Mucormycosis with oral involvement (OIM) is a rare opportunistic and lethal mycosis, which has increased in the last decade and is generally associated with uncontrolled diabetes and neutropenia.
MethodsA retrospective study of cases with OIM was carried out in a tertiary-care center. Mycological and histological examinations were performed, and the isolated organisms were identified by morphology and molecular biology.
ResultsFifty-five OIM patients were included, with a median age of 38 years (61.8% males). The most frequent associated disease was type-2 diabetes mellitus (61%), followed by neutropenia due to acute lymphocytic leukemia (27%). The main presentation was palatal and mandibular ulcers (92.7%) and, to a lesser extent, gingival and lingual necrosis. The diagnosis was established by mycological and histopathological studies. The most frequent fungi isolated was Rhizopus arrhizus (67.2%).
ConclusionOIM is a rapidly progressing disease, therefore, an early diagnosis and the proper control of predisposing factors is necessary, and consequently, contributing to improve the outcome of mucormycosis.
La mucormicosis con afectación oral (MAO) es una micosis oportunista, letal y poco frecuente, pero que ha aumentado en la última década y que generalmente se asocia a diabetes descontrolada y neutropenia.
MétodosSe realizó un estudio retrospectivo de casos con MAO en un centro de tercer nivel. Se realizaron examen micológico e histológico, y los organismos aislados se identificaron por morfología y biología molecular.
ResultadosSe incluyeron 55 pacientes con MAO, con una mediana de edad de 38 años (61,8% varones). La enfermedad asociada más frecuente fue la diabetes mellitus tipo 2 (61%), seguida de la neutropenia por leucemia linfocítica aguda (27%). La presentación principal fueron úlceras palatinas y mandibulares (92,7%) y, en menor medida, necrosis gingival y lingual. El diagnóstico se estableció con estudios micológicos e histopatológicos. El hongo más frecuentemente aislado fue Rhizopus arrhizus (67,2%).
ConclusiónLa MAO es una enfermedad de rápida evolución, por lo que es necesario un diagnóstico precoz y un adecuado control de los factores predisponentes y, en consecuencia, contribuir a mejorar la evolución de la mucormicosis.
Mucormycosis is a rare, aggressive, and opportunistic mycosis, with increased morbidity in the last decade.1–3 It is caused by Mucorales, classified within the Mucoromycotina subphylum with more than thirty species of nine genera, with a predominance of Rhizopus arhizus (formerly R. oryzae).3,4 Rhinocerebral mucormycosis (RCM) is the most common in up to 88% of cases, associated with several underlying diseases like uncontrolled diabetes, with or without ketoacidosis, and leukemia-related neutropenia.4 The first one is usually observed in developing countries such as India, Iran, and Mexico,4–6 and the second is often reported in Europe and developed countries.1,3
Oral involvement in mucormycosis (OIM) has been reported in 20–50% of cases of mucormycosis, mostly associated with RCM, disseminated forms, or as the primary site, mostly related to rapidly growing palatal ulcers.7 The control of the underlying diseases is necessary (diabetes, immunosuppression, underlying tumor), as well as an early diagnosis and an effective and multidisciplinary therapy.7,8
Here we evaluate a series of OIM patients with a culture- and molecular-proven diagnosis, and analyze their clinical characteristics and outcomes.
Material and methodsThe present retrospective study included 55 OIM cases in a tertiary-level hospital for 35 years (1985–2019). Demographic data and medical records were collected. Mycological studies, including direct examination (20% KOH) and cultures from various clinical materials were also recorded.
Genomic DNA of each strain was extracted by the phenol–chloroform method9 and then used as a template for PCR amplification of the non-coding ITS region using the primers IT5 (5′-GGAAGTAAAAGTCGTAACAAGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′).10 Finally, amplicons were purified (Promega, USA) and sequenced by Sanger in the ABI 3130 genetic analyzer (Applied Biosystems, USA). Biopsies were occasionally performed. The institutional review board approved the study at our Hospital.
Data collected from medical records were entered and analyzed using SPSS v25 (USA). The demographic, epidemiological and clinical characteristics were categorized and described.
The statistical analysis was performed with the description of the demographic, epidemiological, clinical and diagnosis profile of OIM patients.
ResultsFifty-five OIM cases were selected from a total of 162 cases (33.9%) with RCM. Demographic, epidemiological, clinical, and mycological data are shown in Table 1.
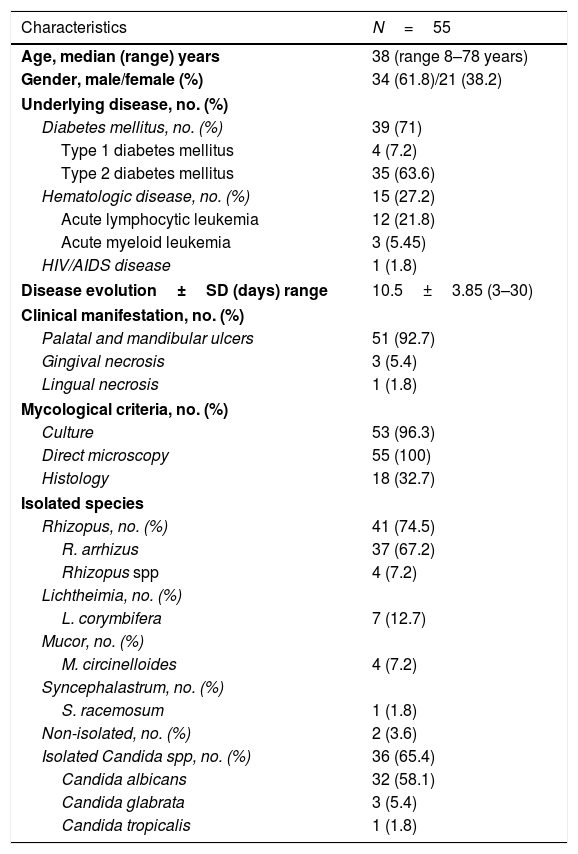
Demographic, epidemiological, clinical, and mycological data of the OIM patients (1985–2019).
| Characteristics | N=55 |
|---|---|
| Age, median (range) years | 38 (range 8–78 years) |
| Gender, male/female (%) | 34 (61.8)/21 (38.2) |
| Underlying disease, no. (%) | |
| Diabetes mellitus, no. (%) | 39 (71) |
| Type 1 diabetes mellitus | 4 (7.2) |
| Type 2 diabetes mellitus | 35 (63.6) |
| Hematologic disease, no. (%) | 15 (27.2) |
| Acute lymphocytic leukemia | 12 (21.8) |
| Acute myeloid leukemia | 3 (5.45) |
| HIV/AIDS disease | 1 (1.8) |
| Disease evolution±SD (days) range | 10.5±3.85 (3–30) |
| Clinical manifestation, no. (%) | |
| Palatal and mandibular ulcers | 51 (92.7) |
| Gingival necrosis | 3 (5.4) |
| Lingual necrosis | 1 (1.8) |
| Mycological criteria, no. (%) | |
| Culture | 53 (96.3) |
| Direct microscopy | 55 (100) |
| Histology | 18 (32.7) |
| Isolated species | |
| Rhizopus, no. (%) | 41 (74.5) |
| R. arrhizus | 37 (67.2) |
| Rhizopus spp | 4 (7.2) |
| Lichtheimia, no. (%) | |
| L. corymbifera | 7 (12.7) |
| Mucor, no. (%) | |
| M. circinelloides | 4 (7.2) |
| Syncephalastrum, no. (%) | |
| S. racemosum | 1 (1.8) |
| Non-isolated, no. (%) | 2 (3.6) |
| Isolated Candida spp, no. (%) | 36 (65.4) |
| Candida albicans | 32 (58.1) |
| Candida glabrata | 3 (5.4) |
| Candida tropicalis | 1 (1.8) |
SD=standard deviation.
Thirty-four patients were male (61.8%), and 21 were female (38.2%) with a median age of 38 years (range 8–78 years). Disease evolution ranged from 3 to 30 days (mean 10.5±3.85 days). The most frequent clinical sign was palatal and mandibular ulcers (51 cases, 92.7%). All patients initially present headaches, facial swelling, fever, and pain. The most associated underlying condition was type 2 diabetes (35 cases, 63.6%), followed by acute lymphocytic leukemia (12 patients (21.8%).
Direct microscopic examination was positive in all 55 cases and revealed broad coenocytic or aseptate fungal hyphae with a right-angle branching (90°), consistent with the morphological features of Mucorales. Histology showed positive findings in 18 cases (32.7%). Fungal culture was the main method to confirm the diagnosis, and correlated with the presence of fungal elements in direct examination (53 patients, 96.3%). The most commonly isolated species was Rhizopus arrhizus in 37 cases (67.2%), followed by Lichtheimia corymbifera in 7 cases (12.7%). Disease severity was assessed by computed tomography scanning. We did not find any cerebral cases.
Amphotericin B combined with surgical debridement achieved clinical and mycological cure in 14 cases (25.4%). No serious adverse events were reported in the clinical records of the included patients secondary to the use of amphotericin B; 41 patients (74.6%) developed a more severe form of the disease and died.
DiscussionMucormycosis is generally considered an emerging disease that has increased in frequency in the last two decades.1–4 OIM usually develops as an extension of RCM, or as the primary site of mucormycosis. The disease is rapidly progressing and often depends on the metabolic and immune status of the patient.2,4–7,11 The present study involves a large case series of OIM; our patients had a median age of 38 years, with a slight male predominance (61%); the patients had a marked association with diabetes (71%), mostly type 2, similar to a previous study in Mexican patients (72%)6,7 and higher than the most extensive meta-analysis report including 929 patients,12 reporting diabetes in 39% of cases; 48% and 40% of cases have been reported by others.2,4,13
The second most associated comorbidity found in our study was neutropenia (27%), mainly related to acute lymphocytic leukemia, unlike previous studies reporting acute myeloid leukemia as the most frequent cause of neutropenia in mucormycosis.2,4,14 We also observed a single case with HIV-AIDS; this association has been rarely reported, often in chronic cases with leukopenia (neutropenia and lymphopenia).2,12,14
The most common clinical presentation included palatal ulcers; maxillary involvement was also observed; the sporangiospores enter through the nasal and palatal mucosa, invading the palatine and sphenopalatine arteries due to the angioinvasive ability of the Mucorales, and may progress to cerebral cases.7,8,13 The palate usually develops black or pale necrotic ulcers, with well-defined edges; disease progression depends on the underlying metabolic comorbidities (diabetes with or without ketoacidosis), the severity of neutropenia, and either immunosuppression by corticosteroids or cytotoxic drugs. Differential diagnoses may include aspergillosis, squamous cell carcinoma, or lymphomas.7,13
OIM usually develops lysis of the maxilla and adjacent structures like the alveolar ridge, mandible, lips, and cheeks.7,8,13 In our study, most cases developed palatal and often mandibular involvement. We observed a single case with lingual mucormycosis, being this manifestarion rarely reported in the literature. In 8 cases (14.5%), the primary site was the oral mucosa, five as palatal ulcers, and three with gingival involvement, rapidly progressing to cerebral mucormycosis; therefore, OIM may be the first manifestation of RCM, often with poor prognosis.7,11,13
Early diagnosis of OIM is critical to prevent RCM. Direct microscopic examination with 20% KOH, shows fungal structures as thick, coenocytic (not septate) hyaline hyphae, with branches often arising non-dichotomously in right angles (90°), different from aspergillosis.7,10,14 Biopsies are often useful, mainly in cases with negative direct microscopic examination; histopathological analysis with hematoxylin–eosin staining reports edema, necrosis, polymorphonuclear cells, plasma cells, and intense angioinvasion; silver methenamine stains (Gomori-Grocott) highlights fungal structures.7,10,14
Cultures grow in the usual media, after 3–5 days of incubation.7,10 We observed a predominance of R. arrhizus, the most reported agent worldwide (>80% of cases)2,4,5,14,15; L. corymbifera (formerly Absidia corymbifera), Mucor circineloides, and Syncephalastrum racemosum are less frequent14; S. racemosum has not been previously reported as a cause of OIM.
Our study showed candidiasis in 65% of cases; this was probably due to the underlying diseases (diabetes and neutropenia); the most frequently isolated species was Candida albicans. The presence of Candida spp., may interfere with the growth of the Mucorales, hindering the mycological findings, and thus, it may delay the diagnosis of mucormycosis.7
The Global Guideline for the treatment of mucormycosis recommends the control of the underlying conditions (uncontrolled diabetes and immunosuppression),15 as well as an early diagnosis and an effective treatment including systemic antifungal with extensive surgical debridement. The first choice is liposomal amphotericin B that exhibits better safety profile and effectiveness than deoxycholate and can be used at higher doses and for more extended periods. It can be effectively combined with posaconazole, and isavuconazole.15 Liposomal amphotericin B and the new triazoles are more expensive than conventional systemic antifungal and not accessible in low-income countries.
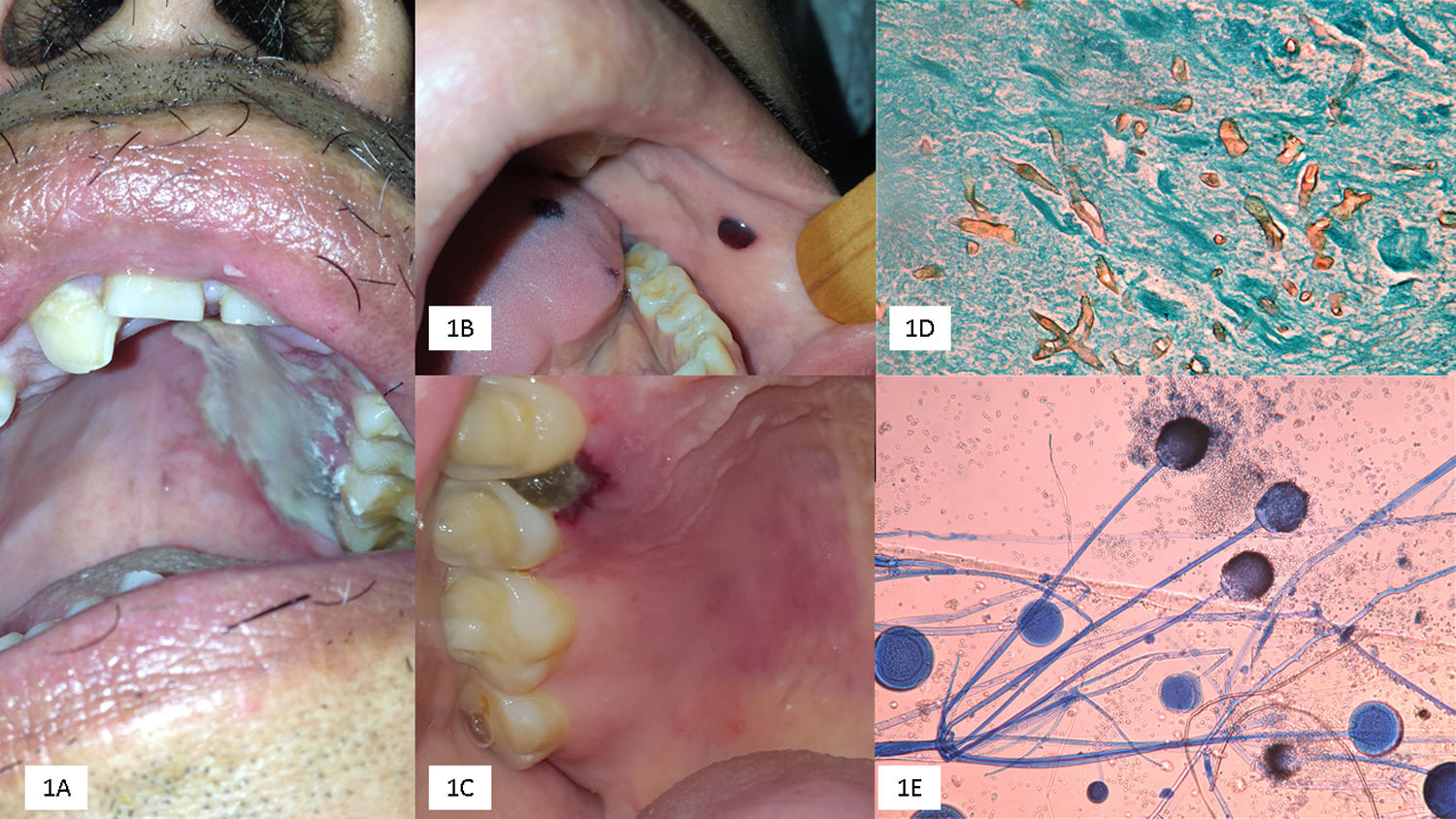
We emphasize that OIM may be the primary site of mucormycosis or developed as an extension of RCM. OIM represents one-third of the cases, mainly in the form of rapidly progressing palatal ulcers, increasing the risk of cerebral mucormycosis; however, this was not observed in our study (Fig. 1).
(A) Palatal ulcer in an uncontrolled diabetic patient. (B) Necrosis of the cheek lining and lingual in a patient with acute myeloid leukemia. (C) Necrotic lesion on the soft palate and onset of palatal ulcer. (D) Biopsy with multiple thick and coenocytic hyphae (Grocott, 40×). (E) Rhizopus arrhizus (cotton blue 10×).
Bonifaz A created and designed the study; Tirado-Sánchez A created, edited and statistical analysis of the study; Paredes-Farrera F was involved in the stomatology study; Hernández-Medel ML & Moreno-Moreno J, studies of infectology and final review and Araiza J the mycology studies; González GM molecular identification.
Conflict of interestNone to declare.