This study aimed to present real-life data on the use, efficacy, and safety of administering antibiotic therapy through portable elastomeric pumps (pEP) in the outpatient setting.
MethodsThis retrospective observational cohort study was conducted from January 2020 to May 2023 in a large academic hospital in Rome, Italy. All patients receiving antibiotic therapy via pEP were included up to a follow-up period of 90 days after the end of antibiotic therapy.
The primary outcome was the treatment response. Secondary endpoints were adverse events attributable to the drug administered, the vascular catheter, or the infection itself.
ResultsOf the 490 patients referred to our outpatient parenteral antibiotic therapy (OPAT) unit, 94 (19.2%) received antibiotic therapy via pEP and were included in the final analysis. The most frequently treated infections were those involving bone and prosthetics, including spondylodiscitis (n=27; 28.8%). Most infections were due to Pseudomonas aeruginosa (n=55; 48.3%). Cefepime (n=32; 34.0%), piperacillin/tazobactam (n=29; 30.9%), ceftolozane/tazobactam (n=7; 7.5%), and oxacillin (n=7; 7.5%) were the most frequently administered antibiotics. The infection cure rate reached 88.3% (n=83). 12 patients (12.8%) reported adverse events, of which half (6.4%) were drug-related and half (6.4%) were line-related.
ConclusionsOPAT through portable elastomeric infusion pumps proved to be safe and effective. It also contributed to the reduction of healthcare costs, fully respecting the principles of personalized medicine. This strategy has emerged as a promising tool for antibiotic stewardship and infection control.
El objetivo de este estudio es presentar datos sobre el uso, la eficacia y la seguridad de administrar terapia antibiótica a través de bombas elastoméricas portátiles (BEP) en pacientes ambulatorios.
MétodosEste estudio observacional retrospectivo se realizó entre enero de 2020 y mayo de 2023 en un gran hospital académico de Roma, Italia. Se incluyó a todos los pacientes que recibieron tratamiento antibiótico mediante BEP hasta un período de seguimiento de 90 días después del final de la terapia antibiótica. El resultado primario fue la respuesta al tratamiento. Los criterios de valoración secundarios fueron los efectos adversos atribuibles al fármaco administrado, al catéter vascular o a la propia infección.
ResultadosDe los 490 pacientes remitidos a nuestra unidad de tratamiento antimicrobiano domiciliario endovenoso (TADE), 94 (19,2%) recibieron tratamiento antibiótico vía BEP. Las infecciones tratadas más frecuentemente fueron aquellas de huesos y prótesis, incluyendo espondilodiscitis (n=27; 28,8%). La mayoría de las infecciones se debieron a Pseudomonas aeruginosa (n=55; 48,3%). Cefepima (n=32; 34,0%), piperacilina/tazobactam (n=29; 30,9%), ceftolozano/tazobactam (n=7; 7,5%) y oxacilina (n=7; 7,5%) fueron los antibióticos más frecuentemente administrados. La tasa de curación de la infección alcanzó el 88,3% (n=83). Doce pacientes (12,8%) notificaron eventos adversos, de los cuales la mitad (6,4%) estaban relacionados con el fármaco y la otra mitad (6,4%) estaban relacionados con el catéter.
ConclusionesEl TADE utilizando BEP demostró ser seguro y efectivo. También redujo los costes sanitarios, respetando los principios de la medicina personalizada. Esta estrategia es una herramienta prometedora para el uso optimizado de antimicrobianos y el control de las infecciones.
Patient-centered outcomes, patient satisfaction and optimization of healthcare resources represent the main goals for modern and future medicine. In this context, society and healthcare systems are working to develop solutions that allow optimal patient care in familial and less expensive settings. For example, the outpatient management of patients with severe infections that would require long antibiotic therapies perfectly fits in such a model, as it would allow the patient to be discharged earlier, sparing healthcare resources, to limit the risk of hospital-associated infections and to be managed directly at home.
In this context, intravenous (IV) antibiotic therapy using portable elastomeric pumps (pEP) is a relatively new method of administering parenteral antibiotics in outpatient settings. The elastomeric pump is a device that contains a prefilled cartridge of the antibiotic and is attached to an IV line. The pump controls the rate of delivery of the antibiotic, ensuring a consistent and accurate dose, potentially reducing the risk of treatment failure and the development of antibiotic resistance.1 This method of administration allows for high-dose, long-term antibiotic treatment, making this technique a promising option for the outpatient treatment of infections that are difficult to cure with oral antibiotics.2 In addition, the pEP is a small, disposable, and easily portable device that can be worn by the patient, therefore reducing the need for frequent injections, and increasing patient comfort and satisfaction, making this tool ideal to treat outpatients requiring long antibiotic therapies.3–6 Therefore, the implementation of this technique may have potential cost-effectiveness benefits, as it requires fewer healthcare provider interventions and reduces hospitalization costs.7,8 Altogether, the mentioned characteristics explain why IV therapy using pEP is increasingly used in both hospital and home settings.9,10
However, it is important to note caveats that need to be addressed about this new technique. First, the use of elastomers is possible for antibiotics that are stable after dilution.11–13 Second, although studies have shown that IV therapy with an elastomeric pump is effective in treating a wide range of infections, including skin and soft tissue infections, osteomyelitis, and endocarditis, these data are based on small, local experiences that cannot be routinely translated in different clinical settings so that further implementation of guidelines and protocols is needed.14,15 Third, although therapy with pEP is usually well tolerated, the burden of adverse events is not fully elucidated. In theory, these devices can be prone to infections or to catheter-associated complications, including thrombosis and ruptures. Additionally, specific environmental conditions (e.g., hot temperatures) might impair the stability of the antibiotics and, therefore, their effectiveness so that special attention to storage is required.16 Last, as patients would be in outpatient settings, it is possible that some complications, either associated directly with the infection itself or to the device, may be diagnosed later than in an inpatient setting, negatively impacting the prognosis.17
Therefore, considering promising benefits and current uncertainties, we analyzed the efficacy and safety of intravenous antibiotic therapy via pEP for the treatment of various infections in a large and well-characterized cohort of patients in a large Italian University Hospital, aiming to provide more evidence and data from clinical practice and expand the use of this new methodology of antibiotic administration.
MethodsWe conducted a retrospective observational cohort study over a period from January 2020 to May 2023. All adult patients aged ≥18 years from our outpatient parenteral antibiotic therapy (OPAT) clinic who received antibiotic treatment via pEP were included.
The course of admission, care and follow-up of patients is shown in Fig. 1.
Structure and organization of the treatment course in our OPAT unit. OPAT=outpatient parenteral antimicrobial therapy; ID=infectious disease; PICC=peripherally inserted central catheter; pEP=portable elastomeric pumps. a pEP are prepared daily by the nurses of our OPAT clinic, at the moment when the patient shows up. The infusion of the same day is started at the clinic, while the elastomeric pumps for the daily therapy until next access to the clinic are delivered to the patient who transports them inside a cooled thermal bag up to his home where they are then stored at the temperature of 5°C.
Data collected from hospital medical records and laboratory tests included patient demographics, type of infection, responsible microorganism, antibiotics administered, and outcomes. All information was entered on a case report form and recorded in a specific database.
Stability data for antimicrobial therapy were assessed together with a specialized clinical pharmacist, part of the OPAT team, and obtained through the consultation of clinical pharmacology studies, the technical data sheet of each antibiotic and a specific database provided by the producers of pEP.
The outcome measured was the infection response (as defined below) up to a follow-up period of 90 days after the end of antibiotic therapy.
The infection was defined as cured when complete recovery from it was observed. Improved infection was defined as infection that showed an initial response to antibiotic treatment but whose recovery could not be ascertained due to complications (related to the patient's comorbidities or antibiotic therapy) that led to early discontinuation of antibiotic therapy and exit from the study observation. The lack of clinical response to antibiotic therapy defined a therapeutic failure. Finally, we defined relapse as the recurrence of infection, previously classified as cured and within 28 days of the end of antibiotic treatment with pEP, characterized by a new positivity for the same pathogen in microbiological cultures.
Any adverse events attributable to the drug administered, the vascular catheter, the infection itself or the patient's comorbidities were recorded during the same period.
Statistical analysis with calculation of frequencies and percentages for description of qualitative variables and means, medians, standard deviation (SD), and interquartile range (IQR) for presentation of quantitative variables according to their distribution type was conducted with Microsoft Excel® for Microsoft 365 MSO (Version 2306 Build 16.0).
ResultsStudy populationOver the study period, 490 patients were referred to our OPAT Unit, and 94 of them received antibiotic therapy via an elastomeric continuous infusion pump.
The demographic and clinical characteristics of our cohort are shown in Table 1.
Demographic and clinical characteristics of patients.
| Characteristics | N=94 |
|---|---|
| Male,n(%) | 53 (56.4) |
| Age in years, median (IQR) | 61 (46–75) |
| Charlson index comorbidities, median (IQR) | 2 (0–4) |
| Underlying diseases,n(%) | |
| Solid malignancy | 21 (22.3) |
| Gynecologic cancer | 10 (47.6) |
| Breast cancer | 5 (23.8) |
| Gastric cancer | 2 (9.5) |
| Nasopharyngeal cancer | 2 (9.5) |
| Prostatic cancer | 1 (4.8) |
| Pulmonary cancer | 1 (4.8) |
| Cardiovascular disease | 13 (13.9) |
| Ischemic cardiomyopathy | 5 (38.5) |
| Peripheral artery disease | 5 (38.5) |
| Essential hypertension | 3 (23.1) |
| Valvular heart disease | 1 (7.7) |
| Chronic heart failure | 1 (7.7) |
| Arrhythmia | 1 (7.7) |
| Diabetes mellitus | 9 (9.6) |
| Immunosuppression | 5 (5.3) |
| Therapy with antirejection drugs | 2 (40.0) |
| Therapy with TNF inhibitors | 2 (40.0) |
| HIV infection | 1 (20.0) |
| Chronic lung disease | 4 (4.3) |
| Chronic obstructive pulmonary disease | 2 (50.0) |
| Bronchiectasis | 2 (50.0) |
| Hematological malignancy | 4 (4.3) |
| Non-Hodgkin lymphoma | 2 (50.0) |
| Hodgkin lymphoma | 1 (25.0) |
| Multiple myeloma | 1 (25.0) |
| Neurological disease | 1 (1.1) |
IQR=interquartile range; TNF=tumor necrosis factor.
Of the 94 patients, just over half were males (n=53; 56.4%), with a median age of 61 years (IQR 46–75). The most represented comorbidities were solid organ tumors (n=21; 22.3%), cardiovascular diseases (n=13; 13.9%) and diabetes mellitus type II (n=9; 9.6%). The median of Charlson Index Comorbidities score was 2 (IQR 0–4).
Table 2 shows the types of infectious diseases treated and the causative pathogens.
Infection types and pathogens.
| N=94 | |
|---|---|
| Infection types,n(%) | |
| Bone and prosthetics infection | 27 (28.8) |
| Vertebral osteomyelitis | 8 (29.6) |
| Other osteomyelitis | 8 (29.6) |
| Total knee arthroplasty infection | 5 (18.5) |
| Total hip arthroplasty infection | 3 (11.1) |
| Osteosynthesis-associated infection | 3 (11.1) |
| Acute bacterial skin and skin structure infection | 14 (14.9) |
| Cellulitis | 7 (50.0) |
| Surgical site infection | 4 (28.6) |
| Diabetic foot ulcer | 3 (21.4) |
| Bloodstream infection | 14 (14.9) |
| Central-line related | 4 (28.6) |
| From unknown source | 4 (28.6) |
| From skin and skin structure source | 4 (28.6) |
| From abdominal source | 1 (7.1) |
| From urinary source | 1 (7.1) |
| Head and neck infection | 11 (11.8) |
| Otomastoiditis | 4 (36.4) |
| Eye infection | 3 (27.3) |
| Skull base infection | 2 (18.2) |
| Epidural empyema | 2 (18.2) |
| Intra-abdominal infection | 9 (9.6) |
| Intra-abdominal abscess | 5 (55.6) |
| Cholangitis | 2 (22.3) |
| Cholecystitis | 2 (22.3) |
| Pneumonia | 9 (9.6) |
| Lobar pneumonia | 4 (44.4) |
| Pulmonary abscess | 3 (33.3) |
| Pleural empyema | 1 (11.1) |
| COPD exacerbation | 1 (11.1) |
| Urinary tract infection | 7 (7.5) |
| Pyelonephritis | 3 (42.9) |
| Cystitis | 2 (28.6) |
| Prostatitis | 2 (28.6) |
| Endocarditis | 3 (3.2) |
| PVE (aortic) | 2 (66.7) |
| NVE (mitral) | 1 (33.3) |
| Pathogens,n(%) | |
| P. aeruginosa | 55 (48.3) |
| MSSA | 13 (11.4) |
| Proteus spp. | 8 (7.0) |
| E. coli | 5 (4.4) |
| E. cloacae | 4 (3.5) |
| E. faecalis | 3 (2.6) |
| Providencia spp. | 3 (2.6) |
| Bacteroides spp. | 3 (2.6) |
| Othersa | 4 (3.5) |
| Polymicrobial | 27 (25.4) |
| No etiology | 15 (13.2) |
COPD=chronic obstructive pulmonary disease; PVE=prosthetic valve endocarditis; NVE=native valve endocarditis; MSSA=methicillin-susceptible S. aureus.
The most frequently found infections were those involving bone and prostheses, including spondylodiscitis (n=27; 28.8%), followed by skin and soft tissue infections (n=14; 14.9%) and bloodstream infections, including those associated with central venous catheters (n=14; 14.9%), head and neck infections (n=11; 11.8%), complicated intra-abdominal infections (n=9; 9.6%), pneumonia (n=9; 9.6%) and complicated urinary tract infections (n=7; 7.5%). Finally, 3 cases (3.2%) of endocarditis were also included.
The most frequently isolated pathogen was Pseudomonas aeruginosa (n=55; 48.3%), followed by methicillin-susceptible Staphylococcus aureus (n=13; 11.4%). Twenty-seven infectious episodes (25.4%) were caused by more than one pathogen, while in 15 cases (13.2%), no microorganisms were isolated.
Antibiotic therapies performedIn Table 3, we report data on antibiotics administered with details on the type of elastomer used, dosages, dilution, frequency and timing of administration and storage conditions.
Characteristics of OPAT treatment with pEP and data on antibiotics administered.
| Antimicrobial agents used | N (%) | pEP type (material and nominal fill volume) | Concentration (diluent) | Infusion time | Temperature and duration of storagea |
|---|---|---|---|---|---|
| Cefepime | 32 (34.0) | Polyisoprene – 240ml | 25mg/ml (NS or DW5%) | 24h every 24h | 5°C: 3 days – RT: 24h |
| Piperacillin/Tazobactamb | 29 (30.9) | Polyisoprene – 240ml | 56mg/ml (NS or DW5%) | 24h every 24h | 5°C: 5 days – RT: 24h |
| Ceftolozane/Tazobactam | 7 (7.5) | Polyisoprene – 240ml | 18.75–37.5mg/ml (NS) | 24h every 24h | 5°C: 3 days – RT: 24h |
| Oxacillin | 7 (7.5) | Polyisoprene – 240ml | 50mg/ml (NS or DW5%) | 24h every 24h | 5°C: 5 days – RT: 24h |
| Meropenem | 6 (6.4) | Polyisoprene – 250ml | 8mg/ml (NS) | 2.5h every 8h | 5°C: 2 days – RT: 3h |
| Ceftazidime | 3 (3.2) | Polyisoprene – 240ml | 25mg/ml (NS or DW5%) | 24h every 24h | 5°C: 5 days – RT: 24h |
| Cefazolin | 3 (3.2) | Polyisoprene – 240ml | 25mg/ml (NS or DW5%) | 24h every 24h | 5°C: 5 days – RT: 24h |
| Colistin | 3 (3.2) | Polyisoprene – 240ml | 37.000IU/ml (NS) | 24h every 24h | 5°C: 2 days – RT: 24h |
| Ceftazidime/Avibactam | 2 (2.1) | Polyisoprene – 100ml | 25mg/ml (NS) | 2h every 8h | 5°C: 2 days – RT: 2h |
| Acyclovir | 2 (2.1) | Polyisoprene – 240ml | 2.9–3.3mg/ml (NS) | 24h every 24h | 5°C: 3 days – RT: 24h |
pEP=portable elastomeric pumps; NS=normal saline solution; DW5=5% dextrose in water; h=hours; RT=room temperature.
The most frequently administered antibiotic was cefepime (n=32; 34.0%), followed by piperacillin/tazobactam (n=29; 30.9%), ceftolozane/tazobactam (n=7; 7.5%), oxacillin (n=7; 7.5%), and meropenem (n=6; 6.4%). In 2 cases (2.1%), ceftazidime/avibactam was administered. Almost all antibiotics, except for meropenem and ceftazidime/avibactam, were administered by continuous 24-h infusion.
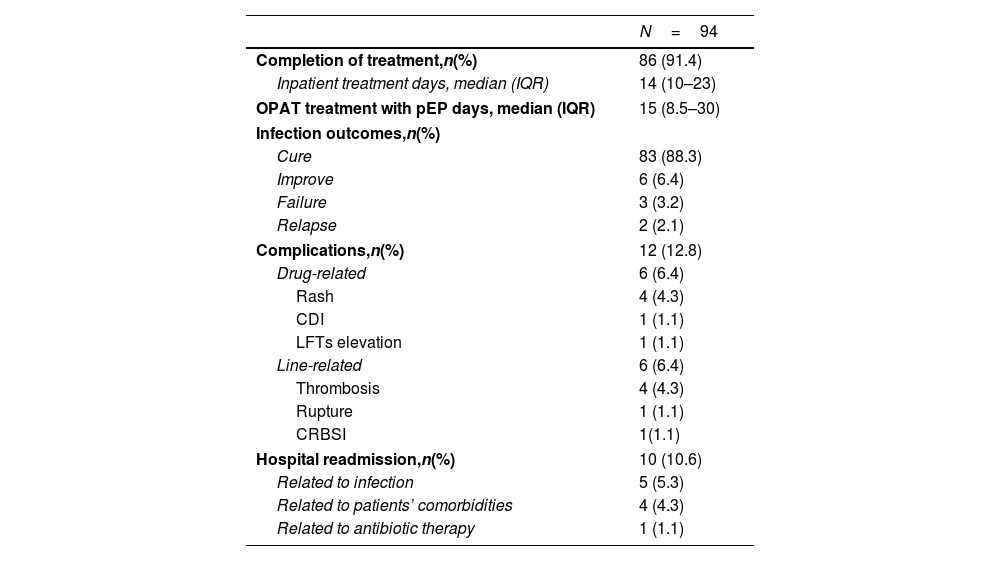
Patients’ outcomesTable 4 shows the characteristics of initial admission to OPAT treatment and the main outcomes recorded.
Outcomes.
| N=94 | |
|---|---|
| Completion of treatment,n(%) | 86 (91.4) |
| Inpatient treatment days, median (IQR) | 14 (10–23) |
| OPAT treatment with pEP days, median (IQR) | 15 (8.5–30) |
| Infection outcomes,n(%) | |
| Cure | 83 (88.3) |
| Improve | 6 (6.4) |
| Failure | 3 (3.2) |
| Relapse | 2 (2.1) |
| Complications,n(%) | 12 (12.8) |
| Drug-related | 6 (6.4) |
| Rash | 4 (4.3) |
| CDI | 1 (1.1) |
| LFTs elevation | 1 (1.1) |
| Line-related | 6 (6.4) |
| Thrombosis | 4 (4.3) |
| Rupture | 1 (1.1) |
| CRBSI | 1(1.1) |
| Hospital readmission,n(%) | 10 (10.6) |
| Related to infection | 5 (5.3) |
| Related to patients’ comorbidities | 4 (4.3) |
| Related to antibiotic therapy | 1 (1.1) |
CDI=C. difficile infection; LFTs=liver function tests; CRBSI=catheter-related bloodstream infection.
Half of the 94 patients (n=47; 50%) entered the OPAT program after the inpatient treatment period, which had a median duration of 14 days (IQR 10–23); the other half (n=47; 50%) was directly admitted without having carried out previous intravenous antibiotic therapy. The median duration of OPAT treatment was 15 days (IQR 8.5–30).
Eighty-six (91.4%) patients regularly completed the entire therapeutic course, and 83 (88.3%) achieved therapeutic success with the treatment of the infection. In 6 cases (6.4%), there was an improvement in infectious disease; in 3 cases (3.2%), there was a recurrence of infection; and in 2 cases (2.1%), there was therapeutic failure.
Twelve patients (12.8%) had complications, of which 6 (6.4%) were related to the drug and 6 (6.4%) were related to the central line. The most frequent drug-related complication was the appearance of rash (n=4, 4.3%), while thrombotic events represented the most frequent central line-related adverse event (n=4, 4.3%).
Hospital readmissions during OPAT treatment or within 90 days from the end of it were 10 (10.6%), of which only one (1.1%) was due to an adverse event related to antibiotic therapy for the onset of infection by Clostridioides difficile, 5 (5.3%) because of events related to infection under treatment, and 4 (4.3%) due to complications depending on patients’ comorbidities.
Economic considerationsThe total number of OPAT therapy days was 2029, with an equivalent savings in bed days. More precisely, given that (according to a report by the Italian Ministry of Economy) a day of hospital stay in Italy has an average per capita cost of 650 €, and that instead the average daily cost of the OPAT program was estimated to be 165 €, the healthcare cost savings amount to approximately 984.000 €.18
DiscussionThis study reports on the safety and efficacy of the OPAT system in patients treated with continuous antibiotic infusion by pEP at a university hospital in Rome, Italy.
In line with Voumard et al., and Ferreiro et al., the total rate of infection cure reached was almost 95% considering the 83 cases in which the infections were completely cured and the 6 cases in which initial infection improvement was observed.19,20
Of these 6 patients, 4 had a complication due to their comorbidities that led to hospital readmission so that therapy was continued as an inpatient, 1 patient had a rash on the third day of treatment, and 1 patient developed a C. difficile infection.
Infection relapse was observed after completing the therapeutic course with apparent cure of the infection in three patients for whom adequate source control could not be performed immediately. In a series of 39 patients with osteomyelitis by Bernand et al., inadequate control of the source of infection prior to initiation of antibiotic therapy with elastomeric pumps was also the main cause of relapse, so complete eradication of septic sources should always be considered among the criteria for admission to antibiotic treatment with pEP.7
Regarding the two patients who presented with therapeutic failure, one patient did not meet the criteria for admission to the OPAT protocol and probably required further therapy as an inpatient, and the other patient presented with a septic source that was not controlled.
Of the 12 complications observed, six were due to the drug administered, and six were due to the central line.
The percentage of drug-related adverse events is in line with other studies, such as that of Karimaghaei et al. (6.6% vs 6.8%). However, compared to their cohort, we observed a lower incidence of line-related complications (14.3% vs 6.4%). This could be attributed to the periodicity of clinical follow-up and above all to the periodic check and verification of the integrity, positional and hygienic conditions of the vascular catheter, which in our unit took place at the same time as the medical re-evaluation by experienced nurses belonging to the PICC team that was an integrated part of our Infectious Diseases Unit.21
Of the six drug-related complications, 4 were rashes, two of which occurred at the end of the therapeutic cycle so that the drug was discontinued, and two were totally regressed by replacement with another drug. One patient presented with a C. difficile infection that required hospitalization, and one patient presented elevated liver function tests (LFTs) for which antibiotic therapy was modified. However, without observing an improvement in liver function, diagnostic investigations were carried out, and hepatitis C virus acute infection was found.
All 6 central line-related complications were managed at our OPAT center thanks to the support of the PICC team, in two cases with the replacement of the vascular catheter and in 4 cases with the introduction of anticoagulant therapy so that none of these complications led to the discontinuation of antibiotic treatment.
Compared to Gardiol et al., who reported an adverse event rate of 9%, in our cohort, it was 12.8%. The slightly higher rate we observed can be attributed to the different characteristics of the two cohorts of patients, such as median age (58 vs. 61), median days of pEP treatment (9 vs. 15) and the main infections treated (bone and urinary tract infections vs. bone, bloodstream and skin and soft tissue infections), reflecting a greater clinical complexity and an older age of our cohort, which is therefore more prone to complications.22
In fact, García-Queiruga et al. reported a cure rate of 83%, which is lower than in the other studies, compared to which patients had a higher median age (70.5±17 years), a median treatment days of 13 and were mostly suffering from respiratory infections, outlining more severe clinical features of their cohort.23
Of the 10 hospital readmissions, only one was due to a major adverse event secondary to OPAT therapy (C. difficile infection), 5 were represented by 3 cases of recurrence and two cases of failure, and 4 were due to their own pathologies.
The cumulative hospital readmission rate we observed (10.8%) was higher than that reported by Zikri et al. (8.5%) and Hase et al. (4.5%). It should be noted, however, that the median age of the patients presented by Zikri was 53 years, which were presumably healthier than our patients, although it is not possible to stratify by clinical severity due to the unavailability of a comorbidity score. On the other hand, the study by Hase et al. showed that the majority of patients entered OPAT after a period of treatment as inpatients, suggesting that the most critical infectious phase had already been overcome.24,25
OPAT protocols in our institution allowed the saving of 2029 admission days and an estimated cost of approximately 984.000 €, suggesting that its further implementation can have huge repercussions on the sustainability of the national healthcare systems. Not unexpectedly, during the period of the study, there was a progressive annual increase in patients undergoing treatment with pEP (19 patients in 2020, 25 in 2021 and 41 in 2022), denoting the satisfaction of clinical staff and the good application of procedures, as well as the appreciation of the institutional administrative perspective, which supported our unit in a process of gradual implementation, aiming to further save healthcare costs.
In addition, the Charlson Index showed a growing trend from a median of 2 (IQR 1.25–4.25) in 2020 to a median of 4 (IQR 2–5) in 2023. This could suggest that over time, the self-confidence of health care professionals toward elastomeric pumps has increased, as well as the credibility of the OPAT system in our hospital, allowing the extension of clinical and demographic admission criteria to the OPAT protocol with pEP.
A strength of pEP systems also lies in allowing continuous infusion of antibiotics by optimizing the PK/PD ratio, especially for beta-lactams, which exhibit time-dependent bactericidal activity, thus benefitting from a higher plasma concentration and a longer temporary concentration interval exceeding the MIC.26
Outpatient administration of antibiotics such as piperacillin/tazobactam, cefepime and ceftolozane/tazobactam is a good practice of antimicrobial stewardship and carbapenem-sparing because it allows the reduction of the prescription of ertapenem, which in contexts where the use of elastomeric pumps is not routine, remains the only alternative for the outpatient treatment of infections caused by extended-spectrum beta-lactamase-producing pathogens, as already described in a previous work.27
An additional strength of pEP administration lies in minimizing the number of hospital accesses, allowing a lower rate of colonization by multidrug resistant bacteria and therefore of nosocomial infections, although this hypothesis would be verified through a randomized trial in which a population such as ours but that receives inpatient therapy is chosen for comparison.
The use of elastomeric pumps has allowed the treatment at home of infections for which intermittent antibiotic infusion would require from 3 to 4 accesses at home of a nursing team, services that not all territorial health services can guarantee, especially in a metropolitan area such as Rome with a large number of patients.
To date, only a few studies related to antibiotic therapy administered through pEP are available. This study is one of the most up-to-date real-life studies with a large cohort of patients, a higher median age, and a greater variety of infections.
An element of absolute novelty of this study consists of presenting data that are the first representative of the use through pEP of new molecules such as ceftolozane/tazobactam (7 patients) and ceftazidime/avibactam (2 patients).
This study is not without limitations. First, it is a retrospective study with intrinsic limitations. However, our OPAT protocol has been implemented on a rigorous protocol, and our institution only has electronic charts, allowing collection of all information we needed for this study. Second, the lack of an inpatient control population does not allow a precise estimation of the costs saved, so that economic considerations have been calculated on the basis of averages of health expenditure shared by health authorities and therefore do not return an exact valuation, but allow to deduce that in any case there were significant monetary savings.
One of the most challenging aspects in OPAT is switching from intravenous to oral antibiotic therapy, a practice recommended by some studies when the right conditions exist to do so.2 However, none of the patients in our cohort received sequential oral maintenance therapy, as effective oral therapy was not available for microbiological reasons (antibiotic resistance pattern of the pathogens) or PK/PD reasons (need for higher concentrations of antimicrobial agents at the target site, ongoing or potential absorption problems).
Healthier populations have better outcomes, but the greater the confidence of the health professionals toward the OPAT system through pEP, the greater they can take charge of clinically more complex patients, possibly increasing the frequency of room visits. For further optimization of therapeutic success, it is also essential to work in synergy with other figures of the OPAT team as clinical pharmacists and nurses with experience in the management of vascular catheters for better assistance to the needs of patients.
Subsequent real-life studies are needed to evaluate the use of pEPs in different healthcare settings and for the administration of new antibiotic molecules such as meropenem–vaborbactam, imipenem–relebactam, sulbactam–durlobactam and cefiderocol as soon as data on the chemical stability of these molecules become available.
In conclusion, the present study confirms the safety and efficacy of outpatient parenteral antibiotic therapy (OPAT) through portable elastomeric continuous infusion pumps, attests to a considerable impact on the reduction of hospitalization days and thus an economic saving on care costs and its usability as an excellent antibiotic stewardship tool that could contribute to the reduction of colonization by multiresistant bacteria.
Ethical approvalThe study's protocol was approved on 11/11/2021 by the ethics committee of Fondazione Policlinico Gemelli with protocol number 4582.
Authors’ contributionG. Giuliano: data curation, formal analysis, investigation, writing-original draft.
F. Raffaelli: data curation, investigation, writing-original draft.
E. Tamburrini: conceptualization, methodology, data curation, investigation, validation, writing-review & editing.
D. Tarantino: resources.
M. C. Nurchis: conceptualization, methodology.
G. Scoppettuolo: conceptualization, methodology, validation, supervision.
FundingThis study was carried out as part of our routine work.
Conflict of interestNone to declare.
Our greatest thanks go to the nursing staff of our OPAT clinic, the PICC team and colleagues of our clinic from the departments of Infectious Diseases 03U and 04U, the outpatient unit, and the consulting unit.