |
| Flu | Recommended | Annually |
| Hepatitis A | Only in case of risk factors | 1st dose on diagnosis; 2nd dose after 6–12 months |
| Hepatitis B | Recommended in unimmunised patients | 1st dose: month 0; 2nd dose: 1st month; 3rd dose: 6th month |
| Tetanus-diphtheria-acellular pertussis (Tdap) | Booster TD or Tdap dose if no previous vaccine against whooping cough | – |
| Human papillomavirus (HPV) | If due on immunisation schedule | 1st dose: month 0; 2nd dose: 1st or 2nd month; 3rd dose: 6th month |
| Meningococcus | Only in case of risk factors | – |
Patients with active solid tumours and those undergoing treatment with chemotherapy should receive an annual flu vaccine.2 It is recommended that patients be vaccinated against pneumococcus according to the guidelines established for immunocompromised patients.
Depending on the aforementioned characteristics (type and duration of chemotherapy, clinical situation of the patient), a tetanus-diphtheria booster dose is advisable. Patients who have not been vaccinated against whooping cough are recommended to have the tetanus-diphtheria-pertussis vaccine (Tdap). Likewise, vaccination against HPV, meningococcus and HAV should be considered provided there is a specific indication. The administration of the HBV vaccine should be considered in unimmunised patients after evaluating their serological and clinical situation.
Patients should receive the indicated vaccines before beginning chemotherapy. As for inactivated vaccines, their administration is recommended at least two weeks before the start of treatment (except the flu vaccine, which will be administered annually, even during the chemotherapy regimen), while live attenuated vaccines should be given at least four weeks prior to commencing treatment.3
Preventing hepatitis BHBV screening is especially important in patients who are deemed high-risk (e.g. those treated with everolimus, temozolomide, rituximab, etc.) and should be considered, according to medical judgement, in other patients. Screening will be carried out by detecting the surface antigen (HBsAg), the hepatitis B core antibody (anti-HBc) and the hepatitis B surface antibody (anti-HBs). If they all come back negative, there is no infection and the patient should be vaccinated before beginning immunosuppressive therapy. If a patient tests positive for HbsAg, the study should be completed with the determination of the viral load, e antigen (HBeAg), liver function tests and a liver biopsy, if applicable. Based on the results, it can be determined whether the patient has chronic hepatitis, is in the immunotolerant phase or is an inactive carrier of HBV. In case of chronic hepatitis, the patient should receive antiviral treatment with entecavir or tenofovir. In the other two cases, the patient should receive antiviral prophylaxis.
If HBsAg is negative and anti-HBc positive, this indicates resolved hepatitis B. In this case, regardless of the anti-HBs result, viral deoxyribonucleic acid (DNA) levels should be determined. If the viral load is positive, the infection is occult and the patient should therefore receive prophylaxis. If the viral load is negative, the possibility of reactivation should be checked periodically throughout the immunosuppressive treatment for early detection and in order to initiate treatment as soon as possible. This monitoring will be performed by determining the liver biochemistry, HbsAg and/or viral load. In high-risk patients, most authors feel a prophylaxis regimen should be instituted immediately.4,5 For patients with no HBV risk factors, cancer treatment is not expected to activate the risk of disease; current evidence does not support HBV detection before starting anti-cancer treatment.6
Preventing tuberculosisOnce active disease has been ruled out, all patients who meet one or more of the following criteria should receive prophylaxis against TB4–10: (1) positive purified protein derivative (PPD) skin test (≥5mm); (2) positive IGRA test; (3) history of incorrectly-treated TB; (4) radiological findings suggestive of residual TB lesions, such as apical fibronodular lesions, pleural thickening, etc.; or (5) contact with a patient with active TB. The routine regimens are to be used, with the known precautions.
Preventing central venous catheter infectionAt present, there is not enough evidence to recommend a specific type of long-term central venous catheter (CVC)–either a tunnelled CVC (Hickman), “port-a-cath” (PAC) or a peripherally inserted CVC (PICC)–and there is no specific insertion site, although femoral access is generally discouraged due to a greater risk of infection.8
The most important measures in the prevention of CVC infections are: (i) education and training of healthcare professionals; (ii) strict hand hygiene; and (iii) the use of aseptic techniques when setting and changing dressings.9 Routine CVC changes and the application of topical antimicrobial agents at the insertion site are not recommended, as they may encourage fungal infections and the emergence of resistances. The use of CVCs impregnated or coated with antimicrobial/antiseptic agents such as chlorhexidine and silver sulfadiazine or minocycline/rifampicin and/or heparin-impregnated catheters may reduce the risk of infections, although their benefit is relative and the cost high.10 The prophylactic administration of antibiotics before the placement of a CVC has not been shown to reduce the incidence of infections.11
Preventing infection after endoscopic proceduresThe administration of prophylactic antibiotics is not generally recommended before an endoscopic procedure in order to avoid the development of bacterial endocarditis as cases are rare and there are insufficient data supporting their relationship and the utility of antibiotics in this context.12
In case of an endoscopic retrograde cholangiopancreatography (ERCP), the administration of prophylactic antibiotic therapy should be considered to cover Gram-negative enteric bacilli and enterococci in patients with a blockage in whom full biliary drainage may not be possible. If the procedure does not resolve the blockage, continued antibiotic therapy is advised.12 In percutaneous endoscopic gastrostomies (PEG), the administration of antibiotics (cefazolin, 1g iv; 30min before the procedure) has been proven to significantly reduce the risk of infection.13
Preventing infection by Pneumocystis jiroveciProphylaxis should be considered in the face of Pneumocystis jiroveci (P. jiroveci) in patients who are due to receive: (1) temozolomide with radiotherapy; (2) drugs that cause profound T-cell lymphocytopaenia; and (3) steroids at a dose equivalent to ≥20mg/day of prednisone for four or more weeks.14
The regimen of choice is co-trimoxazole (800/160mg, one tablet, three times per week). In case of an allergy to co-trimoxazole, the possibility of desensitisation should be considered.15,16 Alternatively, atovaquone (1.5g/day)17 or dapsone (100mg/day) may be used, although glucose-6-phosphate dehydrogenase deficiency (G6PD)18 should be ruled out, or pentamidine (300mg, four times per week or monthly iv).19,20 Prophylaxis should be maintained at least throughout the chemotherapy treatment, and prolonging it for at least two months–or until the CD4 lymphocyte count is above 200U/mm3–is recommended.
Special situationsGiven the current characteristics of the resident Spanish population and frequent links between different geographical areas, the prevention of Strongyloides stercoralis hyperinfestation20 and Chagas disease (Trypanosoma cruzi)21 should be taken into account.
Prevention with granulocyte-colony stimulating factorsThe prophylactic administration of granulocyte-colony stimulating factors (G-CSF) reduces the incidence, duration and severity of neutropenia and prevents associated infections.22 As such, the risk of febrile neutropenia (FN) should be estimated before initiating chemotherapy, taking into account different factors, such as the type of tumour, the chemotherapy regimen applied, the patient's characteristics and the treatment intention. The prophylactic use of G-CSFs is recommended in patients with a estimated FN risk of over 20%.23,24 If the estimated risk lies between 10% and 20%, an individual assessment is advised, with G-CSF administration primarily being considered if the treatment intention is curative, in order to avoid delays and dosage reductions, or in high-risk patients, such as those over 65 years of age with previous episodes of FN, extensive bone marrow involvement or who have recently undergone extensive surgery, especially if this includes an intestinal resection. Prophylactic use is more controversial in patients with very advanced tumours, a fragile general or nutritional status, significant comorbidities or in those in whom the benefit of chemotherapy and maintaining a dose intensity is dubious. Routine G-CSF use is not indicated in patients with a risk below 10%, except in specific circumstances that may entail serious consequences in the event of FN.
In FN, treatment with G-CSFs reduces the length of the patient's hospital stay and the neutrophil recovery time. However, it is not associated with a patient survival benefit.25,26 As such, administration should be considered in cases associated with a high complication risk, as occurs with severe neutropenia (neutrophils <100/mm3) or when a long duration is expected (>10 days). Moreover, the use of G-CSFs should be considered in patients aged over 65 years, in cases of sepsis, pneumonia, invasive fungal infections, hospitalisation at the time of fever onset or previous episodes of FN.27
Antibiotic prophylaxisPatients with solid tumours receiving conventional chemotherapy are thought to have a low risk of suffering infectious complications.25,28 In these patients, fluoroquinolones have a protective effect29,30 but do not reduce mortality. In high-risk patients, fluoroquinolones have demonstrated their efficacy in preventing infections in the neutropenic phase,30 particularly in the first chemotherapy cycle.31 Given the high number of patients requiring treatment to prevent an infection, the cost, the adverse effects, the onset of superinfections and the range of resistances,32–37 antibacterial prophylaxis is contraindicated in low-risk patients receiving conventional chemotherapy with or without biological agents.34,36 In specific situations, such as during the first chemotherapy cycle, when profound and prolonged neutropenia is expected, with very aggressive cytostatic regimens, when there is a high base morbidity or in elderly patients, its administration will be considered on an individual basis.38,39
Febrile neutropeniaAssessing infection risk in a patient with febrile neutropeniaThe rate of infectious complications in patients with FN is 25–30%, with mortality reaching 11% in some groups.27 However, this risk is not homogeneous, so the overtreatment of low-risk episodes is common.39 The objective of assessing the infection risk in these patients is to predict the risk of serious complications and thus the need for hospital admission and parenteral therapy. The initial assessment should include the evaluation of: (1) systemic inflammatory response data, by means of checking vital signs such as temperature, heart and respiratory rate; (2) severe sepsis data such as hypotension, signs of tissue hypoperfusion or acute organ dysfunction; and (3) existence of primary or secondary infection foci, taking into account the clinical and epidemiological context.
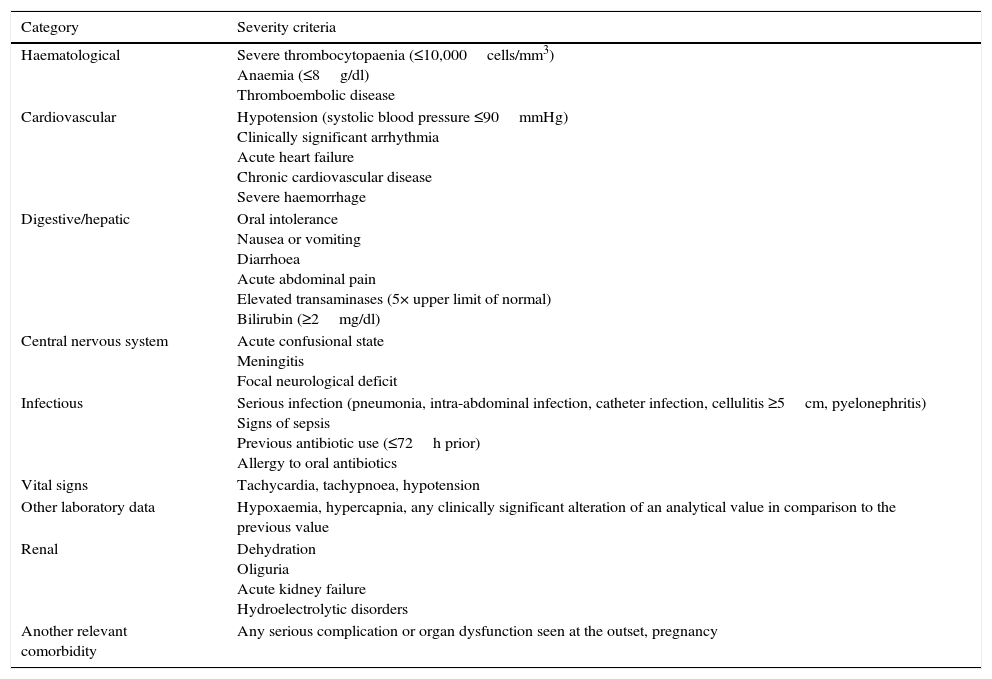
The most widely validated prognostic tool is the Multinational Association of Supportive Care in Cancer (MASCC)40 score, although this is not specific to patients with solid tumours, and infectious complications may develop in 9–15% of episodes classified as low-risk.40–42 The selection of patients in clinical trials on oral/outpatient treatments has been based on exclusion criteria, with the results deemed to have been satisfactory.43 Patients defined as empirically “low-risk” are those with neutropenia (<500neutrophils/mm3) lasting less than seven days, with no complications at the initial assessment and no acute organ dysfunction43,44 (Table 3).
Risk criteria for complications that exclude the patient from oral/outpatient management.
| Category | Severity criteria |
|---|---|
| Haematological | Severe thrombocytopaenia (≤10,000cells/mm3) Anaemia (≤8g/dl) Thromboembolic disease |
| Cardiovascular | Hypotension (systolic blood pressure ≤90mmHg) Clinically significant arrhythmia Acute heart failure Chronic cardiovascular disease Severe haemorrhage |
| Digestive/hepatic | Oral intolerance Nausea or vomiting Diarrhoea Acute abdominal pain Elevated transaminases (5× upper limit of normal) Bilirubin (≥2mg/dl) |
| Central nervous system | Acute confusional state Meningitis Focal neurological deficit |
| Infectious | Serious infection (pneumonia, intra-abdominal infection, catheter infection, cellulitis ≥5cm, pyelonephritis) Signs of sepsis Previous antibiotic use (≤72h prior) Allergy to oral antibiotics |
| Vital signs | Tachycardia, tachypnoea, hypotension |
| Other laboratory data | Hypoxaemia, hypercapnia, any clinically significant alteration of an analytical value in comparison to the previous value |
| Renal | Dehydration Oliguria Acute kidney failure Hydroelectrolytic disorders |
| Another relevant comorbidity | Any serious complication or organ dysfunction seen at the outset, pregnancy |
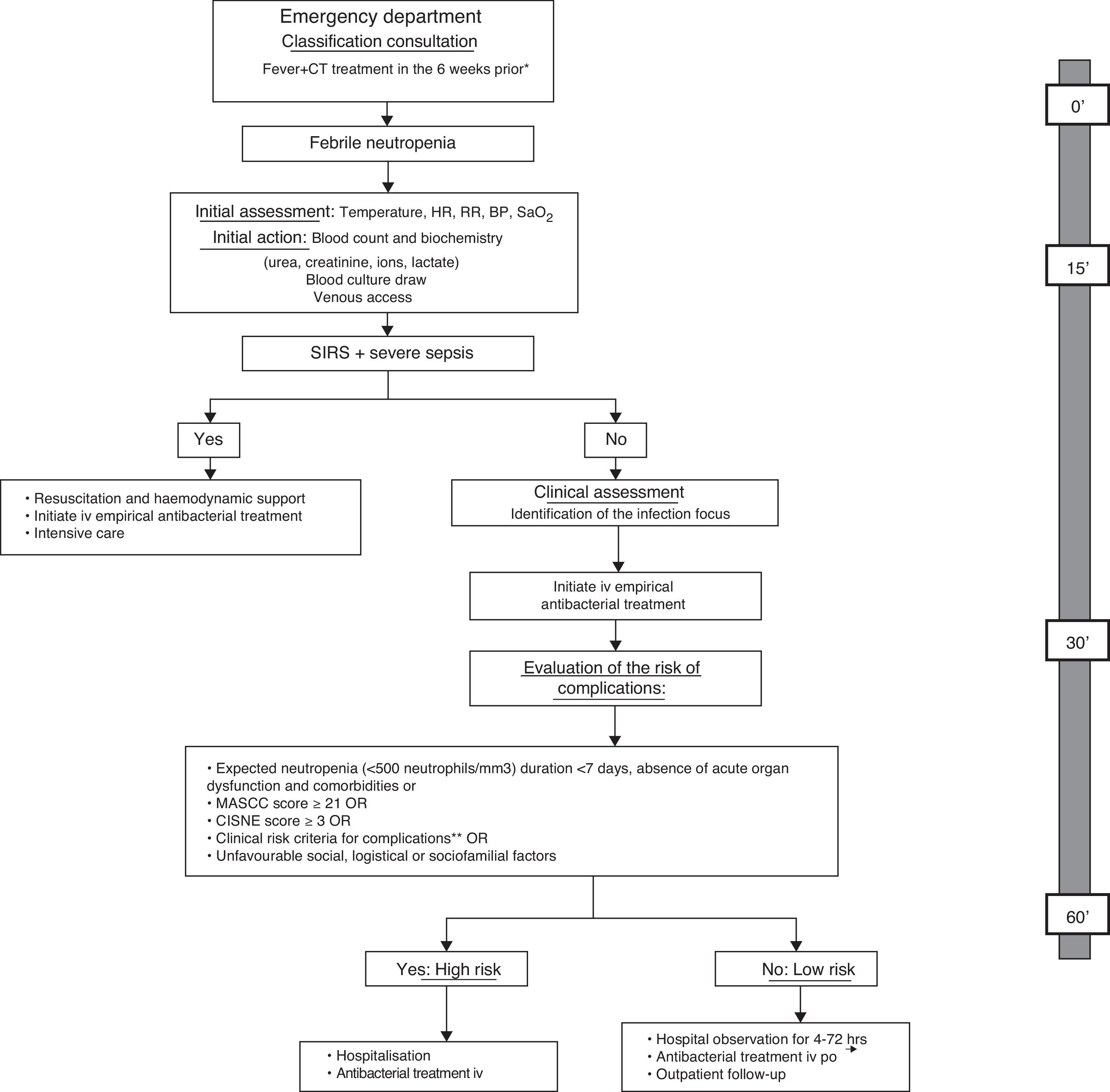
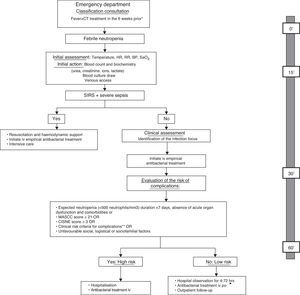
The American Society of Clinical Oncology (ASCO) recommends avoiding the outpatient management of patients who meet any of the clinical risk criteria summarised in Table 3, regardless of the patient's classification on one risk scale or another.33 Moreover, the first prognostic index was published recently in order to predict the incidence of severe complications in patients with solid tumours and apparently stable FN episodes.45 The Clinical Index of Stable Febrile Neutropenia (CISNE) includes six predictors that are independently associated with the incidence of severe complications (ECOG PS≥2 [2 points], chronic bronchitis [1 point], cardiovascular disease [1 point], NCI grade ≥2 mucositis [1 point], monocytes <200/mm3 [1 point] and stress-induced hyperglycaemia [2 points]). These factors are added to a scale ranging from 0 to 8, allowing patients to be classified into three prognostic groups: low risk (0 points), moderate risk (1–2 points) and high risk (≥3 points). The ultimate goal of this index is to prevent the early discharge of patients who, despite their apparent clinical stability, have a high risk of complications (≥3 points). Other social, psychological and logistical factors must be taken into account in order to decide upon the method of treatment. Fig. 1 proposes an action algorithm for treating patients with FN at the Emergency Department that helps the clinician to choose the method of treatment.
Action algorithm for the initial care of febrile neutropenia patients at the Emergency Department and evaluation of the risk of complications and treatment method, including the maximum desirable time for each of the actions. Adapted from: Bell MS, Scullen P, McParlan D, et al. Neutropenic sepsis guideline. In edition Northern Ireland Cancer Network 2010; 1–11. *It is not necessary to wait for an analytical confirmation of neutropenia in order to begin the evaluation; **clinical risk criteria: onset or deterioration of organ dysfunction, comorbidity, altered vital signs, clinical signs or symptoms, documented focal infection or analytical/imaging data. BP, blood pressure; CT, chemotherapy; HR, heart rate; iv, intravenous; MASCC, Multinational Association for Supportive Care in Cancer; po, per os; RR, respiratory rate; SaO2, oxygen saturation; SIRS, systemic inflammatory response syndrome.
Although hospitalisation and the intravenous treatment of FN patients has achieved a significant reduction in mortality, hospitalisation may in itself cause multiple problems, such as toxicity due to intravenous treatments, increased costs, exposure to nosocomial pathogens and a loss of patient quality of life. For this reason, hospital and outpatient treatment strategies have been developed, according to the individual risk stratification.
Empirical antibiotic therapy should be started as soon as possible, as a delay may compromise the patient's prognosis. Before initiating antibiotic therapy, blood cultures should be collected (if the patient has a CVC, take one of the draws through the catheter) as well as samples of possible infection foci based on clinical data (urine, sputum, exudate, mucous or skin lesions, faeces, cerebrospinal fluid, urinary antigens for pneumococcus and/or Legionella, nasal smears for flu in the seasonal period, etc.).
Oral outpatient treatmentLow-risk patients are candidates for outpatient treatment, provided the patient tolerates the oral route and has good social and familial support. The most frequent combination is ciprofloxacin with amoxicillin/clavulanic acid and, in patients allergic to β-lactam antibiotics, ciprofloxacin with clindamycin.46 In a recently-published randomised, double-blind, multicentre clinical trial, moxifloxacin was proven to be as effective as the combination of amoxicillin/clavulanic acid and ciprofloxacin, with fewer gastrointestinal adverse effects.47 However, moxifloxacin has a lower antipseudomonal activity and a greater risk of hepatotoxicity. It is also necessary to take rates of local quinolone resistance in Gram-negative bacilli into consideration. Patients who are receiving prophylaxis with fluoroquinolones should not receive empirical treatment with these antibiotics due to the risk of an infection being caused by bacteria that have become resistant to this treatment.
Patients who are discharged under oral outpatient treatment should undergo a check-up after 48h in order to verify their positive clinical evolution and microbiology results, and in order to adjust the antibiotic treatment and determine its duration. In the event of a clinical deterioration, further diagnostic tests should be considered, as well as hospital admission with intravenous antibiotic therapy.
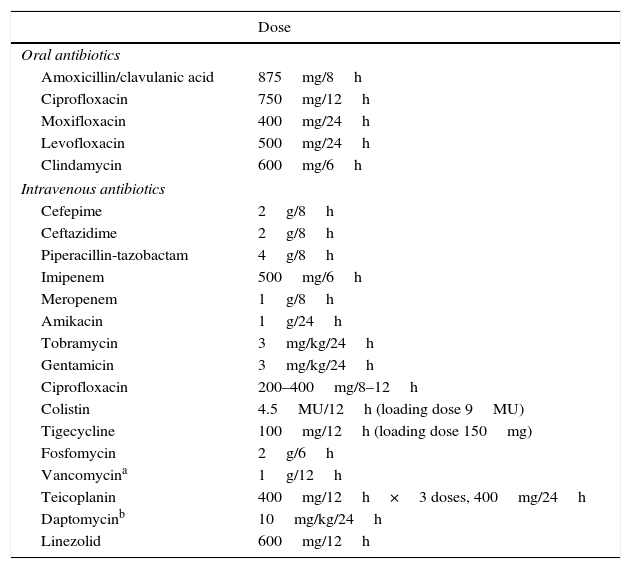
Intravenous treatmentHigh-risk FN patients require hospital admission and intravenous antibiotics. Treatment options include antipseudomonal β-lactam antibiotics such as piperacillin in combination with tazobactam, cefepime, meropenem or imipenem with cilastatin. At many centres, ceftazidime is no longer considered adequate in monotherapy due to its low activity against many Gram-positive microorganisms, such as streptococcus. In case of allergy to β-lactam antibiotics, the alternative is vancomycin in combination with aztreonam (and metronidazole if there is an abdominal focus). In patients who present complications or those in whom an infection caused by resistant pathogens is suspected, the use of other drugs should be considered, such as aminoglycosides, quinolones and glycopeptides, and more occasionally daptomycin, linezolid, fosfomycin, tigecycline and rifampicin. The empirical addition of a glycopeptide to the initial antibiotic regimen does not generally improve the prognosis of these patients,48 although it may be considered in specific cases (mucositis, catheter infection, colonisation by methicillin-resistant Staphylococcus aureus [S. aureus], positive blood cultures for Gram-positive cocci pending an antibiogram, etc.). Table 4 depicts routine doses of oral and intravenous antibiotics.
Dose of routine oral and intravenous antibiotics.
| Dose | |
|---|---|
| Oral antibiotics | |
| Amoxicillin/clavulanic acid | 875mg/8h |
| Ciprofloxacin | 750mg/12h |
| Moxifloxacin | 400mg/24h |
| Levofloxacin | 500mg/24h |
| Clindamycin | 600mg/6h |
| Intravenous antibiotics | |
| Cefepime | 2g/8h |
| Ceftazidime | 2g/8h |
| Piperacillin-tazobactam | 4g/8h |
| Imipenem | 500mg/6h |
| Meropenem | 1g/8h |
| Amikacin | 1g/24h |
| Tobramycin | 3mg/kg/24h |
| Gentamicin | 3mg/kg/24h |
| Ciprofloxacin | 200–400mg/8–12h |
| Colistin | 4.5MU/12h (loading dose 9MU) |
| Tigecycline | 100mg/12h (loading dose 150mg) |
| Fosfomycin | 2g/6h |
| Vancomycina | 1g/12h |
| Teicoplanin | 400mg/12h×3 doses, 400mg/24h |
| Daptomycinb | 10mg/kg/24h |
| Linezolid | 600mg/12h |
MU, million units.
The recently-published guidelines of the Infectious Diseases Society of America (IDSA) recommend the use of an antipseudomonal β-lactam drug in monotherapy as initial antibiotic therapy in FN.25 A meta-analysis found monotherapy to be significantly more beneficial than the combination of a β-lactam antibiotic and aminoglycoside, with fewer adverse effects, lower morbidity and similar survival rates.49 However, it cannot be completely ruled out that certain patient subgroups may benefit from the initial use of antibiotic combinations, and two types of strategies have thus been used pragmatically: escalation and de-escalation. Specifically, in recent decades, we are seeing an increase in Gram-negative bacterial infections in cancer patients, whilst we also observe a multidrug resistance emergency among these microorganisms.50,51 In this context, it is doubtful as to whether initial empirical treatment with a β-lactam antibiotic in monotherapy is sufficiently safe in patients with FN,52 particularly when there are associated severity criteria.
The escalation strategy involves beginning an intravenous treatment in monotherapy and, if the patient deteriorates or a resistant pathogen is isolated, the treatment will be escalated to an antibiotic or a combination of broader-spectrum antibiotics. The advantages of this strategy are that it avoids the early use of some broad-spectrum antibiotics, generates less toxicity, has a lower financial cost and a lower risk of resistance selection, fundamentally to carbapenem antibiotics. In contrast, the patients’ prognosis may be compromised if the resistant microorganisms are not adequately covered from the outset.
This escalation strategy should be used in high-risk patients in the following situations: (1) uncomplicated clinical presentation; (2) no risk factors for resistant bacterial infections; and (3) at centres with a low prevalence of resistant microorganisms.
The initial therapeutic options include a non-carbapenem antipseudomonal β-lactam antibiotic such as cefepime, ceftazidime or piperacillin in combination with tazobactam. Carbapenems should be avoided in patients with no complications who lack risk factors for resistant bacteria. Nevertheless, they may be the most suitable option in patients who have had a recent hospital admission (<1 month), previous antibiotic use or previous invasive procedures, who might have a greater risk of Gram-negative bacilli infections with extended-spectrum beta-lactamases.
In the de-escalation strategy, on the other hand, the initial antibiotic treatment administered covers even the most resistant pathogens. Subsequently, therapy is de-escalated to a narrower-spectrum treatment, once the presence of resistant pathogens is ruled out or if a pathogen is identified and its antibiotic sensitivity profile is defined. The main advantage of de-escalation is that it is more likely to achieve an adequate initial antibiotic coverage. However, this strategy often leads to an unnecessary use of broad-spectrum antibiotics, with physicians generally not tending to de-escalate when they have the opportunity to do so, and there is a higher risk of resistance selection.
The de-escalation strategy should be used: (1) in complicated clinical presentations; (2) when there are risk factors for resistant bacterial infections; and (3) at centres with a high prevalence of resistant microorganisms.
The initial therapeutic options include: (1) monotherapy with meropenem or imipenem in seriously ill patients or when there is a previous history of colonisation/infection by extended-spectrum β-lactamase producing enterobacteriaceae; (2) an antipseudomonal β-lactam in combination with aminoglycoside or quinolone in seriously ill patients or if the presence of resistant nonfermenting Gram-negative bacilli (Pseudomonas aeruginosa or Acinetobacter spp.) is suspected; (3) a β-lactam alongside colistin with or without aminoglycoside, fosfomycin or tigecycline if an infection caused by carbapenemase-producing Gram-negative bacilli or multidrug-resistant nonfermenting Gram-negative bacilli is suspected; (4) a β-lactam in combination with co-trimoxazole if an infection caused by Stenotrophomonas maltophilia is suspected. In any case, if there are risk factors for an infection caused by a resistant Gram-positive microorganism or there is a serious infection related to a venous catheter, the skin and soft tissues, a glycopeptide, daptomycin or linezolid may be added to the initial therapy.
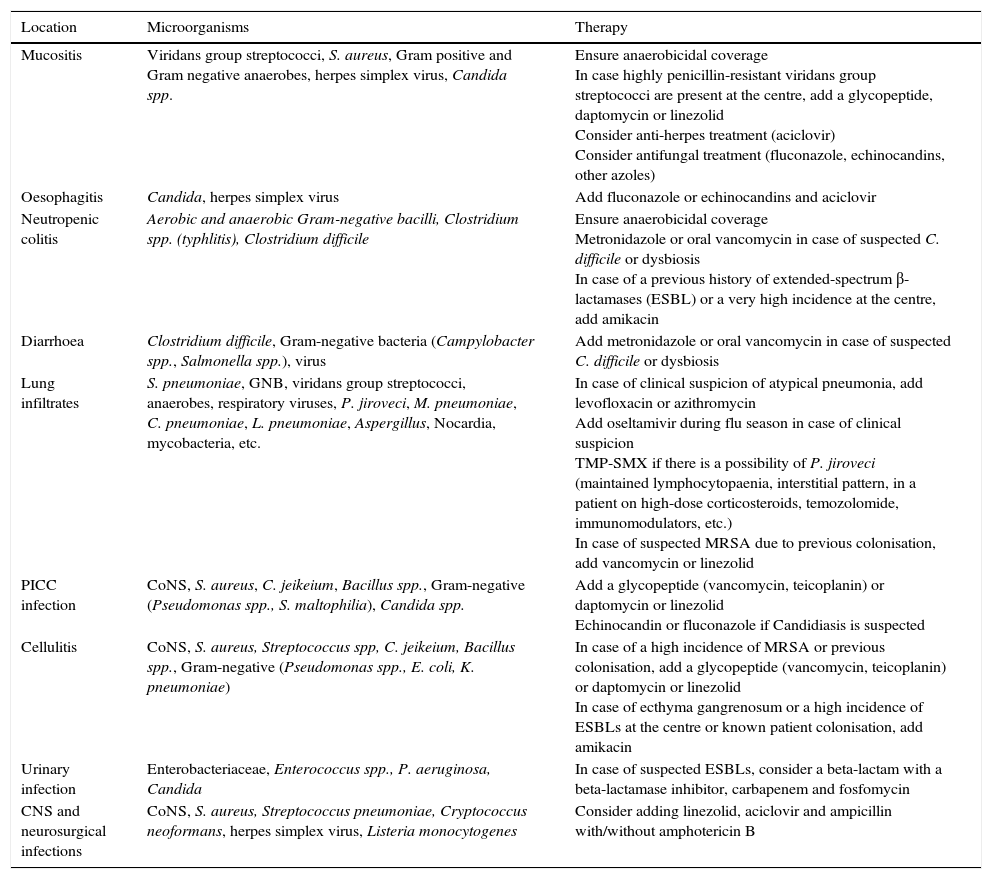
Clinical follow-up once empirical treatment has begun48–72h after starting the empirical treatment, the patient's clinical evolution and results should be assessed from a microbiological point of view. In case an aetiological agent or clinical focus is isolated, treatment should be simplified and adjusted to the antibiotic sensitivity profile of each microorganism or type of infection, as reflected in Table 5.
Recommendations for treating febrile neutropenia when there is an evident clinical focus.
| Location | Microorganisms | Therapy |
|---|---|---|
| Mucositis | Viridans group streptococci, S. aureus, Gram positive and Gram negative anaerobes, herpes simplex virus, Candida spp. | Ensure anaerobicidal coverage In case highly penicillin-resistant viridans group streptococci are present at the centre, add a glycopeptide, daptomycin or linezolid Consider anti-herpes treatment (aciclovir) Consider antifungal treatment (fluconazole, echinocandins, other azoles) |
| Oesophagitis | Candida, herpes simplex virus | Add fluconazole or echinocandins and aciclovir |
| Neutropenic colitis | Aerobic and anaerobic Gram-negative bacilli, Clostridium spp. (typhlitis), Clostridium difficile | Ensure anaerobicidal coverage Metronidazole or oral vancomycin in case of suspected C. difficile or dysbiosis In case of a previous history of extended-spectrum β-lactamases (ESBL) or a very high incidence at the centre, add amikacin |
| Diarrhoea | Clostridium difficile, Gram-negative bacteria (Campylobacter spp., Salmonella spp.), virus | Add metronidazole or oral vancomycin in case of suspected C. difficile or dysbiosis |
| Lung infiltrates | S. pneumoniae, GNB, viridans group streptococci, anaerobes, respiratory viruses, P. jiroveci, M. pneumoniae, C. pneumoniae, L. pneumoniae, Aspergillus, Nocardia, mycobacteria, etc. | In case of clinical suspicion of atypical pneumonia, add levofloxacin or azithromycin Add oseltamivir during flu season in case of clinical suspicion TMP-SMX if there is a possibility of P. jiroveci (maintained lymphocytopaenia, interstitial pattern, in a patient on high-dose corticosteroids, temozolomide, immunomodulators, etc.) In case of suspected MRSA due to previous colonisation, add vancomycin or linezolid |
| PICC infection | CoNS, S. aureus, C. jeikeium, Bacillus spp., Gram-negative (Pseudomonas spp., S. maltophilia), Candida spp. | Add a glycopeptide (vancomycin, teicoplanin) or daptomycin or linezolid Echinocandin or fluconazole if Candidiasis is suspected |
| Cellulitis | CoNS, S. aureus, Streptococcus spp, C. jeikeium, Bacillus spp., Gram-negative (Pseudomonas spp., E. coli, K. pneumoniae) | In case of a high incidence of MRSA or previous colonisation, add a glycopeptide (vancomycin, teicoplanin) or daptomycin or linezolid In case of ecthyma gangrenosum or a high incidence of ESBLs at the centre or known patient colonisation, add amikacin |
| Urinary infection | Enterobacteriaceae, Enterococcus spp., P. aeruginosa, Candida | In case of suspected ESBLs, consider a beta-lactam with a beta-lactamase inhibitor, carbapenem and fosfomycin |
| CNS and neurosurgical infections | CoNS, S. aureus, Streptococcus pneumoniae, Cryptococcus neoformans, herpes simplex virus, Listeria monocytogenes | Consider adding linezolid, aciclovir and ampicillin with/without amphotericin B |
CNS, central nervous system; CoNS, coagulase-negative staphylococci; MRSA, methicillin-resistant Staphylococcus aureus; PICC, peripherally inserted central catheter; TMP-SMX, trimethoprim and sulfamethoxazole.
In situations where no clinical focus or aetiological agent has been documented and the patient is stable, the antibiotic treatment should be de-escalated to a narrower-spectrum agent and/or the drugs administered in combination should be withdrawn (aminoglycoside, quinolone, colistin, etc.). If the initial presentation was not serious, and the patient has been afebrile for over 72h and is asymptomatic, the possibility of suspending the treatment may be considered. However, if the patient was in a serious or unstable clinical situation, modifying the initial antibiotic treatment is not advisable.
In most documented infections, 10–14 days of antibiotic treatment is usually sufficient. In some cases, treatment may be extended beyond the resolution of the fever and neutropenia, if necessary. If a catheter infection is documented, its withdrawal or sealing with antimicrobial agents should be considered, depending on the patient's characteristics and the microorganism isolated. In patients with persistent fever, a full reassessment should be performed, with an active search for possible infection foci or other causes of fever such as drug toxicity, tumour fever, etc.
Biomarkers are analytical parameters that may complement other clinical and microbiological variables in the assessment of a FN episode and its severity. Likewise, normalised values support the treatment response. Of the biochemical parameters, those of greatest interest are stress hyperglycaemia as an acute phase reactant and hypoalbuminaemia as a marker of malnutrition and fragility.42 Of the serum markers specific to the inflammatory/infectious process, those most frequently used are lactate, procalcitonin and C-reactive protein. Their utility is not well defined due to the heterogeneity of the populations studied and the few patients included in published clinical trials.53 Procalcitonin (value>0.5ng/ml) is a more useful and earlier marker than C-reactive protein (value≥90mg/dl), especially in the diagnosis of bacteraemia, as it is not elevated in viral infections, and for predicting the severity of FN complications. The addition of procalcitonin to clinical risk scores could increase the sensitivity and negative predictive value for detecting bacteraemia and the failure of the antibiotic treatment.54 Interleukin 6, 8 and 10 could be better predictors of severity and complications but are less used due to their high cost, lack of availability and low specificity. The lipopolysaccharide binding protein, interleukin 2 and tumour necrosis factor, among others, have no current application in the context of cancer patients with FN.
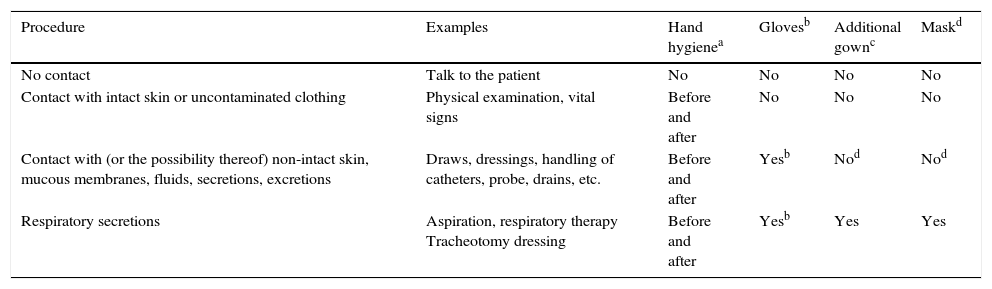
Specific precaution measuresThe objective of these measures is to avoid the transmission of certain pathogens from a colonised patient or one with an active infection to other patients or healthcare personnel. One key aspect is that the application of such measures should not affect the quality of the healthcare received by the patient, and that these measures should be added to the so-called standard precautions, such as hand hygiene and decontamination, the use of gloves, gowns and/or a mask, according to cases, situations and indications, which are shown in Table 6.
Application of standard precautions and types of specific precautions to be adopted according to the infectious disease or microorganism, and their transmissibility period.
| Procedure | Examples | Hand hygienea | Glovesb | Additional gownc | Maskd |
|---|---|---|---|---|---|
| No contact | Talk to the patient | No | No | No | No |
| Contact with intact skin or uncontaminated clothing | Physical examination, vital signs | Before and after | No | No | No |
| Contact with (or the possibility thereof) non-intact skin, mucous membranes, fluids, secretions, excretions | Draws, dressings, handling of catheters, probe, drains, etc. | Before and after | Yesb | Nod | Nod |
| Respiratory secretions | Aspiration, respiratory therapy Tracheotomy dressing | Before and after | Yesb | Yes | Yes |
| Disease or microorganisms | Types of precautions or isolation measures | Possible transmission period |
|---|---|---|
| Multidrug-resistant bacterial pathogens (MRSA, VRE, ESBL+ Enterobacteriaceae, Acinetobacter baumannii, MDR Pseudomonas aeruginosa) | Contact | Cross-species transmission during colonisation or infection by the corresponding microorganism. In case of a prolonged hospital stay, carry out weekly epidemiological surveillance cultures for three consecutive weeks; if they are negative, lift precautions. Short hospital stays: throughout the stay. |
| Adenovirus | Droplets and contact | Adenovirus infections may be transmitted for up to 14 days after their onset. |
| Flu (Influenza) | Droplets | From 3 to 5 days after the onset of symptoms in adults. In children, transmissibility may extend to 7 days. |
| Respiratory syncytial virus | Contact | The period immediately before the active disease and the entire duration of the active disease. |
| Human parainfluenza virus | Droplets-fomites | Prior to the onset of symptoms up until their resolution (it may be transmitted by asymptomatic carriers). |
| Measles | Airborne | From 4 days prior to the exanthema to 4 days after (minimum contagion after the 2nd day of the exanthema). |
| Rubella (congenital) | Contact | Virus can be transmitted for months in infants. |
| Rubella | Droplets | From 1-week before the exanthema to 7 days after. |
| Mumps | Droplets | The virus is isolated in the saliva from 7 days before to 9 days after the onset of clear symptoms. The maximum contagion risk covers the period from 2 days before the onset of the disease to 4 days afterwards. |
| Hepatitis A | Contact (Faecal – oral) | Infectivity period: From 2 to 3 weeks prior to the onset of symptoms and one week after symptom onset. |
| Rotavirus | Contact (Faecal – oral) | During the acute phase and while virus excretion persists. |
| Parvovirus B19 | Droplets | If the patient only presents an exanthema, transmissibility is at its highest before the rash and unlikely thereafter. In case of an aplastic crisis, the transmissibility period is up to one week post-onset. |
| Varicella-Zoster | Airborne and contact | 4–5 days before the rash and up until the lesions have scabbed over (around 7 days) |
| Salmonella | Contact (Faecal – oral) | From the first week up to the end of convalescence (1–2 weeks). In S. Typhi, assess chronic carriers. |
| Tuberculosis | Airborne | Lasts for as long as tuberculosis bacilli are expelled in sputum. Effective antimicrobial chemotherapy eliminates transmissibility within 2–4 weeks |
| Impetigo | Contact | Until the lesions have completely healed (usually 1–2 weeks). |
| Mycoplasma (Primary Atypical Pneumonia) | Droplets-(recently contaminated fomites or respiratory secretions) | Duration of under 20 days. Treatment does not eradicate the microorganism from the airways, where it may persist for up to 13 weeks. |
| Whooping cough | Droplets | Persists for up to 5 days after effective treatment. |
| Type B H. influenzae | Droplets | Stops being transmissible 24–48h after the onset of effective antibiotic treatment. |
| Neisseria meningitidis | Droplets | Persists until the live meningococci disappear from nose and mouth secretions, that is, 24h after starting an appropriate treatment. |
| Scarlet fever | Droplets | Persists for up to 24h after effective treatment. |
| Clostridium difficile | Contact | May persist for weeks and months in its non-vegetative or spore forms. |
| Scabies | Contact | Very persistent up until the mites and eggs have been destroyed. Not transmitted 24h after effective treatment (Permethrin 5%). |
ESBL, extended-spectrum beta-lactamases; MDR, multidrug-resistant; MRSA, methicillin-resistant Staphylococcus aureus; VRE, vancomycin-resistant enterococci.
Specific precaution measures are classified based on the microorganisms’ modes of transmission as follows: (1) respiratory precautions, the objective of which is to avoid the airborne dissemination of particles under 5μ, which may remain suspended in the air for prolonged periods of time as in the case of respiratory TB, disseminated VZV, measles, etc.; (2) precautions against droplets, aimed at avoiding the transmission of pathogenic microorganisms through larger droplets and which requires close contact between the source of exposure and susceptible host, as in meningococcal infection, flu, etc.; and (3) contact precautions, which seek to avoid transmission through direct or indirect contact by means of objects or contaminated surfaces. Table 6 summarises the recommendations and specific measures to be adopted according to the infectious disease or microorganism in question.
Contact precautions are those needed most frequently in cancer patients, and are indicated in the following situations: (1) respiratory, gastrointestinal or skin infections and/or wounds colonised or infected by multidrug-resistant pathogens; (2) diarrhoeal infections, including infections caused by Clostridium difficile; (3) infections caused by respiratory viruses; and (4) skin or mucous membrane infections.
As regards multidrug-resistant microorganisms, the Infection Control Committee or Team of each centre should decide which are the most important and susceptible to be subject to the implementation of contact precautions, based on the existing recommendations, and always considering the local epidemiology and capacity for transmission between patients of each of the multidrug-resistant pathogens assessed. To that effect, it may be necessary to perform epidemiological surveillance cultures.
In most hospitals, the application of contact precautions is recommended in the following scenarios: (1) all cases of methicillin-resistant S. aureus; (2) vancomycin-resistant Enterococci; (3) extended-spectrum beta-lactamase producing enterobacteriaceae; (4) carbapenemase-producing enterobacteriaceae; (5) nonfermenting Gram-negative bacilli, such as Pseudomonas aeruginosa or Acinetobacter baumannii with multidrug or pandrug resistance patterns.
Reverse isolation measures would only be indicated in those solid cancer patients who are receiving chemotherapy regimens that lead to profound and prolonged neutropenia. Reverse isolation rooms must fulfil a series of special characteristics that reduce environmental contamination by circulating microorganism-stripped air and preventing the penetration of microorganisms into the room by means of positive pressure.
Conflicts of interestThe authors declare that they have no conflicts of interest.
The Boards of Directors of the SEIMC and SEOM have approved the final wording and content of this Document.
Please cite this article as: Aguado JM, Cruz JJ, Virizuela JA, Aguilar M, Carmona A, Cassinello J, et al. Manejo de la infección y la neutropenia febril en el paciente con cáncer sólido. Enferm Infecc Microbiol Clin. 2017;35:451–460.
All members of the Spanish Society of Infectious Diseases and the #Spanish Society of Medical Oncology.