We have recently documented a case of tropical spastic paraparesis by HTLV-I in a Spanish patient. HTLV-I infection is rare in Europe, and hardly ever is accompanied by symptoms, but if it does it could trigger a major health issue. This case is presented here, as well as a discussion on the situations in which HTLV-I detection is justified. An analysis was made of the HTLV diagnostic requests at our centre during 2014–2015 (n=123). The diagnostic algorithm was: 1) enzyme immunoassay, 2) reverse hybridisation, and 3) proviral DNA detection by PCR. The results showed several situations of HTLV screening, emphasising those related to paraparesis (22%). Seven cases of HTLV-I infection were found: five in patients from endemic regions, one in an HIV-infected patient, and the case of TSP mentioned above. HTLV-I surveillance in non-endemic regions is a challenging issue, as the cost-benefit ratio is not well-established. This case report emphasises the importance of including HTLV within the differential diagnosis of insidious spastic paraparesis.
Recientemente hemos documentado un caso de paraparesia espástica tropical por HTLV-I en un paciente de nacionalidad española. Este retrovirus infrecuente en Europa rara vez produce sintomatología, pero cuando lo hace supone un grave problema sanitario. Aquí presentamos dicho caso y discutimos situaciones clínicas que justifiquen su detección. Se analizaron las peticiones de cribado de HTLV que recibimos durante 2014-2015 (n=123). El algoritmo diagnóstico fue: 1) Enzimoinmunoanálisis, 2) Hibridación reversa y 3) PCR de ADN proviral. Los resultados mostraron diversas situaciones de cribado de HTLV, destacando el estudio de paraparesia (22%). Se detectaron 7 casos de infección por HTLV-I: 5 pacientes de zona endémica, un paciente VIH+ y por último el caso de paraparesia mencionado. La vigilancia de HTLV-I en regiones no endémicas supone un reto sanitario al no estar bien establecido su balance coste-beneficio. Este caso apoya la inclusión de HTLV-I dentro del diagnóstico diferencial de paraparesia espástica de evolución insidiosa.
The human T-lymphotropic virus (HTLV) belongs to the Retroviridae family and includes four phylogroups (HTLV I/II/III/IV). It is present throughout the world, with its greatest prevalence (0.5–50%)1 being in endemic regions such as Japan, the Caribbean, Sub-Saharan Africa and Central and South America. It is very uncommon in Europe and is primarily observed in imported cases. In Spain, recent studies confirm this low prevalence, which stands at less than 0.23% even among risk groups.2,3 258 cases of HTLV-I infection had been reported by December 2013, along with 769 HTLV-II cases.4 No cases of HTLV-III or HTLV-IV have been reported to date. HTLV transmission generally occurs through intercourse, from mother-to-child (during breastfeeding), via blood products/organs and through the consumption of parenteral administration drugs, with the latter being a characteristic mechanism of HTLV-II.5
Clinical manifestations in HTLV infection are uncommon and carriers are generally asymptomatic. Nevertheless, there are two incurable aetiologies caused by HTLV-I: tropical spastic paraparesis (TSP) or HTLV-I-associated myelopathy, and adult T-cell leukaemia. Both diseases amount to a global incidence of 5% among infected patients.6 In Spain, 27 cases of TSP/HTLV-I-associated myelopathy had been reported by December 2012, along with 17 cases of adult T-cell leukaemia.3
This article describes a case of non-endemic TSP diagnosed in Spain, the only risk factor of which was having sexual relations with people from endemic areas. We also present the HTLV-I/II screening results from our hospital over the past year and discuss the associated risk factors.
Material and methodsAll HTLV screening requests between January 2014 and March 2015 were studied. HTLV-I/II IgG antibodies were detected by means of enzyme immunoassays (EIA) in the automatic Architect system (Abbott Laboratories, Abbott Park, IL, USA). Positive results were confirmed by reverse hybridisation (RH) using the INNO-LIA™ HTLV-I/II Score test (Fujirebio Europe, Gent, Belgium). Finally, the PCR was performed at the Instituto de Salud Carlos III in Madrid, by amplifying the pX region of the virus.7,8
Epidemiological data such as patient age, gender and origin, coinfection with other viruses, requesting department, suspected diagnosis and other relevant clinical information were also obtained.
ResultsCase report34-Year-old Spanish male admitted for an assessment of progressive abnormal gait, which has been ongoing for two years. His medical history included systemic mastocytosis diagnosed at 14 years of age (currently asymptomatic), incompletely resolved idiopathic peripheral facial paralysis seven years prior and severe autoimmune pancreatitis two and a half years prior, which required surgery and a transfusion of two packed red blood cell units. On admission, a neurological examination showed symmetrical spastic paraparesis, with hyperreflexia in the lower limbs and bilateral Babinski reflex. Superficial and deep sensitivity were normal. He also presented urinary urgency and occasional incontinence. Magnetic resonance imaging of the spine revealed no abnormalities. Cerebrospinal fluid (CSF) analysis showed mild lymphocytic pleocytosis (5cells/mm3), with normal protein and glucose levels. Moreover, his blood work was normal and he tested negative for syphilis and HIV. HTLV-I/II antibodies (anti-HTLV) tested by EIA were positive, and this was confirmed by RH (HTLV-I). His CSF also tested positive for HTLV-I antibodies, with viral replication detected in both serum and CSF by PCR. The transfusion donor's serological results were repeatedly negative. The patient had not taken any trips outside of Spain, but had had sexual relations with at least two men of South American origin; he did not specify their countries of origin. He was treated with methylprednisolone for five days, with no clinical improvement. He has also received physiotherapy treatment and botulinum toxin for his spasticity, with a partial improvement in gait.
ScreeningDuring the study period, 123 samples were received for the detection of HTLV IgG antibodies. 60% were from men and 40% from women, with a mean age of 43.4 years. The departments to request screening most frequently were Infectious Diseases (n=46; 37.4%), Neurology (n=26; 21.1%), Haematology (n=11; 8.9%) and Internal Medicine (n=9; 7.3%).
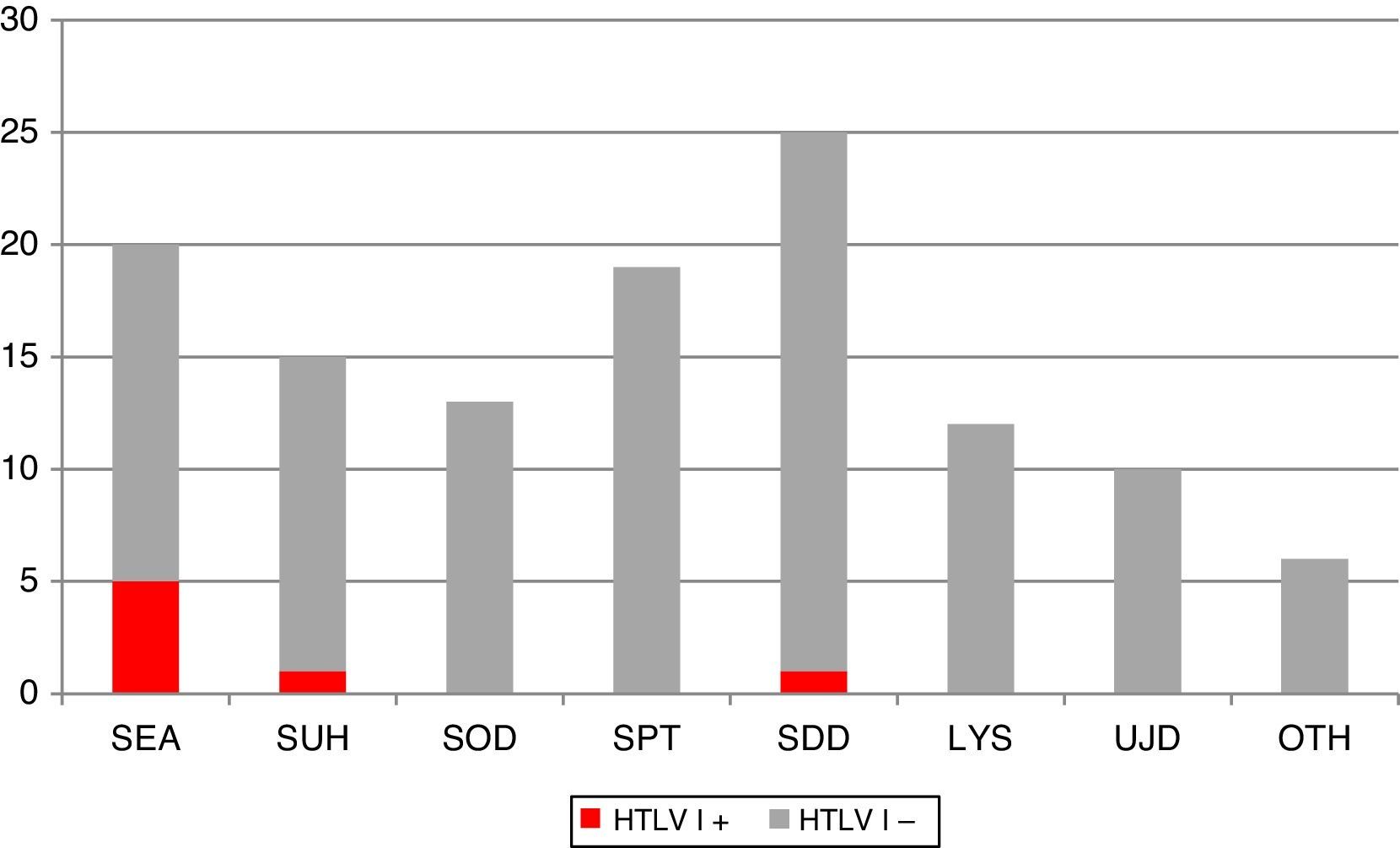
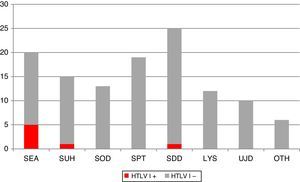
Fig. 1 shows the clinical and/or epidemiological criteria followed for the screening of HTLV-I/II. Of all the requests, 8.1% (n=10) did not meet any of these criteria and they were therefore considered unjustified requests. Conversely, among the justified requests, the most common reasons were: paraparesis assessment (n=25; 22.1%); screening in patients from an endemic area (n=20; 17.7%); pre-transplant screening (n=19; 16.8%); screening in parenteral drug users and/or HIV+ patients (n=18; 15.9%); screening in organ donors (n=13; 11.5%); suspected lymphoproliferative syndrome (n=12; 10.6%) and others (n=6; 5.3%). The latter category included clinical contexts that do not justify HTLV suspicion per se, but which are accompanied by some form of risk factor.
Distribution of different clinical and epidemiological criteria that motivate the screening of HTLV-I/II IgG antibodies at our centre. Positive HTLV-I cases are highlighted in the darker colour. LYS: lymphoproliferative syndrome; OTH: others; PDU: parenteral drug users; SDD: suspected demyelinating disease; SEA: routine screening in patients from endemic areas; SOD: screening in organ donors with risk factor; SPT: screening in pre-transplant patients with risk factor; SUH: routine screening in PDU and/or HIV+ patients; UJD: unjustified.
In total, 11 positive results were observed by EIA (8.9% of the total) and seven were confirmed as positive for HTLV-I by RH (Table 1). A PCR was performed on whole blood in six. It was not possible to locate the remaining patient. Viral replication was seen in all of them.
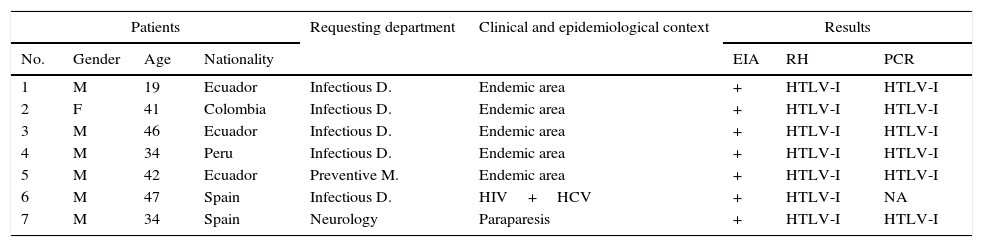
Analytical, clinical and epidemiological description of the 7 positive HTLV-I patients.
| Patients | Requesting department | Clinical and epidemiological context | Results | |||||
|---|---|---|---|---|---|---|---|---|
| No. | Gender | Age | Nationality | EIA | RH | PCR | ||
| 1 | M | 19 | Ecuador | Infectious D. | Endemic area | + | HTLV-I | HTLV-I |
| 2 | F | 41 | Colombia | Infectious D. | Endemic area | + | HTLV-I | HTLV-I |
| 3 | M | 46 | Ecuador | Infectious D. | Endemic area | + | HTLV-I | HTLV-I |
| 4 | M | 34 | Peru | Infectious D. | Endemic area | + | HTLV-I | HTLV-I |
| 5 | M | 42 | Ecuador | Preventive M. | Endemic area | + | HTLV-I | HTLV-I |
| 6 | M | 47 | Spain | Infectious D. | HIV+HCV | + | HTLV-I | NA |
| 7 | M | 34 | Spain | Neurology | Paraparesis | + | HTLV-I | HTLV-I |
EIA: enzyme immunoassay; F: female; HCV: hepatitis C virus; HIV: human immunodeficiency virus; HTLV: human T-lymphotropic virus; M: male; NA: not available; PCR: polymerase chain reaction; RH: reverse hybridisation.
Finally, the clinical and epidemiological characteristics of these patients were analysed: Five came from endemic areas, while the two remaining patients were Spanish; one was coinfected with HIV and HCV while the other was the TSP case described above.
DiscussionThe TSP/HTLV-I-associated myelopathy case presented herein includes all the typical characteristics of this disease: progressive evolution, weakness and spasticity in the lower limbs and impaired urethral sphincter.5 It is worth noting that the HTLV study was performed as part of the assessment of spastic paraparesis of unknown origin, despite the fact there had been no epidemiological suspicion of HTLV-I infection initially.
The criteria for performing HTLV-I screening at our centre during the study period were fairly homogeneous. It is striking that the most common reason (22%) was to rule out this virus as the cause behind spastic paraparesis. Moreover, the yield obtained in screening was fairly heterogeneous if we take into account that we only detected positive cases in 3 of the 7 clinical and epidemiological profiles that motivated the request. Testing for HTLV-I in patients from endemic areas (5/20), parenteral drug users and/or HIV+ patients (1/18), and patients with suspected tropical spastic paraparesis (1/25) proved most efficient.
In Spain, HTLV-I surveillance has traditionally focussed on blood donors. Various studies have recently established the prevalence of HTLV-I in new donors within Europe at 0–0.0048%.9–11 Countries such as Norway and Finland cancelled HTLV screening at their blood banks after 7 and 13 years of screening with no positive cases.9 However, in Spain the latest data generate a prevalence of 0.01%6 in new donors, which may warrant the continuation of the current strategy. As regards screening for HTLV-I in pregnant women, there is no consensus in regions of low prevalence. The most common indication in Spain could be the selective screening of pregnant women from endemic areas or who have had risky sexual relations.3 With respect to organ donors and recipients, a similar strategy could be applied, although the high risk of false positives and the consequent loss of a valid organ may end up contraindicating such screening in areas of low prevalence.12 On the other hand, as can be seen from the case presented above, HTLV analysis is a useful diagnostic tool in cases of chronic myelopathy or lymphoproliferative syndrome of unknown origin. Another group that may be included in screening is HIV+ patients, particularly if they are parenteral drug users or from an endemic area. HIV/HTLV coinfection—particularly HTLV-II—is common as they share the same transmission mechanisms.13
HTLV-I is uncommon in Spain. Its range of transmission mechanisms, low prevalence and typically asymptomatic presentation hinder its detection. The reported case of indigenous TSP compels us to include this disease within the differential diagnosis of progressive myelopathy. A clear consensus should also be established for performing HTLV-I screening in non-endemic countries such as Spain, which may be considered in the following groups: blood donors, organ recipients and donors from endemic areas, suspected spastic paraparesis or lymphoproliferative syndrome with some form of associated risk factor, pregnant women from endemic areas, HIV+ patients or parenteral drug users and individuals that have sexual relations with people from endemic areas. In other clinical situations, the efficiency of different HTLV-I screening strategies is minimal.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Please cite this article as: Ponce-Alonso M, del Corral-Corral I, Ortiz-Rivera M, Anciones-Martín C, Mateos-Lindemann ML. Paraparesia espástica tropical autóctona en la Comunidad de Madrid. Experiencia en el cribado del virus linfotrópico de células T humanas tipo I. Enferm Infecc Microbiol Clin. 2017;35:441–443.