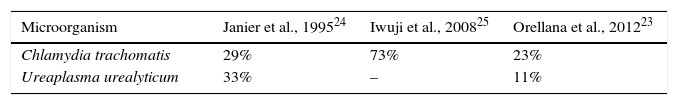Sexually transmitted infections (STIs) are responsible for an enormous burden of morbidity and mortality. Worldwide, millions of cases of STIs, such as syphilis, chlamydia, or gonorrhoea occur every year, and there is now an increase in antimicrobial resistance in pathogens, such as gonococcus. Delay in diagnosis is one of the factors that justifies the difficulty in controlling these infections. Rapid diagnostic tests allow the introduction of aetiological treatment at the first visit, and also leads to treating symptomatic and asymptomatic patients more effectively, as well as to interrupt the epidemiological transmission chain without delay. The World Health Organisation includes these tests in its global strategy against STIs.
Las infecciones de transmisión sexual (ITS) suponen una importante carga de morbimortalidad. A nivel mundial todos los años se producen millones de casos de ITS como sífilis, infección por clamidias o gonococia, y actualmente se asiste a un incremento de la resistencia a los antimicrobianos en patógenos como el gonococo. La demora en el diagnóstico es uno de los factores que justifica la dificultad para controlar estas infecciones. Las pruebas de diagnóstico rápido permiten instaurar el tratamiento etiológico en la primera consulta, lo que lleva a tratar a más pacientes, tanto sintomáticos como asintomáticos, de forma más efectiva, e interrumpir sin demoras la cadena epidemiológica de transmisión. La OMS incluye estas pruebas en su estrategia mundial contra las ITS.
Worldwide, sexually transmitted infections (STIs) pose a significant morbidity and mortality burden as they compromise quality of life, sexual and reproductive health and newborn and child health. They also indirectly facilitate the transmission of the human immunodeficiency virus (HIV) and cause cell changes that precede some forms of cancer. The WHO provides data on a reality that seems far-removed from developed countries. Syphilis causes over 300,000 foetal and neonatal deaths per year and exposes another 215,000 children to premature death. It also estimates that 357 million new cases of four types of curable STIs are recorded every year: 131 million Chlamydia trachomatis (CT) infections, 78 million Neisseria gonorrhoeae (NG) infections, 6 million Treponema pallidum (TP) infections and 142 million Trichomonas vaginalis (TV) infections. Similarly, the human papillomavirus (HPV) is believed to be responsible for 530,000 cases of cervical and uterine cancer as well as 264,000 deaths. We also cannot forget the emergence of multidrug-resistant gonococcal strains that threaten us with untreatable gonorrhoea.
While it is true that progress has been made, such as the reduction of mother-to-child syphilis transmission in developing countries, globally the prevalence of STIs remains the same or is even on the rise. Numerous factors contribute to this, but the treatment delay that arises while patients await a diagnostic result is particularly important. By means of a mathematical model, it has been shown that using a rapid diagnostic test with a sensitivity as low as 63% successfully improves the percentage of treated patients, compared to waiting for the result of a high-sensitivity test available at a second consultation, which many patients fail to attend.1 As such, diagnostic tests that provide immediate results will facilitate the administration of aetiological treatments to a larger number of infected patients and new transmissions will be avoided by breaking the chain of infection.
To that effect, it is necessary to reinforce the capacity of the laboratories and to also proceed with designing and implementing diagnostic tests at the point of care to facilitate systematic and early STI diagnosis of all suspected individuals, even if they are asymptomatic.
In this review, we will present the latest rapid diagnostic techniques for the main STI- and vulvovaginitis-causing pathogens, though we will leave viral hepatitis and HIV to one side.
History, concept and characteristics of the rapid tests in sexually transmitted infectionsIn STIs, diagnostic tests can serve several purposes: (a) diagnosis; (b) screening of high-risk groups; (c) treatment monitoring; (d) epidemiological surveillance; (e) investigation of outbreaks; (f) validation of syndrome management in countries with few resources; (g) detection of resistance patterns; (h) ensuring quality in laboratory tests; and (i) research.2
The diagnostic tests used in STIs are: (a) direct microscopy; (b) culture; (c) antigen detection; (d) serology; (e) detection of microbial metabolites (whiff test, for example), and (f) molecular methods. All of these may be considered rapid at least in some of their forms, except culture.
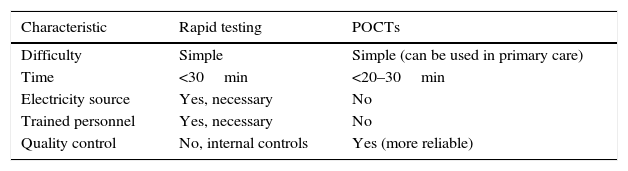
Rapid tests in STIs can be considered as such, or as point-of-care tests (POCTs). This review considers both types indiscriminately. POCTs can be defined as diagnostic tests that allow a diagnosis to be obtained and a treatment indicated at the same visit. The differences between them are detailed in Table 1 (adapted from Muralidhar3).
Differences between rapid tests and POCTs.
| Characteristic | Rapid testing | POCTs |
|---|---|---|
| Difficulty | Simple | Simple (can be used in primary care) |
| Time | <30min | <20–30min |
| Electricity source | Yes, necessary | No |
| Trained personnel | Yes, necessary | No |
| Quality control | No, internal controls | Yes (more reliable) |
POCTs: point-of-care tests.
Source: Adapted from Muralidhar.3
Rapid tests/POCTs aimed at STI diagnosis must meet the following requirements established by the WHO4:
ASSURED
- •
Affordable.
- •
Sensitive.
- •
Specific.
- •
User-friendly (few steps and minimum training).
- •
Rapid and robust (storable at room temperature, results in <30min).
- •
Equipment-free.
- •
Deliverable to end-users.
POCTs must also comply with quality controls included in the tests and be environmentally safe and low cost.
An important aspect is what users think the qualities of rapid tests/POCTs should be in STIs. In a study on focus groups, it was found that the qualities of a rapid test should be high sensitivity and specificity, a short procedure time and a low cost.5
For POCTs, various types of technology are used6:
- a)
Precipitation/agglutination reactions such as the RPR test for syphilis.
- b)
Immunochromatography in different test formats: (b1) lateral flow; (b2) multiple, e.g. HIV+syphilis, HIV+syphilis+HBV/HCV, treponemal test+non-treponemal test; (b3) using a flow assay such as dot-blot; (b4) with readers/scanners to eliminate observer bias, thereby increasing sensitivity and facilitating quantification.
- c)
Emerging technologies such as microfluidic assays (which detect multiple analytes such as HIV and syphilis) and loop-mediated isothermal amplification technology (LAMP), which achieves amplification using four primers and polymerase enzyme in a constant temperature reaction (60–65°C) and obtains results in less than 1h.
POCTs were developed to complement centralisation in “core” laboratories, allowing for decentralised determinations that are available 24h a day, 365 days a year. They are usually combinations of syndrome-based microorganisms, can be collected by the patients themselves, do not require trained personnel and are rapid so as to enable decision-making. Of all of them, the STI tests are among those that have been proven cost-effective.7
Irrespective of the terminology and discourse that may surround rapid tests or POCTs, in this review we include the types of tests we consider to be interchangeable, whether these are with or without equipment at the point of care or in a nearby laboratory, and which try to provide the fastest results possible.
The rapid response laboratory in sexually transmitted infections and needs in different settings: primary care, emergency departments, STI clinics, reference laboratoriesLaboratory centralisation makes the presence of a rapid response laboratory difficult in our setting, but the following methods may be available in various working environments:
- a)
Primary care: e.g. Gram stain and Amsel criteria for bacterial vaginosis.
- b)
Emergency departments: normally have a support laboratory.
- c)
STI clinic: the above methods, dark-field microscopy, urine sediment.
- d)
Reference laboratory: all rapid techniques.
In developing countries, rapid tests/POCTs generate huge interest as they help to reduce the enormous disease burden that they endure. These tests allow a move away from syndromic treatments (targeted blindly and simultaneously at various pathogens causing the same syndrome) and a progression towards aetiological treatments (targeted specifically at the causal pathogen). The unnecessary use of antimicrobials and the emergence of resistances are avoided, costs are reduced and the chain of infection is broken more effectively. However, there are drawbacks, as aspects such as cost, the need for the refrigeration of reagents or electrical supply requirements (e.g. for a microscope) can mean that tests which prove very useful in one setting may be useless in another. As such, all of the ASSURED requirements are equally important.
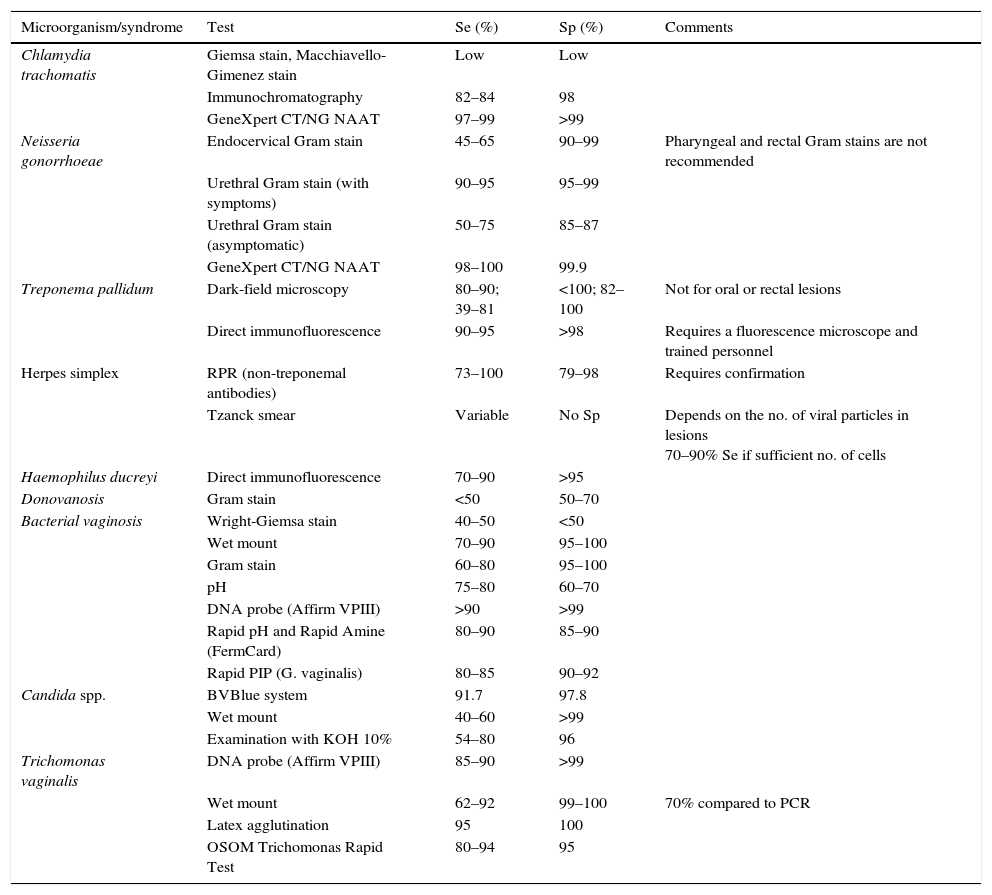
Rapid diagnostic tests in sexually transmitted infections and their evidenceTable 2 shows the rapid techniques for STIs (adapted from Aznar Martín et al.8).
List of rapid tests for sexually transmitted infections.
| Microorganism/syndrome | Test | Se (%) | Sp (%) | Comments |
|---|---|---|---|---|
| Chlamydia trachomatis | Giemsa stain, Macchiavello-Gimenez stain | Low | Low | |
| Immunochromatography | 82–84 | 98 | ||
| GeneXpert CT/NG NAAT | 97–99 | >99 | ||
| Neisseria gonorrhoeae | Endocervical Gram stain | 45–65 | 90–99 | Pharyngeal and rectal Gram stains are not recommended |
| Urethral Gram stain (with symptoms) | 90–95 | 95–99 | ||
| Urethral Gram stain (asymptomatic) | 50–75 | 85–87 | ||
| GeneXpert CT/NG NAAT | 98–100 | 99.9 | ||
| Treponema pallidum | Dark-field microscopy | 80–90; 39–81 | <100; 82–100 | Not for oral or rectal lesions |
| Direct immunofluorescence | 90–95 | >98 | Requires a fluorescence microscope and trained personnel | |
| Herpes simplex | RPR (non-treponemal antibodies) | 73–100 | 79–98 | Requires confirmation |
| Tzanck smear | Variable | No Sp | Depends on the no. of viral particles in lesions 70–90% Se if sufficient no. of cells | |
| Haemophilus ducreyi | Direct immunofluorescence | 70–90 | >95 | |
| Donovanosis | Gram stain | <50 | 50–70 | |
| Bacterial vaginosis | Wright-Giemsa stain | 40–50 | <50 | |
| Wet mount | 70–90 | 95–100 | ||
| Gram stain | 60–80 | 95–100 | ||
| pH | 75–80 | 60–70 | ||
| DNA probe (Affirm VPIII) | >90 | >99 | ||
| Rapid pH and Rapid Amine (FermCard) | 80–90 | 85–90 | ||
| Rapid PIP (G. vaginalis) | 80–85 | 90–92 | ||
| Candida spp. | BVBlue system | 91.7 | 97.8 | |
| Wet mount | 40–60 | >99 | ||
| Examination with KOH 10% | 54–80 | 96 | ||
| Trichomonas vaginalis | DNA probe (Affirm VPIII) | 85–90 | >99 | |
| Wet mount | 62–92 | 99–100 | 70% compared to PCR | |
| Latex agglutination | 95 | 100 | ||
| OSOM Trichomonas Rapid Test | 80–94 | 95 |
Se: sensitivity; Sp: specificity.
Source: Adapted from Aznar Martín et al.8
Microscopy and culture are the tests that should routinely be performed in symptomatic women (level of evidence III, grade B).9 The microscopic observation of yeast in vaginal discharge by means of a wet mount or Gram stain examination has the advantage of being rapid, but has a low sensitivity (50%).
Criteria for diagnosing vaginal candidiasis (level of evidence III, grade B)10:
- -
Absence of fishy odour in the “whiff test”, using a potassium hydroxide solution on a speculum, and amine odour test on a slide, are supportive, since candidiasis does not usually coexist with bacterial vaginosis or TV infection, but they are not diagnostic.
- -
Presence of yeasts or pseudohyphae on the wet mount examination of vaginal discharge (40–60% sensitivity).
- -
Presence of yeasts or pseudohyphae on the Gram stain of vaginal discharge (up to 65% sensitivity).
The BD Affirm VPIII™ system (Becton Dickinson, Franklin Lakes, New Jersey, United States) uses hybridisation to determine the presence of the genetic material of TV, Gardnerella vaginalis (GV) and certain Candida species (Candida albicans, Candida glabrata, Candida kefyr, Candida krusei, Candida parapsilosis and Candida tropicalis), with a Candida sensitivity of 85–90% and a specificity of >99%. Results are obtained in less than 1h.
ChancroidAt present, the use of the PCR technique is indicated for the detection of Haemophilus ducreyi (HD), due to the low sensitivity of Gram staining (typical in “schools of fish” with small pleomorphic Gram-negative bacilli). In comparison to culture, microscopy has a sensitivity of 50%, and false positives are also common.11 A multi-format PCR is available on the market for HD and TP.
ChlamydiaThe majority of the diagnostic tests available are carried out in the laboratory and there may be a delay between collection and the result.
The urethral Gram stain is the standard diagnostic test for nongonococcal urethritis, but is dependent on the observer and has a low specificity. As such, the use of flow cytometry is also currently being introduced to detect leukocyturia in urine as an inflammatory marker of nongonococcal urethritis (NGU) caused by CT and Mycoplasma genitalium (MG).12
EIA-based POCTs have a low sensitivity, although new forms present sensitivities of 82–84% compared to nucleic acid amplification tests (NAATs).13,14
NAATs are the diagnostic benchmark but are generally carried out in the laboratory, taking several hours. New formats like isothermal NAATs (LAMP) are commercially available, offer good sensitivity (97.1%) and specificity (97.9%), and have an execution time of less than 1h. However, performing the techniques is complex and should be done by trained personnel; they therefore cannot be used as POCTs.
New NAAT-based techniques (GeneXpert CT/NG, Cepheid, Sunnyvale, CA, United States) are appropriate for genital samples and reduce the time required to issue a result.15 Extra-genital samples require additional validations.16 Their sensitivity is very high (97–99%) as is their specificity (>99%), for endocervical, vaginal and urine samples.17
Lymphogranuloma venereum (LGV)Diagnosis requires clinical suspicion and very specialised laboratory tests are necessary for confirmation. Firstly, the presence of CT must be seen in the lesions, usually using NAATs. The samples used are both genital and extra-genital: rectal, pharyngeal and urine.18 Chlamydia-positive samples should be assessed a second time using a specific PCR reaction for LGV-producing chlamydia DNA. Typing techniques are also used, by means of RFLP or sequencing. In light of the above, no rapid detection method exists at present, although the GeneXpert PCR could meet these criteria. However, this is usually used in a second step, when the presence of chlamydia has already been detected.
GonorrhoeaGonorrhoea diagnosis is established with the detection of NG at the infection site. The method to be used will depend on the patient's symptoms, transport conditions to the laboratory and the available tests.
- a)
Gram stain for urethritis: Gram staining is a rapid technique that is equally as sensitive as culture in symptomatic urethritis among men, though it has limited sensitivity in other areas. Examinations should be performed with a 1000× objective lens under oil immersion for at least 2min, checking for the presence of polymorphonuclear (PMN) leukocytes, a pink nucleus and a colourless cytoplasm, usually >4–5 PMN leukocytes per immersion field. If a urinary Gram stain is performed, it is advisable to collect the first 10–15ml from the first part of urination as well as to observe the sediment after centrifugation to check for the presence of ≥10 PMN leukocytes. Gonorrhoea appears as kidney-shaped, oval, Gram-negative cocci, in intra and extracellular pairs.8 In urethral samples from symptomatic men, Gram staining offers a high sensitivity (90–95%) and facilitates immediate diagnosis (III, C).19 The sensitivity of the same samples in asymptomatic men is distinctly lower (50–75%). Gram staining of rectal samples is not recommended in symptomatic patients or in urethral samples from women (III, C). It should also not be used on pharyngeal samples.
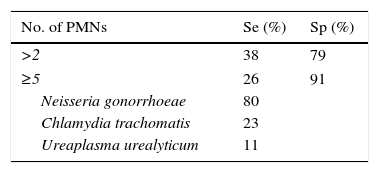
The traditional criterion for determining the existence of urethritis is the observation of 4–5 PMN leukocytes with an immersion field objective lens and the presence or absence of Gram-negative diplococci, in both gonococcal or nongonococcal urethritis.20 This criterion was based on the best sensitivity and specificity established with NG and CT cultures. However, with NAAT systems, it has been seen to be too strict for NGU, also considering variations between microscopists and the fact that sensitivity varies according to the grade of urethritis (low-grade cases present more false negatives). Considering there to be no urethritis with the presence of <5 PMN leukocytes causes 32% of CT-induced cases to go undetected, along with 37% caused by MG, 38% by adenovirus and 44% by herpes virus; it is thus deduced that this is not a good cut-off point for ruling out infection.21 Various studies22–26 highlight that, in order to determine the existence of urethritis, a cut-off point of ≥5 PMN leukocytes is not very sensitive (Table 3), and that lowering it to ≥2 PMN leukocytes increases sensitivity (Table 4).
- b)
Methylene blue stain for urethritis: another aspect to take into account in stains is whether the Gram stain is superior to others. The Gram stain has been compared to the methylene blue stain and a mixture of methylene blue and crystal violet (4:1 parts),27,28 which favours the intra or extracellular examination of diplococci.
The main advantage is that it takes less time to perform: 4min (Gram) versus 10–15s (methylene blue), with a sensitivity of 97.3%, a specificity of 99.6% and a concordance of 100%. Although the bacterial morphology cannot be seen with its stain characteristics in methylene blue, this is not important for examining the presence of PMN leukocytes and, thus, urethritis.
- c)
Gram stain for cervicitis: this is performed on cervical discharge and >10 PMN leukocytes with a 1000× objective lens is deemed to be suggestive of infection.
- d)
NAATs: NG detection with NAATs is more sensitive than culture and may be performed on a wide range of samples: urethral or urine in men, and endocervical or vaginal in women. Urine samples in women have a lower sensitivity and they are not an optimal sample (II, B).29
In the rectum and pharynx, NAATs are more sensitive than culture, but positive samples should be confirmed with another NAAT that uses a different target (III, C).
In any case, in their current formats, NAATs cannot be considered rapid tests, although the incorporation of the LAMP technique renders it adequate in terms of its rapidness, sensitivity and specificity. A LAMP technique by the same manufacturer is available on the market, with an identical format and characteristics to the one mentioned previously for chlamydia. However, although rapid (<1h), it cannot be used as a POCT due to its complexity.
The GeneXpert CT/NG NAAT, with the characteristics cited in the “Chlamydia” section, presents excellent sensitivity (98–100%) and specificity (99.9%) in vaginal and endocervical samples as well as male urine, with lower results in urine samples from women.17
Rapid tests for diagnosing syphilis through the detection of antibodies have shown excellent results in prenatal syphilis screening, particularly in developing countries. Opportunities for accessing pregnant women are rare, and diagnosis—and, where applicable, treatment—have to be given at the time of the consultation. It is estimated that in Sub-Saharan Africa, where there is a syphilis seroprevalence of 8.3%, only 38% of pregnant women have access to neonatal screening, so half a million children are believed to die every year due to congenital syphilis sequelae.6 With regard to the serological laboratory techniques used in developed countries that require a centrifuge, new immunochromatography techniques may be considered authentic POCTs given that they can be performed on whole blood obtained by finger prick, the reagents do not require refrigeration and they obtain results in 15–20min. Although they are easy to perform, the manufacturer's instructions must be strictly adhered to in order to guarantee accuracy. These techniques use recombinant antigens and capture specific treponemal antibodies, so the exclusive performance of this test leads to unnecessary treatments in previously treated and cured patients who continue to present lifelong treponemal antibodies. In such cases that come back positive during the initial screening period, referrals to reference laboratories would be ideal in order to determine the disease activity with non-treponemal tests. A dual POCT system that separately determines the presence of treponemal and non-treponemal antibodies is available on the market, offering diagnostic confirmation when both are positive. However, the lack of non-treponemal antibody quantification inhibits the determination of the initial titre for treatment control. Dual determination is also possible in the same antibody assay for syphilis and HIV.
Things are very different in our setting, and serological tests are usually carried out in the context of other laboratory tests. An initial positive non-treponemal test result would be confirmed with another treponemal test, and the same is true in reverse; if we carry out an initial treponemal test (usually an EIA), we confirm a positive result with a non-treponemal test (reverse algorithm). These tests are performed in a scheduled manner in the laboratory.
However, it is clear that in certain situations where an immediate diagnosis prevails over other considerations, the performance of an RPR test only requires one centrifuge, the reagent and the person responsible for performing the technique having the knowledge to do so.
Dark-field microscopy requires a microscope with the aforementioned characteristics (which not all laboratories have) and a microscopist who is an expert in the technique, which is considerably complex for the observer. Non-genital samples, especially oral samples, may contain nonpathogenic treponemes that lead to false positives, so their use is not recommended. There is a IIA recommendation for performing dark-field microscopy on chancre samples, provided an expert microscopist and adequate equipment are available.30
PCR techniques, on the other hand, may be used on oral and other extra-genital and genital samples, with a IA recommendation,30 since commensal treponemes do not interfere with the technique. These techniques are only available at referral centres, but are not considered “rapid response” unless they are of the LAMP variety.
TrichomoniasisA wet mount examination is easy to perform, rapid, low-cost and highly specific (98%), but has a low sensitivity (62–92%) and is dependent on the observer. To perform this test, a drop of urethral or vaginal discharge is mixed on a slide with a drop of 0.5% physiological saline solution at 37°C. A coverslip is then applied and it is observed under a microscope to check for the characteristic movements of trichomonas. The preparation should be examined within 10min of taking the sample, as the characteristic movement of the trichomonas parasite reduces after this time, thus hindering its identification.31
As mentioned previously in the “Candidiasis” section, the BD Affirm VPIII™ system also determines the presence of TV, in this case with a sensitivity of 89.2% and a specificity of 99.3% compared to culture.
Moreover, there are POCTs such as the OSOM Trichomonas Rapid Test (Sekisui Diagnostic, Hartwell Place, Lexington, United States), which determines the presence of the antigen and has shown a high sensitivity (80–94%) and specificity (95%). No instruments are required and it provides results in 30min, although it must be taken into account that false positives are possible (IIb, B).32
Herpes simplex virus (HSV)There are various rapid techniques:
- a)
Optical microscopy. This technique is inadequate. Direct examination using the Tzanck or Papanicolaou smear has a limited sensitivity and is nonspecific. It does not differentiate between HSV-1 and 2, and Cowdry type A intranuclear inclusions are not exclusive to the HSV. Moreover, a negative result does not rule out the possibility of genital herpes.
- b)
Electron microscopy. This has fallen out of use as it requires concentrations of over 108virions/ml. It provides rapid results but has a limited sensitivity and requires equipment that is not widely available in clinical laboratories.
- c)
Direct immunofluorescence. HSV antigens can be detected with immunofluorescence or immunoenzyme techniques. These are rapid techniques with a sensitivity and specificity ranging from 70% to 90% in symptomatic patients. On direct samples, sensitivity reduces as the lesion develops over time. The use of monoclonal antibodies provides good specificity and facilitates the differentiation of HSV-1 and 2. It is currently used more as a complement to identify viruses in cell cultures.
- d)
NAATs are recommended for the diagnosis of genital herpes (Ib, A).33 Detecting HSV DNA using PCR techniques increases detection by 11–71% compared to culture. It also allows for more lenient conditions than culture with regard to sample storage and transportation. It allows for the differentiation of HSV-1 and 2, which should be established in all new diagnoses (III, B). Positive results do not require confirmation.
Vaginosis may be diagnosed on the basis of three sets of criteria (Amsel, Nugent or Ison) (II, B).10
The Amsel clinical criteria34 are based on the presence of three of the following signs or symptoms: (a) thin, homogeneous, white, uniform vaginal discharge that adheres to the vaginal walls; (b) vaginal pH >4.5; (c) fishy odour after adding 10% KOH to the sample; and (d) more than 20% clue cells in wet mount preparations (microscope with a 40× objective lens).
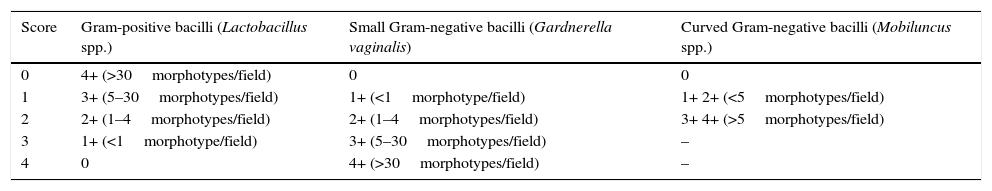
It may be confirmed by objective microscopic criteria viewed in a Gram stain of vaginal discharge using the Nugent35 score (Table 5). This is considered the gold standard. The microbiological diagnosis of bacterial vaginosis is carried out using a Gram stain of vaginal discharge, determining the relative quantity of morphotypes characteristic of abnormal vaginal microbiota (Gram-positive and Gram-negative bacilli and curved bacteria) and the presence of clue cells (epithelial cells coated with Gram-positive and Gram-negative morphotypes with obscured borders). Stained cervical smears using the Papanicolaou method are not appropriate due to their low sensitivity.
Interpretation of the Nugent criteria.
| Score | Gram-positive bacilli (Lactobacillus spp.) | Small Gram-negative bacilli (Gardnerella vaginalis) | Curved Gram-negative bacilli (Mobiluncus spp.) |
|---|---|---|---|
| 0 | 4+ (>30morphotypes/field) | 0 | 0 |
| 1 | 3+ (5–30morphotypes/field) | 1+ (<1morphotype/field) | 1+ 2+ (<5morphotypes/field) |
| 2 | 2+ (1–4morphotypes/field) | 2+ (1–4morphotypes/field) | 3+ 4+ (>5morphotypes/field) |
| 3 | 1+ (<1morphotype/field) | 3+ (5–30morphotypes/field) | – |
| 4 | 0 | 4+ (>30morphotypes/field) | – |
However, the BASHH guidelines recommend (grade C) the use of the Ison and Hay criteria,36 as they reflect real microbiota possibilities better than the Nugent criteria.
Grade 0: Unrelated to bacterial vaginosis; epithelial cells only, with no Lactobacillus morphotypes. May indicate recent antibiotic use.
Grade 1 (normal): Lactobacillus morphotypes predominate.
Grade 2 (intermediate): mixed flora with some lactobacilli present, but Gardnerella or Mobiluncus morphotypes also present.
Grade 3 (bacterial vaginosis): predominantly Gardnerella and/or Mobiluncus morphotypes. Few or absent lactobacilli.
Grade 4: unrelated to bacterial vaginosis; only Gram-positive cocci observed, no lactobacilli.
Commercial systems also exist, although some are not marketed in Spain:
OSOM BVBlue (Sekisui Diagnostic, Hartwell Place, Lexington, United States) measures sialidase levels.
Pip Activity TestCard (Litmus Concepts Inc, Santa Clara, California, United States) assesses proline aminopeptidase.
BD Affirm VPIII (Becton Dickinson, Franklin Lakes, New Jersey, United States) uses a DNA probe to detect high concentrations of GV, with a sensitivity of over 90% and a specificity of over 99%.
PCR-based systems have also been described but are not available on the market.
In relation to POCTs, the most widely implemented tests at present are for HIV, followed by TP. As for gonorrhoea, the same problems regarding cross-reactions continue to arise, and there is limited experience with others, such as those used for the HPV or HSV.
Multiple platforms are being developed, such as the one designed for nine pathogens by FilmArray (BioFire Diagnostics, Salt Lake City, Utah, United States), with promising initial results, though it is yet to be marketed.37
Future of rapid tests in sexually transmitted infectionsSuccessfully controlling STIs will largely depend on the availability of aetiological diagnoses which, as well as being sensitive and specific, are rapid and allow immediate aetiological treatment. This is the n of the WHO in the Global health sector strategy on sexually transmitted infections, 2016–2021.38
A rapid diagnosis can be achieved if there is a laboratory close to the point of care, with rapid techniques and personnel willing to perform these immediately. This may be possible at certain highly-specialised centres, but laboratory centralisation trends are heading in the opposite direction. Multiple PCRs that provide results in 1–2h may be an alternative here, including GeneXpert (Cepheid, Sunnyvale, CA, United States).
It can also be achieved by providing points of care with basic laboratory materials (stain reagents, centrifuges and microscopes) and by training the clinic on their use. Although this is in place at some centres, its widespread implementation seems to be a challenge.
Instead, the future of rapid diagnosis lies in the development and implementation of POCTs that allow the diagnosis of as many pathogens as possible (ideally in multiple formats) and that meet most of, if not all, the ASSURED criteria proposed by the WHO. We have rapid tests for syphilis and HIV but still lack genuinely rapid point-of-care tests that enable the diagnosis of highly prevalent and curable infections, such as those caused by CT, TV and NG, within a matter of minutes. Generally speaking, the available methods do not reach the sensitivity and specificity levels attained by PCR techniques.
Among the most promising tests described in the literature, we can site the assay for CT with microwave accelerated metal enhanced fluorescence (MAMEF) technology, which provides results in 9min with a sensitivity of 82% and a specificity of 93%.39
Moreover, hopes are high regarding microfluidic techniques, which are microsystems that incorporate assay operations and sample preparation on a chip, with the advantages being that they require very small quantities of sample, have a faster response time and are portable. Generally speaking, these techniques work well for antibody detection due to the abundance of target molecules. For other samples, such as urine or those obtained with a swab, the sample will require prior processing before it is added to the microsystem. This presents limitations for antigen detection due to few target molecules being found in the sample.
The future also lies in determining the presence of antimicrobial resistances, which is especially important for NG and MG. Work on array and microfluidic formats is currently only being conducted at highly-specialised laboratories, although it would be ideal if they could be used at the point of care.
Conflicts of interestNone.
Please cite this article as: Otero-Guerra L, Fernández-Blázquez A, Vazquez F. Diagnóstico rápido de las infecciones de transmisión sexual. Enferm Infecc Microbiol Clin. 2017;35:444–450.