Data regarding outpatient parenteral antimicrobial therapy (OPAT) with continuous infusion of meropenem (CIM) remain scarce and controversial. We aimed to analyze its outcomes.
MethodsWe conducted a retrospective analysis of a cohort of patients who received OPAT with CIM during a three-year period at a single center in northwest Spain. Demographics, clinical data and OPAT outcomes were recorded.
ResultsSince January 2017–December 2019, 34 patients received 35 OPAT episodes with CIM. The median age was 75 years, and 18 (51.4%) had a Charlson comorbidity index>2. Twelve (34.3%) had respiratory infection, 11 (31.4%) urinary tract infection, and 12 (34.3%) other infections. Twenty-one (60%) received a dose of 6g/day, and 27 (77.1%) received combined antibiotic therapy. The duration of OPAT with CIM was 10 median days. Pseudomonas aeruginosa was the most frequently (34.3%) isolated microorganism and 10 (28.6%) infections were polymicrobial. During OPAT and hospital at home unit admission, 4 (11.4%) patients had any adverse reaction that required CIM withdrawal, 2 (5.7%) were readmitted, and 3 (8.8%) died (2 infection-related deaths). After 30 days from discharge 6 (18.8%) of 32 not-censored patients had unplanned readmissions (2 infection-related), 6 (18.8%) developed recurrence (3 relapses, 3 reinfections) and 1 (3.1%) died (none-infection-related death). Twenty-three (71.9%) of these 32 patients did not experience unplanned readmission, recurrence or death.
ConclusionCIM can be an option to be administrated in OPAT programs in selected patients. Further studies are warranted to increase evidence regarding its use, and to externally validate our findings.
Los datos sobre el tratamiento antimicrobiano domiciliario endovenoso (TADE) con infusión continua de meropenem (ICM) son escasos y controvertidos. Nuestro objetivo fue analizar sus resultados.
MétodosRealizamos un análisis retrospectivo de una cohorte de pacientes que recibieron TADE con ICM durante tres años en un centro del noroeste de España. Se registraron datos demográficos, clínicos y resultados.
ResultadosDesde enero de 2017 a diciembre de 2019, 34 pacientes recibieron 35 episodios de TADE con ICM. La mediana de edad fue de 75 años y 18 (51,4%) tenían un índice de comorbilidad de Charlson>2. Doce (34,3%) tenían infección respiratoria, 11 (31,4%) urinaria y 12 (34,3%) otras infecciones. Veintiuno (60%) recibieron una dosis de 6g/día y 27 (77,1%) antibioterapia combinada. La duración mediana del TADE con ICM fue de 10 días. Pseudomonas aeruginosa fue el microorganismo aislado más frecuentemente (34,3%) y 10 (28,6%) infecciones fueron polimicrobianas. Durante el TADE, 4 (11,4%) pacientes presentaron alguna reacción adversa que requirió retirada de ICM, 2 (5,7%) reingresaron y 3 (8,8%) fallecieron (2 muertes relacionadas con infección). Tras 30 días desde el alta, 6 (18,8%) de 32 pacientes tuvieron reingresos no programados (2 relacionados con infección), 6 (18,8%) desarrollaron recurrencia (3 recidivas, 3 reinfecciones) y 1 (3,1%) falleció (sin relación con infección). Veintitrés (71,9%) de 32 pacientes no experimentaron reingreso no programado, recidiva o muerte.
ConclusiónLa ICM puede ser una opción para ser administrada en programas de TADE en pacientes seleccionados. Se necesitan más estudios para aumentar la evidencia sobre su uso y validar externamente nuestros hallazgos.
Outpatient parenteral antimicrobial therapy (OPAT) has been established as a safe and effective treatment option, which can be used to treat a wide variety of infections with reduced costs.1–4 However, the continuing worldwide increase of multidrug-resistant (MDR) gram-negative bacteria isolations has limited the role of OPAT in some scenarios.5–7 Continuous infusions with elastomeric pumps may provide OPAT for patients with difficult-to-treat infections, allowing a greater autonomy for the patient and decreasing the burden on the healthcare system.8 Consequently, continuous infusion of meropenem (CIM) could be an interesting OPAT option for selected patients with suspected or confirmed drug-resistant gram-negative bacterial infection. Furthermore, it might be associated with a higher clinical improvement rate and a lower mortality compared with intermittent bolus in patients with severe infection or infected by less sensitive microbial.9 Nevertheless, paucity of data and several concerns about its stability, efficacy, and safety have hampered the instauration of CIM as an OPAT alternative in routine clinical practice.10–14
The RETADE-CHUAC (Registro de Tratamiento Antimicrobiano Domiciliario Endovenoso en el Complexo Hospitalario Universitario de A Coruña) is an ongoing, unicenter, Spanish registry of consecutive patients who received OPAT in the Hospital at Home (HaH) unit of Complexo Hospitalario Universitario de A Coruña since 2015. In the current study, we used data from this registry to analyze the clinical characteristics, treatment patterns and outcomes of patients who received OPAT with CIM.
MethodsPatientsThis in an analysis of retrospectively collected data in the RETADE-CHUAC Registry, from the HaH unit of Complexo Hospitalario Universitario de A Coruña in northwest Spain. Inclusion criteria were to have a confirmed or suspected diagnosis of active infection and to receive OPAT for this reason in this HaH Unit since 2015. In patients with more than one OPAT episode, each episode was recorded. For the current study, only patients who received OPAT with CIM were enrolled consecutively. A confirmed microbiological diagnosis was not mandatory, although all data about microbiological samples collected in each patient and its corresponding isolations were included. Exclusion criteria were a current enrollment in a therapeutic clinical trial with a blinded antimicrobial therapy. The research protocol was conducted in accordance with the 2013 Declaration of Helsinki and was approved by the Galician Drug Research Ethics Committee (registration code 2020/512).
Study design and definitionsCIM became a regular OPAT option in our HaH unit in 2017. Thus, all patients with suspected or confirmed infection who received OPAT with CIM recruited from January 2017 to December 2019 were included in the current study. The clinical characteristics of the patients (demographics, comorbidities, concurrent medications, laboratory tests) for each OPAT episode were recorded at baseline. The characteristics of the infection (location, microbiological information) and treatment patterns (duration, dose, vascular access, coverage and administration modalities) were also collected. Concomitant therapies (i.e. corticosteroids, immunosuppressive drugs, other antibiotics) were considered if they were used during the 30 days prior to the diagnosis of the infection that leaded to meropenem treatment. This period was extended to the previous three months for chemotherapy.
The infection was considered recurrent if an infection in the same location was diagnosed in the previous 90 days. Failure of previous antibiotic was defined as the use of any antibiotic in the prior 30 days because of the same infection which leaded to meropenem use, with no achievement of infection resolution. Previous ICU stay was considered if it occurred prior to OPAT because of the same infection that motivated meropenem use in the HaH unit. The entire meropenem treatment duration was calculated including the time of inpatient treatment and the period of OPAT in the HaH unit. Previous meropenem inpatient treatment could be administered by continuous infusion or by any other modality. Some patients only received meropenem as OPAT, without previous inpatient treatment. Self-administration was considered if the patient or any relative or caregiver administered, at least, one dose of meropenem during the OPAT program. All patients and caregivers who self-administered CIM received prior specific education for this purpose by trained nurses.
CIM was administered through elastomeric pumps (Dosi-Fuser®, 240mL), which release the meropenem dose during 12hours (20mL/h). Hence, the elastomeric pump had to be replaced each 12h. The infusion devices had a poly-isoprene balloon, and an ultraviolet radiation (until 390nm) protecting plastic container. Preparation was performed centrally in the pharmacy service under aseptic conditions, as we reported elsewhere.15 The elastomeric pumps were transported and stored refrigerated at 2–8°C, until administration at room temperature (≤25°C). The annual mean maximum and minimum temperatures in A Coruña are 22.8°C and 8.0°C, respectively.16 We assumed a stability of 12hours at room temperature (5 days refrigerated) at a maximum concentration of 12.5mg/mL of meropenem diluted in 0.9% saline solution, according to previous reported data.10,11,17–21 Concomitant antibiotic during OPAT was considered only when the patient received other antibiotic as combined therapy with meropenem because of the same infection, regardless of its administration route (parenteral, oral or inhaled). Antibiotics used because of concurrent infections were not included for the current analysis. Some patients received more than one concomitant antibiotic and/or this concomitant antibiotic was changed by other antimicrobial agent during OPAT.
The major outcomes were those related with treatment failure during OPAT and 30 days after HaH unit discharge. During OPAT, adverse reaction that required meropenem withdrawal, unplanned readmission, sepsis and death were considered for the analysis. After discharge, the main included outcomes were unplanned readmission, infection recurrence and death. Unplanned readmission was defined as a new unscheduled admission episode since the OPAT beginning. Sepsis was defined according The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3).22 All deaths were included in the analysis regardless of their causes. Infection-related death was defined as any death appearing during the study period due to an unfavorable course of the infection, in the absence of any alternative explanation for death. Recurrence was categorized as relapse when it occurred in the same location by the same microorganism, and as reinfection when it occurred in the same location by different microorganism. Patients were managed according to the common hospital's clinical practice, and there were no standardization or recommendation of treatment. All data were electronically recorded using standardized case report forms, through review of patients’ electronic health records by the study investigators.
Statistical analysisContinuous variables were expressed using the median and interquartile ranges (IQRs). Time zero was the date of the OPAT (with continuous infusion of meropenem) beginning, and participants were censored at the time of death or after 30 days from HaH discharge. SPSS software (v. 23.0; IBM SPSS, Armonk, NY, USA) was used for all analyses.
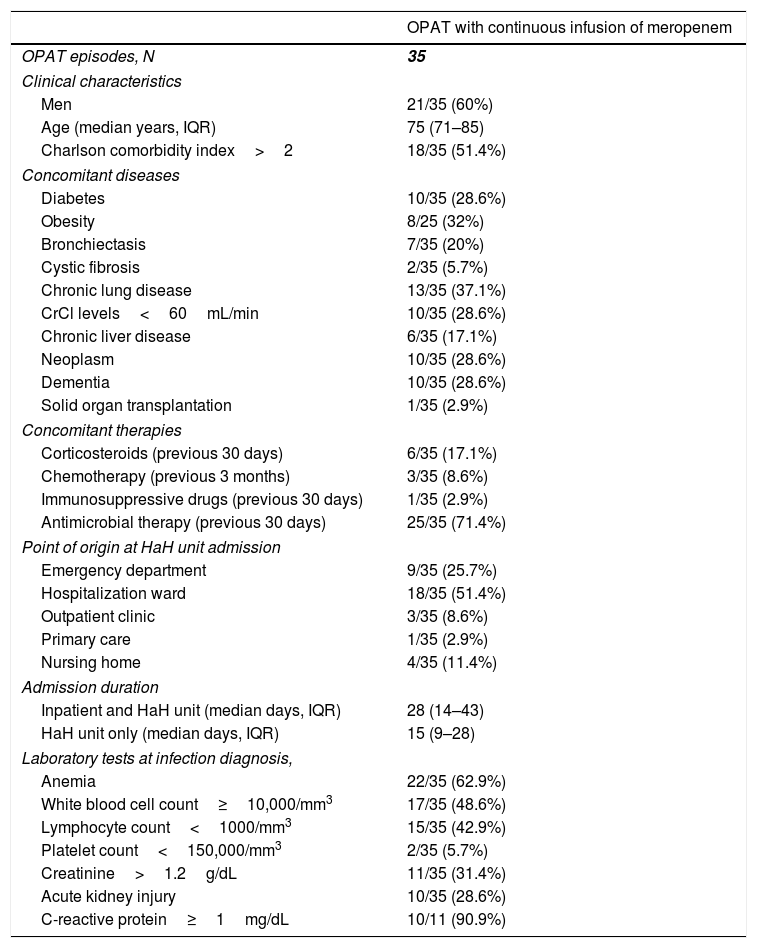
ResultsFrom January 2017 to December 2019, we identified 35 OPAT episodes with CIM in 34 patients. The clinical characteristics of the patients for each OPAT episode are depicted in Table 1. The median age was 75 (IQR, 71–85) years and 18 (51.4%) patients had a Charlson comorbidity index greater than two. Ten (28.6%) patients had cancer and 10 (28.6%) dementia. Twenty-five (71.4%) received antimicrobial therapy 30 days prior to the diagnosis of the infection that leaded to meropenem treatment. The most common point of origin of the patients at HaH unit admission was the hospitalization ward (18 [51.4%]), followed by the emergency department (9 [25.7%]). Only 11 (31.4%) patients had C-reactive protein determination at infection diagnosis (Table 1).
Clinical characteristics of patients who received outpatient parenteral antimicrobial therapy with continuous infusion of meropenem.
| OPAT with continuous infusion of meropenem | |
|---|---|
| OPAT episodes, N | 35 |
| Clinical characteristics | |
| Men | 21/35 (60%) |
| Age (median years, IQR) | 75 (71–85) |
| Charlson comorbidity index>2 | 18/35 (51.4%) |
| Concomitant diseases | |
| Diabetes | 10/35 (28.6%) |
| Obesity | 8/25 (32%) |
| Bronchiectasis | 7/35 (20%) |
| Cystic fibrosis | 2/35 (5.7%) |
| Chronic lung disease | 13/35 (37.1%) |
| CrCl levels<60mL/min | 10/35 (28.6%) |
| Chronic liver disease | 6/35 (17.1%) |
| Neoplasm | 10/35 (28.6%) |
| Dementia | 10/35 (28.6%) |
| Solid organ transplantation | 1/35 (2.9%) |
| Concomitant therapies | |
| Corticosteroids (previous 30 days) | 6/35 (17.1%) |
| Chemotherapy (previous 3 months) | 3/35 (8.6%) |
| Immunosuppressive drugs (previous 30 days) | 1/35 (2.9%) |
| Antimicrobial therapy (previous 30 days) | 25/35 (71.4%) |
| Point of origin at HaH unit admission | |
| Emergency department | 9/35 (25.7%) |
| Hospitalization ward | 18/35 (51.4%) |
| Outpatient clinic | 3/35 (8.6%) |
| Primary care | 1/35 (2.9%) |
| Nursing home | 4/35 (11.4%) |
| Admission duration | |
| Inpatient and HaH unit (median days, IQR) | 28 (14–43) |
| HaH unit only (median days, IQR) | 15 (9–28) |
| Laboratory tests at infection diagnosis, | |
| Anemia | 22/35 (62.9%) |
| White blood cell count≥10,000/mm3 | 17/35 (48.6%) |
| Lymphocyte count<1000/mm3 | 15/35 (42.9%) |
| Platelet count<150,000/mm3 | 2/35 (5.7%) |
| Creatinine>1.2g/dL | 11/35 (31.4%) |
| Acute kidney injury | 10/35 (28.6%) |
| C-reactive protein≥1mg/dL | 10/11 (90.9%) |
Abbreviations: OPAT, outpatient parenteral antimicrobial therapy; IQR, interquartile range; CrCl, creatinine clearance; HaH, hospital at home.
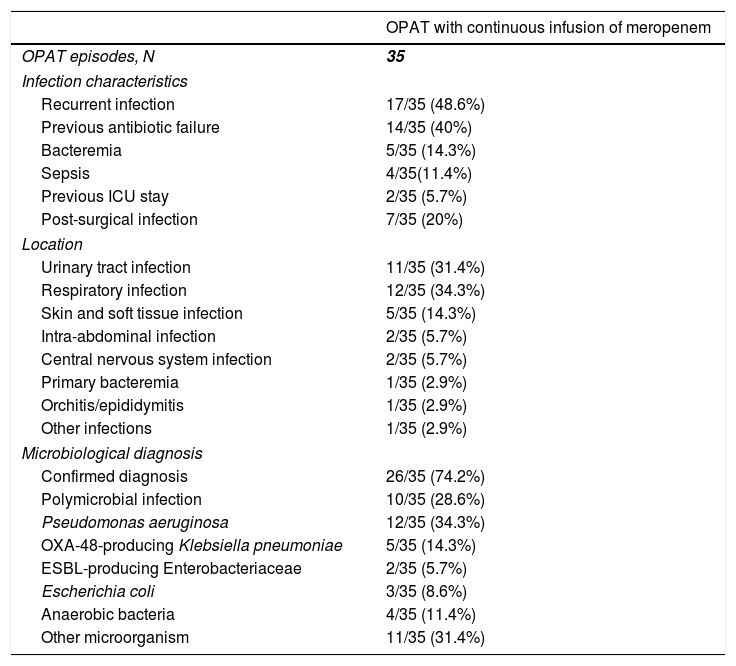
The infection which leaded to meropenem use was recurrent in 17 (48.6%) cases, and previous antibiotic failure was identified in 14 (40%) (Table 2). The most common locations were respiratory infections (12 [34.3%]), urinary tract infections (11 [31.4%]) and skin and soft tissue infections (5 [14.3%]). One (2.9%) patient cataloged as “other infection” had a polymicrobial post-surgical mediastinitis. Microbiological diagnosis was achieved in 26 (74.2%) cases, and Pseudomonas aeruginosa was the most frequent (12 [34.3%]) isolated microorganism, followed by OXA-48-producing Klebsiella pneumonia (5 [14.3%]). The infection was polymicrobial in 10 (28.6%) cases.
Characteristics of the infection in patients who received outpatient parenteral antimicrobial therapy with continuous infusion of meropenem.
| OPAT with continuous infusion of meropenem | |
|---|---|
| OPAT episodes, N | 35 |
| Infection characteristics | |
| Recurrent infection | 17/35 (48.6%) |
| Previous antibiotic failure | 14/35 (40%) |
| Bacteremia | 5/35 (14.3%) |
| Sepsis | 4/35(11.4%) |
| Previous ICU stay | 2/35 (5.7%) |
| Post-surgical infection | 7/35 (20%) |
| Location | |
| Urinary tract infection | 11/35 (31.4%) |
| Respiratory infection | 12/35 (34.3%) |
| Skin and soft tissue infection | 5/35 (14.3%) |
| Intra-abdominal infection | 2/35 (5.7%) |
| Central nervous system infection | 2/35 (5.7%) |
| Primary bacteremia | 1/35 (2.9%) |
| Orchitis/epididymitis | 1/35 (2.9%) |
| Other infections | 1/35 (2.9%) |
| Microbiological diagnosis | |
| Confirmed diagnosis | 26/35 (74.2%) |
| Polymicrobial infection | 10/35 (28.6%) |
| Pseudomonas aeruginosa | 12/35 (34.3%) |
| OXA-48-producing Klebsiella pneumoniae | 5/35 (14.3%) |
| ESBL-producing Enterobacteriaceae | 2/35 (5.7%) |
| Escherichia coli | 3/35 (8.6%) |
| Anaerobic bacteria | 4/35 (11.4%) |
| Other microorganism | 11/35 (31.4%) |
Abbreviations: OPAT, outpatient parenteral antimicrobial therapy; ICU, intensive care unit; ESBL, extended-spectrum beta-lactamase.
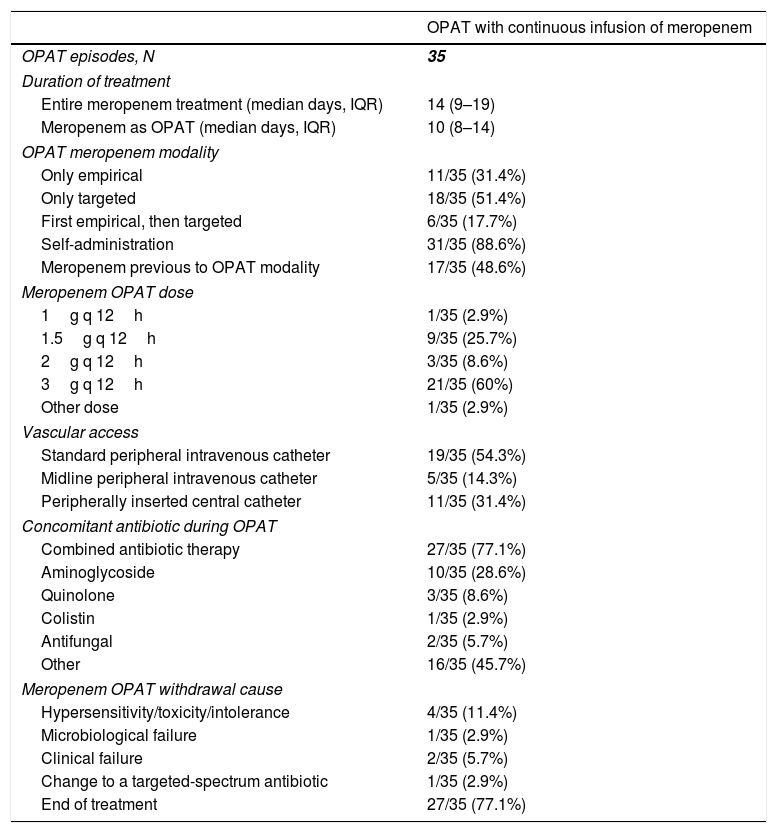
The median duration of the entire meropenem treatment (i.e. including inpatient therapy and OPAT period) was 14 (IQR, 9–19) days (Table 3). When considering meropenem only as OPAT, the median duration of treatment was 10 (IQR, 8–14) days. Meropenem use as OPAT was only targeted (according to microbiological diagnosis) in 18 (51.4%) cases, only empirical in 11 (31.4%) cases, and first empirical with subsequent continuation as targeted therapy in 6 (17.7%). CIM was self-administered in most (31 [88.8%]) of cases, being 3g q 12h and 1.5g q 12h the most common used doses (in 21 [60%] and 9 [25.7%] cases, respectively). A standard peripheral intravenous catheter was the preferred vascular access (13 [54.3%]), followed by peripherally inserted central catheters (11 [31.4%]). Combined antibiotic therapy with CIM was used in 27 (77.1%) cases, 10 (28.6%) of them including an aminoglycoside. Other antibiotics used in the context of suspected or confirmed polymicrobial infections were linezolid (10 cases), daptomycin (3 cases), and trimethoprim/sulfamethoxazole (1 case). The main cause of meropenem OPAT withdrawal was end of treatment (Table 3).
Treatment patterns in patients who received outpatient parenteral antimicrobial therapy with continuous infusion of meropenem.
| OPAT with continuous infusion of meropenem | |
|---|---|
| OPAT episodes, N | 35 |
| Duration of treatment | |
| Entire meropenem treatment (median days, IQR) | 14 (9–19) |
| Meropenem as OPAT (median days, IQR) | 10 (8–14) |
| OPAT meropenem modality | |
| Only empirical | 11/35 (31.4%) |
| Only targeted | 18/35 (51.4%) |
| First empirical, then targeted | 6/35 (17.7%) |
| Self-administration | 31/35 (88.6%) |
| Meropenem previous to OPAT modality | 17/35 (48.6%) |
| Meropenem OPAT dose | |
| 1g q 12h | 1/35 (2.9%) |
| 1.5g q 12h | 9/35 (25.7%) |
| 2g q 12h | 3/35 (8.6%) |
| 3g q 12h | 21/35 (60%) |
| Other dose | 1/35 (2.9%) |
| Vascular access | |
| Standard peripheral intravenous catheter | 19/35 (54.3%) |
| Midline peripheral intravenous catheter | 5/35 (14.3%) |
| Peripherally inserted central catheter | 11/35 (31.4%) |
| Concomitant antibiotic during OPAT | |
| Combined antibiotic therapy | 27/35 (77.1%) |
| Aminoglycoside | 10/35 (28.6%) |
| Quinolone | 3/35 (8.6%) |
| Colistin | 1/35 (2.9%) |
| Antifungal | 2/35 (5.7%) |
| Other | 16/35 (45.7%) |
| Meropenem OPAT withdrawal cause | |
| Hypersensitivity/toxicity/intolerance | 4/35 (11.4%) |
| Microbiological failure | 1/35 (2.9%) |
| Clinical failure | 2/35 (5.7%) |
| Change to a targeted-spectrum antibiotic | 1/35 (2.9%) |
| End of treatment | 27/35 (77.1%) |
Abbreviations: OPAT, outpatient parenteral antimicrobial therapy; IQR, interquartile range.
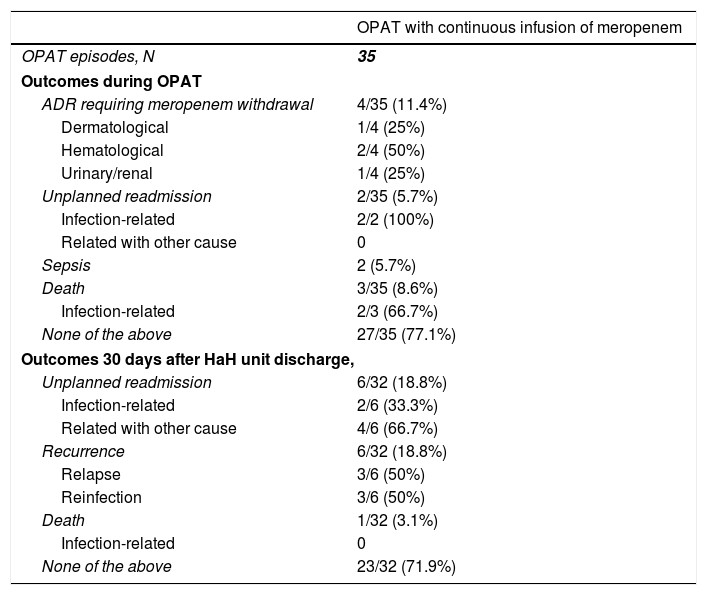
During OPAT, 4 (35%) patients required meropenem withdrawal because an adverse drug reaction (Table 4), which included two patients who developed neutropenia, one patient who presented exanthema, and one patient with renal toxicity. Two patients were readmitted because of clinical infection-related worsening. Two patients developed sepsis, and both were elderly patients with dementia managed only at HaH unit (not readmitted). One of them (man, 80 years, urinary tract infection) survived, while the other one was a 91 year-old woman with a respiratory infection who suffered bronchoaspiration by 7th day, meeting sepsis criteria. Limitation of therapeutic effort was adopted, discontinuing meropenem and initiating palliative sedation (death by 9th day). Other two patients died during OPAT, one of them because of rectum cancer progression, and the other one because of bronchoaspiration. Twenty seven (77.1%) cases had a successful outcome during OPAT, since they did not experience unplanned readmission, recurrence, death or adverse drug reaction requiring meropenem withdrawal.
Clinical outcomes in patients who received outpatient parenteral antimicrobial therapy with continuous infusion of meropenem.
| OPAT with continuous infusion of meropenem | |
|---|---|
| OPAT episodes, N | 35 |
| Outcomes during OPAT | |
| ADR requiring meropenem withdrawal | 4/35 (11.4%) |
| Dermatological | 1/4 (25%) |
| Hematological | 2/4 (50%) |
| Urinary/renal | 1/4 (25%) |
| Unplanned readmission | 2/35 (5.7%) |
| Infection-related | 2/2 (100%) |
| Related with other cause | 0 |
| Sepsis | 2 (5.7%) |
| Death | 3/35 (8.6%) |
| Infection-related | 2/3 (66.7%) |
| None of the above | 27/35 (77.1%) |
| Outcomes 30 days after HaH unit discharge, | |
| Unplanned readmission | 6/32 (18.8%) |
| Infection-related | 2/6 (33.3%) |
| Related with other cause | 4/6 (66.7%) |
| Recurrence | 6/32 (18.8%) |
| Relapse | 3/6 (50%) |
| Reinfection | 3/6 (50%) |
| Death | 1/32 (3.1%) |
| Infection-related | 0 |
| None of the above | 23/32 (71.9%) |
Abbreviations: OPAT, outpatient parenteral antimicrobial therapy; ADR, adverse drug reaction; HaH, hospital at home.
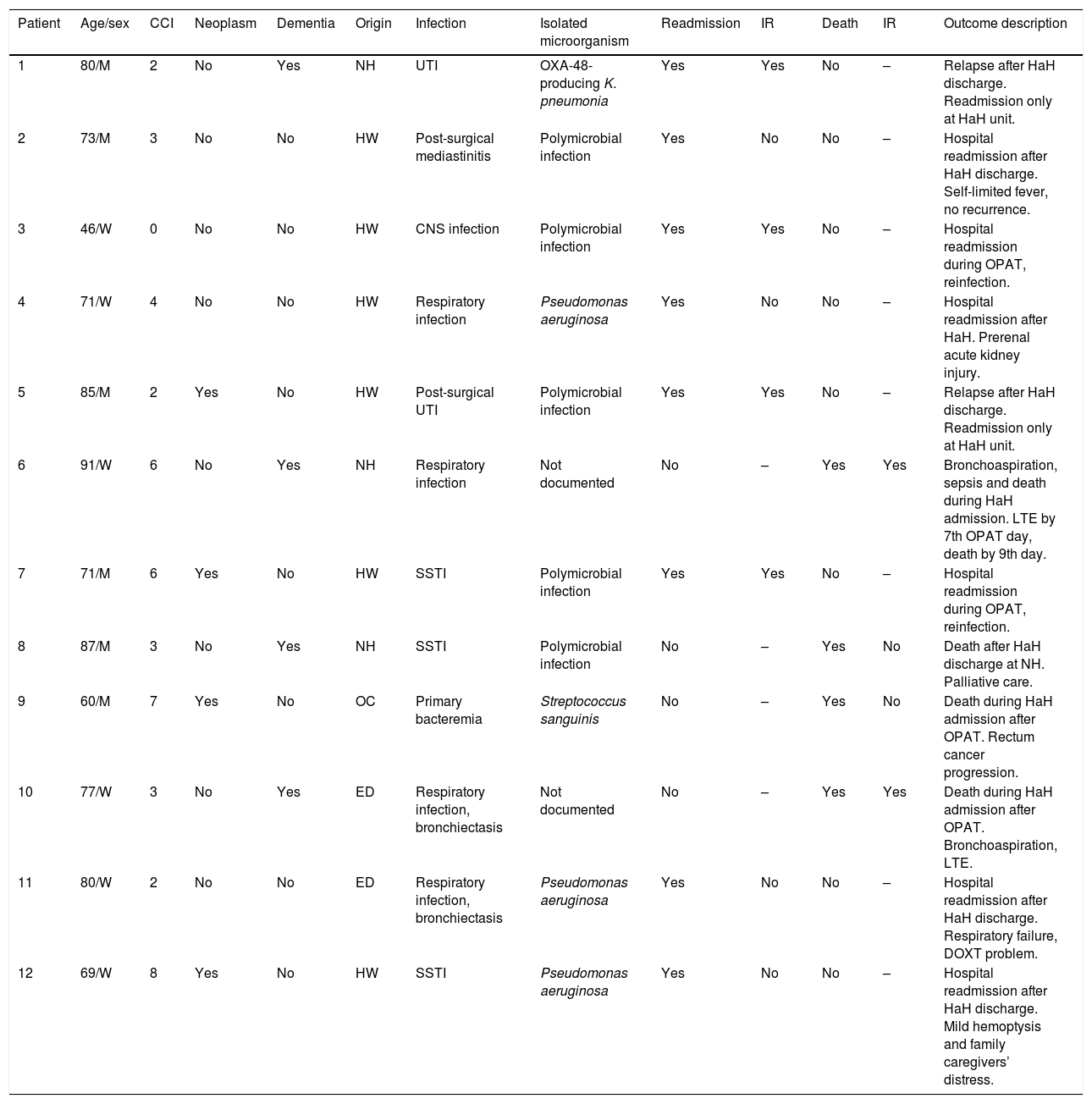
Thirty days after HaH unit discharge 6 (18.8%) of 32 not-censored patients had unplanned readmissions, two of them due to an infection-related cause. Two of these 6 patients were readmitted only at HaH unit (i.e. without conventional hospitalization). Readmissions were planned for a scheduled surgery and to continue with wound care at HaH unit in two other patients. Six recurrences were documented (3 relapses and 3 reinfections). One patient with dementia who was receiving palliative care died due to a none-infection-related cause after 14 days from HaH unit discharge. The clinical characteristics of patients who suffered unplanned readmissions or died during follow-up (including OPAT and 30 days after HaH unit discharge) are depicted in Table 5. Twenty-three (71.9%) of these 32 patients had a successful outcome 30 days after HaH unit discharge, since they did not experience unplanned readmission, recurrence or death.
Clinical characteristics of patients who suffered unplanned readmision or died during follow-up (including OPAT and 30 days after HaH unit discharge).
| Patient | Age/sex | CCI | Neoplasm | Dementia | Origin | Infection | Isolated microorganism | Readmission | IR | Death | IR | Outcome description |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 80/M | 2 | No | Yes | NH | UTI | OXA-48-producing K. pneumonia | Yes | Yes | No | – | Relapse after HaH discharge. Readmission only at HaH unit. |
| 2 | 73/M | 3 | No | No | HW | Post-surgical mediastinitis | Polymicrobial infection | Yes | No | No | – | Hospital readmission after HaH discharge. Self-limited fever, no recurrence. |
| 3 | 46/W | 0 | No | No | HW | CNS infection | Polymicrobial infection | Yes | Yes | No | – | Hospital readmission during OPAT, reinfection. |
| 4 | 71/W | 4 | No | No | HW | Respiratory infection | Pseudomonas aeruginosa | Yes | No | No | – | Hospital readmission after HaH. Prerenal acute kidney injury. |
| 5 | 85/M | 2 | Yes | No | HW | Post-surgical UTI | Polymicrobial infection | Yes | Yes | No | – | Relapse after HaH discharge. Readmission only at HaH unit. |
| 6 | 91/W | 6 | No | Yes | NH | Respiratory infection | Not documented | No | – | Yes | Yes | Bronchoaspiration, sepsis and death during HaH admission. LTE by 7th OPAT day, death by 9th day. |
| 7 | 71/M | 6 | Yes | No | HW | SSTI | Polymicrobial infection | Yes | Yes | No | – | Hospital readmission during OPAT, reinfection. |
| 8 | 87/M | 3 | No | Yes | NH | SSTI | Polymicrobial infection | No | – | Yes | No | Death after HaH discharge at NH. Palliative care. |
| 9 | 60/M | 7 | Yes | No | OC | Primary bacteremia | Streptococcus sanguinis | No | – | Yes | No | Death during HaH admission after OPAT. Rectum cancer progression. |
| 10 | 77/W | 3 | No | Yes | ED | Respiratory infection, bronchiectasis | Not documented | No | – | Yes | Yes | Death during HaH admission after OPAT. Bronchoaspiration, LTE. |
| 11 | 80/W | 2 | No | No | ED | Respiratory infection, bronchiectasis | Pseudomonas aeruginosa | Yes | No | No | – | Hospital readmission after HaH discharge. Respiratory failure, DOXT problem. |
| 12 | 69/W | 8 | Yes | No | HW | SSTI | Pseudomonas aeruginosa | Yes | No | No | – | Hospital readmission after HaH discharge. Mild hemoptysis and family caregivers’ distress. |
Abbreviations: OPAT, outpatient parenteral antimicrobial therapy; HaH, hospital at home; CCI, Charlson comorbidity index; IR, infection-related; M, man; NH, nursing home; UTI, urinary tract infection; HW, hospital ward; W, woman; CNS, central nervous system; LTE, limitation of therapeutic effort; SSTI, skin and soft tissue infection; OC, outpatient clinic; ED, emergency department; DOXT, domiciliary oxygen therapy.
To the best of our knowledge, we presented the largest cohort of patients who received OPAT with CIM in a HaH unit setting, and we analyzed their outcomes during OPAT and 30 days after HaH discharge. A larger previous cohort from Australia was focused on stability of meropenem in OPAT programs that were not developed in the context of HaH units.12 OPAT in Spain was historically carried out by these units, which offer care to patients with acute phase conditions and serious or difficult-to-treat infections, who would otherwise be attended in a hospital. This model require a greater intervention of a multidisciplinary team, including nursing staff and physicians, capable of offering high intensity care at home, and ensuring maximum patient safety.23
This scenario explains the characteristics of our cohort: elderly patients (75 median years), half of them highly comorbid, and many with conditions like dementia (28.6%) or cancer (28.6%). These concomitant diseases determined a poor prognosis, and leaded to a limitation of therapeutic effort with palliative care requirements in advanced disease stages when OPAT outcomes were unfavorable. Although half of our patients came from hospitalization wards, 25% were admitted from the emergency department, and 23% came from other ambulatory settings. Interestingly, we have increased these points of origin (i.e. different than hospitalization wards) in the last years, in an attempt of enhance an admission avoidance HaH modality.24 The complexity of our patients and of their infections justifies a relative long length of stay.
Pseudomonas aeruginosa was the most frequently isolated microorganism, and the presence of other drug-resistant gram-negative bacterial pathogens and polymicrobial infections were common. These findings were not surprising, since the majority (71%) of patients had previous antibiotic exposure, which could facilitate the development of bacterial resistance mechanisms, and/or the selection of resistant strains. Half of the infections which leaded to meropenem use were recurrent, indeed. Microbiological diagnosis was confirmed in three of every four cases. Consequently, OPAT with CIM was initially or finally targeted according isolations in 24 (69%) of cases. None of the patients with confirmed microbiological diagnosis could benefit from a not performed switch from CIM to ertapenem. On the other hand, in 11 (31%) cases OPAT with CIM was maintained empirically, according to previous isolations, colonization patterns, clinical evolution and risk factors for drug-resistant gram-negative bacterial infections. Nevertheless, it is important to highlight that the use of this OPAT option must be implemented prudently and rationally, according the practice antimicrobial stewardship principles.25–27 Misuse should be averted, in order to avoid potential toxicities, development of resistances, and higher costs.
Self-administration has been consolidated as an OPAT option,2,4 constituting our preferred choice when patients and/or caregivers can contribute. The lack of home caregivers support can constitute a limitation for this administration modality, especially considering the high proportion of care-dependent patients represented in our cohort. Self-administration was used in 31 (88.6%) cases, without safety concerns. Generally, it was well valued by patients and caregivers, who did not report relevant problems related to the handling of elastomeric pumps. None of the patients experienced catheter-related bloodstream infection or required readmission due to a vascular access complication. The preparation of the meropenem solution centrally by the pharmacy service under aseptic conditions in laminar flow hoods, minimizes the risk of microbiological contamination, regardless of the mode of administration. Elastomeric infusion devices are ease of handling, light, silent, and they do not require an external power supply for their operation, allowing total mobility of the patients and facilitating their independence. In addition, CIM could be a better option than intermittent bolus for patients with carbapenem-resistant Enterobacteriaceae infections, and for those with severe infection or infected by less sensitive microbial, like many of our patients.9,28 Continuous infusion can enhance the pharmacodynamic target attainment via increasing the amount of time which the free drug concentration remains above the minimum inhibitory concentration.29 Microbiological isolations and polymicrobial infections explain the high rate (77.1%) of combined antimicrobial therapy that we used.
Most of patients had a successful outcome during OPAT (27 [77.8%]) and 30 days after HaH discharge (23 [71.9%]). Nevertheless, our results were not as good as the reported by other OPAT programs in different contexts.23,30–32 Our study was unpowered to search significant associations between the clinical characteristics of the patients and their outcomes. However, previous studies identified a high Charlson comorbidity index, advanced age, recent hospitalization and isolation of MDR microorganisms as risk factors for OPAT failure.30,33,34 These clinical features were highly prevalent in our cohort, which could partially explain our results. Hence, we presented our experience using OPAT with CIM in highly comorbid and relatively elderly patients with difficult-to-treat infections, often polymicrobial or caused by MDR microorganisms, which require combined antimicrobial therapy, including the possibility of different routes of administration (intravenous, oral and inhaled). We hypothesize that the results of this therapy in a more favorable patient profile could be even better.
Our study has several limitations. First, we described a relatively small series of patients who received OPAT with CIM in a HaH unit setting at a single center in northwest Spain. Interestingly, the mean maximum temperature in our region is relatively low compared with other regions of Spain and around the world. Consequently, meropenem solution stability at room temperature could be impaired in warmer climates. Therefore, our findings must be interpreted cautiously and require external validation in larger cohorts and different settings. Second, this was an observational study without a comparable group of inpatients or of patients who received other meropenem administration modalities in the same setting. We could not design the study with a comparator group because of the small sample size that we would reach, since only some few patients were treated with intermittent bolus or 30-minute infusion of meropenem with elastomeric pumps in our HaH unit during the recruitment period. We currently use meropenem in continuous infusion because of the previously mentioned advantages, and we do not use electronic infusion devices. Thus, conclusions comparing our results with other meropenem administration modalities or with inpatient management are lacking. Third, we declined to explore associations with the major outcomes during follow-up using bivariable and multivariable analyses, because of the small sample size and the limited number of cases with adverse outcomes (i.e. the study was unpowered to find significant associations). Fourth, this is a clinical study without a concomitant pharmacological analysis. We assumed a stability of 12hours at room temperature according to previous reported data, as we explained above.10,11,17–21 However, this is a controversial issue with various studies which showed discordant results.14,35–38 Hence, our findings can be interpreted clinically, but other pharmacological considerations are missed. Other authors had reported clinical efficacy, safety and stability of CIM,12 but some new additional evidence would be needed. Finally, although data were included through a concise review of patients’ electronic health records by the study investigators, some data could be not reported or could be missed in these records.
In conclusion, CIM can be an option to be administrated in OPAT programs in selected patients. In highly comorbid patients with difficult-to-treat infections, HaH unit admission or multidisciplinary OPAT programs should be considered, in order to ensure maximum patient safety. Further studies are warranted to increase evidence regarding the use of OPAT with CIM, and to externally validate our findings.
Authors’ contributionsÁlvaro Dubois-Silva conceived the study idea, collected the data, analyzed the data, wrote the manuscript and approved the final version of the manuscript. Lara Otero-Plaza, Leticia Dopico-Santamariña and Ana Mozo-Ríos collected the data, analyzed the data, reviewed and contributed to the manuscript and approved the final version of the manuscript. Leticia Hermida-Porto, Begoña Feal-Cortizas, Marta García-Queiruga, Sonia Pértega-Díaz, Fernando Lamelo-Alfonsín and Luciano Vidán-Martínez analyzed the data, reviewed and contributed to the manuscript and approved the final version of the manuscript. All authors had full access to the data and take responsibility for the integrity of the data and accuracy of the analysis. The corresponding author attests that all listed authors meet authorship criteria. Álvaro Dubois-Silva is the guarantor of the paper, taking responsibility for the integrity of the work as a whole, from inception to published article.
Availability of data and materialThe datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approvalThe research protocol was conducted in accordance with the 2013 Declaration of Helsinki and was approved by the Galician Drug Research Ethics Committee (registration code 2020/512).
Consent to participateFor the retrospective phase of RETADE-CHUAC registry, informed consent was waived, according to the local Ethics Committee requirements. For its prospective phase, written informed consent is obtained from all individual participants (or their health care proxies) included in this registry.
FundingNo specific funding has been received for the present work. This study was conducted as part of our routine activity.
Conflict of interestThe authors declare that they have no conflict of interest regarding the publication of this paper.
We appreciate the support of all staff who is working in the Hospital at Home Unit of Complexo Hospitalario Universitario de A Coruña (A Coruña, Spain).