Outpatient parenteral antimicrobial therapy (OPAT) has been recognised as a useful, cost-effective and safe alternative to inpatient treatment. Nevertheless, the most common antimicrobials used are antibiotics, and there is less information about the use of antifungal therapy (AT). The aim of this study is to analyse a cohort of patients treated with AT administered via OPAT and to compare them with patients from the rest of the cohort (RC) treated with antibiotics.
MethodsProspective observational study with post hoc (or retrospective) analysis of a cohort of patients treated in the OPAT program. We selected the patients treated with antifungals between July 2012 and December 2018. We recorded demographic and clinical data to analyse the validity of the treatment and to compare the differences between the AT and the RC.
ResultsOf the 1101 patients included in the OPAT program, 24 (2.18%) were treated with AT, 12 Liposomal Amphotericin B, 6 echinocandins and 6 fluconazole. This result is similar to other cohorts. There were differences between the AT vs RC in the number of patients with neoplasia (58.3% vs 28%; p=0.001), IC Charlson>2 (58.3% vs 38.8; p=0.053), duration of treatment (15 days vs 10.39 days; p=0.001) and patients with central catheters (54.2% vs 21.7%; p=0.0001). These differences are justified because there were more hematologic patients included in the AT group. Nevertheless, there were no differences in adverse reactions (25% vs 32.3%; p=0.45) or re-admissions (12.5% vs 10%; p=0.686) and OPAT with AT was successful in 21/24 patients (87.5%).
ConclusionsAT can be successfully administered in OPAT programs in selected patients, that are clinically stable and monitored by an infectious disease physician.
El tratamiento antimicrobiano domiciliario endovenoso (TADE) ha sido reconocido como una alternativa al tratamiento hospitalario útil, eficiente y seguro. Sin embargo, los antimicrobianos más utilizados son los antibióticos, y existe menos información sobre el uso de la terapia antimicótica (TA). El objetivo de este estudio es analizar una cohorte de pacientes tratados con TA administrada mediante TADE y compararlos con pacientes del resto de la cohorte (RC) tratados con otros antibióticos.
MétodosEstudio prospectivo observacional con análisis post hoc (o retrospectivo) de una cohorte de pacientes atendidos en el programa TADE. Seleccionamos a los pacientes tratados con antifúngicos entre julio de 2012 y diciembre de 2018. Registramos los datos demográficos y clínicos para analizar la validez del tratamiento y comparar las diferencias entre la TA y el RC.
ResultadosDe los 1.101 pacientes incluidos en el programa TADE, 24 (2,18%) fueron tratados con TA: 12 anfotericina B liposómica, 6 equinocandinas y 6 fluconazol. Este resultado es similar a otras cohortes. Hubo diferencias entre la TA vs. RC en el número de pacientes con neoplasia (58,3 vs. 28%; p=0,001), índice de Charlson>2 (58,3 vs. 38,8; p=0,053), duración del tratamiento (15 vs. 10,39 días; p=0,001) y pacientes con catéteres centrales (54,2 vs. 21,7%; p=0,0001). Estas diferencias están justificadas porque en el grupo TA se incluyeron más pacientes hematológicos. Sin embargo, no hubo diferencias en las reacciones adversas (25 vs. 32,3%; p=0,45) o reingresos (12,5 vs. 10%; p=0,686) y el TADE con TA tuvo éxito en 21/24 pacientes (87,5%).
ConclusionesEn pacientes seleccionados, clínicamente estables y en seguimiento por un médico de enfermedades infecciosas, la TA podría administrarse en programas TADE.
Outpatient parenteral antimicrobial therapy (OPAT) has been recognised as a useful, cost-effective and safe alternative to inpatient treatment.1–3 OPAT presents a series of clinical advantages such as: uses of resources more cost-effective; reduces risks of healthcare acquired infection; achieves high levels of patient acceptability and satisfaction; and improves quality of life. This strategy is of particular interest in the case of patients colonized with multidrug-resistant microorganisms and serves as a tool of infection control.4–7 A systematic review evidences the cost effectiveness of OPAT, highlighting that OPAT is cost-effective without increasing patient complications.7 This care modality should be the reference for patients who require intravenous treatment as soon as they have enough clinical stability to be at home.
The most commonly antimicrobial used are antibiotics, and there is less information about the use of antifungal therapy (AF), perhaps because the use of the later one is mostly seen in hematological patients, with co-morbidities and immune compromise. Nevertheless, in a revision of OPAT published recently, says that OPAT is an underutilised method of delivering therapy for antifungal treatment, and there is little published date of clinical practice, but AF could be useful in a carefully selected cohort of patients with appropriate safety monitoring and follow-up by an infection specialist.8 For selecting suitable patients to receive antifungal by OPAT many factors must be considered such as: site of infection, identified organism(s), co-morbidities, other prescribed medications, age, frailty, clinical stability and home circumstances (including home setting, family support and distance from hospital).9–12
The aim of this study is to analyse our experience with a cohort of patients treated with antifungals administered by OPAT and compare them with patients of the rest of the cohort (RC) treated with antibiotics.
MethodsWe conducted a prospective observational study with post hoc (or retrospective) analysis of a cohort of patients attended in the OPAT program. We selected the patients treated with antifungals between July 2012 and December 2018.
Our OPAT program started in 2012 at the University Hospital Virgen del Rocío and the University Hospital Virgen Macarena (Seville, Spain), two tertiary teaching hospitals with 1279 and 866 beds, respectively. The program focuses on patients over 18 years of age with all kinds of infectious diseases requiring intravenous therapy who are clinically stable enough to be treated at home. The program is coordinated by an infectious disease physician. The activity of this program has the approval of the research ethics committees of both hospitals. Patients are considered to be suitable for OPAT if they are clinically stable enough to be treated at home, the patient has sufficient cognitive capacity to manage venous access at home and/or an electronic infusion pump; there is an adequate family or caregiver support to receive OPAT; the patient has an adequate vascular access for the selected IV antibiotic for the planned duration of therapy, and there is necessary to select an expected date of completion of IV therapy or a scheduled appointment for intermediate evaluation.
Our OPAT program is comprised of two highly knowledgeable nurses experienced in treating these types of patients. The program is coordinated by two infectious disease physicians. Each of them belongs to a different hospital facility in Seville and they both collaborate with one another and with the infectious disease interdisciplinary consultants who assess the candidate patients in each of the hospital services. The OPAT program could include any patient residing in the city of Seville, where the two hospitals are located. The median number of discharges is 246 per year. We have the capacity to attend 10 patients simultaneously.
For those patients that need assistance, there is a medical care telephone service that runs 12hours a day. The nursing team from the infectious disease unit will be answering patients’ enquiries outside those hours. The system used consists of a series of questions that will determine the severity of the incidence and the right protocol to put in place. Patients included in this article (1101) add a total of 14,862 avoided stays.
Patients are assessed by an infectious disease physician on the same day, if clinical deterioration occurs. Patients included in the study were assessed in person during the treatment and continued with follow-up visits after the end of the treatment. The number of follow-up visits of each patient is carried out individually depending on the characteristics of each case.
Patients are visited by the team of nurses in the program 365 days a year to administer antibiotic therapy directly or using an electronic pump for antibiotics given more than once a day. All treatments of antibiotics are prepared by the Pharmacy Service under sterile conditions.
The following data were recorded: sex and age, medical service responsible for the patient, Charlson comorbidity index, diagnosis infection, whether the patient had neoplasia, antifungal used, antifungal's dosage used, microorganism isolated, duration of treatment (inpatient treatment and by OPAT) and vascular access used.
The efficacy variables collected were cause of end of treatment and readmission outcome and mortality. For safety assessment adverse reactions were collected.
The quantitative variables are presented as median (RIQ). The cases of antifungal treatment (CAT) are compared with those of the rest of the cohort (RC) by means of T-Student and Chi-square. PASW Statistics 18 (IBM SPSS, Chicago, IL, USA) was used for statistical analysis.
ResultsOf the 1101 patients included in the OPAT program, 24 (2.18%) were treated with AF, 12 with Liposomal Amphotericin (LAmB), 6 with echinocandins and 6 with fluconazole.
Patients were admitted to the following medical departments: 8 in Infectious Diseases, 7 in Hematology, 5 in Surgery, 2 in Digestive System, 1 in Urology and 1 in Oncology.
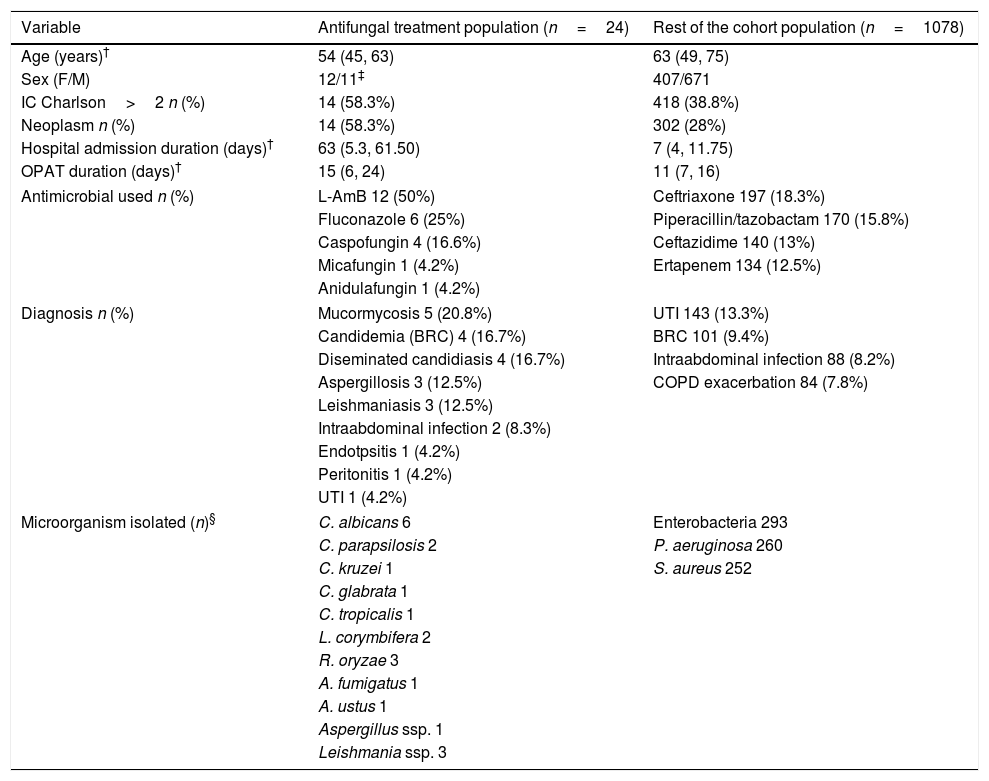
Baseline patient characteristics, diagnosis infectious and microorganisms isolated are shown in Table 1. All the variables selected of each patient of the cohort are listed in Table 2.
Baseline patient characteristics.
| Variable | Antifungal treatment population (n=24) | Rest of the cohort population (n=1078) |
|---|---|---|
| Age (years)† | 54 (45, 63) | 63 (49, 75) |
| Sex (F/M) | 12/11‡ | 407/671 |
| IC Charlson>2 n (%) | 14 (58.3%) | 418 (38.8%) |
| Neoplasm n (%) | 14 (58.3%) | 302 (28%) |
| Hospital admission duration (days)† | 63 (5.3, 61.50) | 7 (4, 11.75) |
| OPAT duration (days)† | 15 (6, 24) | 11 (7, 16) |
| Antimicrobial used n (%) | L-AmB 12 (50%) | Ceftriaxone 197 (18.3%) |
| Fluconazole 6 (25%) | Piperacillin/tazobactam 170 (15.8%) | |
| Caspofungin 4 (16.6%) | Ceftazidime 140 (13%) | |
| Micafungin 1 (4.2%) | Ertapenem 134 (12.5%) | |
| Anidulafungin 1 (4.2%) | ||
| Diagnosis n (%) | Mucormycosis 5 (20.8%) | UTI 143 (13.3%) |
| Candidemia (BRC) 4 (16.7%) | BRC 101 (9.4%) | |
| Diseminated candidiasis 4 (16.7%) | Intraabdominal infection 88 (8.2%) | |
| Aspergillosis 3 (12.5%) | COPD exacerbation 84 (7.8%) | |
| Leishmaniasis 3 (12.5%) | ||
| Intraabdominal infection 2 (8.3%) | ||
| Endotpsitis 1 (4.2%) | ||
| Peritonitis 1 (4.2%) | ||
| UTI 1 (4.2%) | ||
| Microorganism isolated (n)§ | C. albicans 6 | Enterobacteria 293 |
| C. parapsilosis 2 | P. aeruginosa 260 | |
| C. kruzei 1 | S. aureus 252 | |
| C. glabrata 1 | ||
| C. tropicalis 1 | ||
| L. corymbifera 2 | ||
| R. oryzae 3 | ||
| A. fumigatus 1 | ||
| A. ustus 1 | ||
| Aspergillus ssp. 1 | ||
| Leishmania ssp. 3 | ||
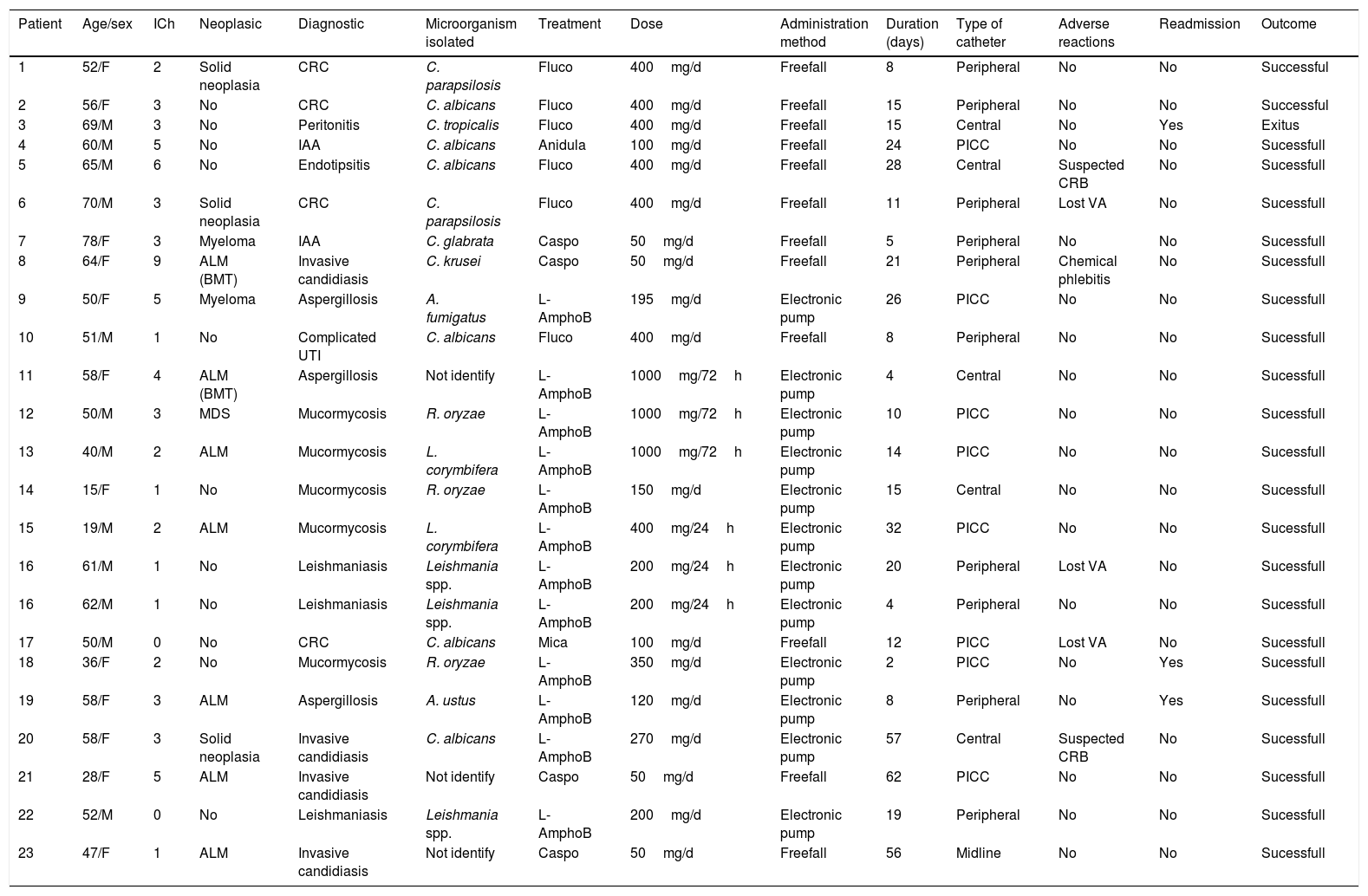
Variables of each patient of the study cohort.
| Patient | Age/sex | ICh | Neoplasic | Diagnostic | Microorganism isolated | Treatment | Dose | Administration method | Duration (days) | Type of catheter | Adverse reactions | Readmission | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52/F | 2 | Solid neoplasia | CRC | C. parapsilosis | Fluco | 400mg/d | Freefall | 8 | Peripheral | No | No | Successful |
| 2 | 56/F | 3 | No | CRC | C. albicans | Fluco | 400mg/d | Freefall | 15 | Peripheral | No | No | Successful |
| 3 | 69/M | 3 | No | Peritonitis | C. tropicalis | Fluco | 400mg/d | Freefall | 15 | Central | No | Yes | Exitus |
| 4 | 60/M | 5 | No | IAA | C. albicans | Anidula | 100mg/d | Freefall | 24 | PICC | No | No | Sucessfull |
| 5 | 65/M | 6 | No | Endotipsitis | C. albicans | Fluco | 400mg/d | Freefall | 28 | Central | Suspected CRB | No | Sucessfull |
| 6 | 70/M | 3 | Solid neoplasia | CRC | C. parapsilosis | Fluco | 400mg/d | Freefall | 11 | Peripheral | Lost VA | No | Sucessfull |
| 7 | 78/F | 3 | Myeloma | IAA | C. glabrata | Caspo | 50mg/d | Freefall | 5 | Peripheral | No | No | Sucessfull |
| 8 | 64/F | 9 | ALM (BMT) | Invasive candidiasis | C. krusei | Caspo | 50mg/d | Freefall | 21 | Peripheral | Chemical phlebitis | No | Sucessfull |
| 9 | 50/F | 5 | Myeloma | Aspergillosis | A. fumigatus | L-AmphoB | 195mg/d | Electronic pump | 26 | PICC | No | No | Sucessfull |
| 10 | 51/M | 1 | No | Complicated UTI | C. albicans | Fluco | 400mg/d | Freefall | 8 | Peripheral | No | No | Sucessfull |
| 11 | 58/F | 4 | ALM (BMT) | Aspergillosis | Not identify | L-AmphoB | 1000mg/72h | Electronic pump | 4 | Central | No | No | Sucessfull |
| 12 | 50/M | 3 | MDS | Mucormycosis | R. oryzae | L-AmphoB | 1000mg/72h | Electronic pump | 10 | PICC | No | No | Sucessfull |
| 13 | 40/M | 2 | ALM | Mucormycosis | L. corymbifera | L-AmphoB | 1000mg/72h | Electronic pump | 14 | PICC | No | No | Sucessfull |
| 14 | 15/F | 1 | No | Mucormycosis | R. oryzae | L-AmphoB | 150mg/d | Electronic pump | 15 | Central | No | No | Sucessfull |
| 15 | 19/M | 2 | ALM | Mucormycosis | L. corymbifera | L-AmphoB | 400mg/24h | Electronic pump | 32 | PICC | No | No | Sucessfull |
| 16 | 61/M | 1 | No | Leishmaniasis | Leishmania spp. | L-AmphoB | 200mg/24h | Electronic pump | 20 | Peripheral | Lost VA | No | Sucessfull |
| 16 | 62/M | 1 | No | Leishmaniasis | Leishmania spp. | L-AmphoB | 200mg/24h | Electronic pump | 4 | Peripheral | No | No | Sucessfull |
| 17 | 50/M | 0 | No | CRC | C. albicans | Mica | 100mg/d | Freefall | 12 | PICC | Lost VA | No | Sucessfull |
| 18 | 36/F | 2 | No | Mucormycosis | R. oryzae | L-AmphoB | 350mg/d | Electronic pump | 2 | PICC | No | Yes | Sucessfull |
| 19 | 58/F | 3 | ALM | Aspergillosis | A. ustus | L-AmphoB | 120mg/d | Electronic pump | 8 | Peripheral | No | Yes | Sucessfull |
| 20 | 58/F | 3 | Solid neoplasia | Invasive candidiasis | C. albicans | L-AmphoB | 270mg/d | Electronic pump | 57 | Central | Suspected CRB | No | Sucessfull |
| 21 | 28/F | 5 | ALM | Invasive candidiasis | Not identify | Caspo | 50mg/d | Freefall | 62 | PICC | No | No | Sucessfull |
| 22 | 52/M | 0 | No | Leishmaniasis | Leishmania spp. | L-AmphoB | 200mg/d | Electronic pump | 19 | Peripheral | No | No | Sucessfull |
| 23 | 47/F | 1 | ALM | Invasive candidiasis | Not identify | Caspo | 50mg/d | Freefall | 56 | Midline | No | No | Sucessfull |
H: male; F: female; ICh: Charlson comorbidity index; ALM: acute myeloid leukemia; BMT: bone marrow transplantation; MDS: myelodysplastic syndrome; CRC: catheter-related candidemia; Candidiasis D: candidiais diseminada; IAA: intra-abdominal abscess; CRB: catheter related bacteraemia; Fluco: fluconazole; Anidula: anidulafungin; Caspo: caspofungin; Mica: micafungin; L-AmphoB: Liposomal Amphotericin; UTI: urinary tract infection; VA: vascular access.
58.3% of patients in CAT had a neoplasm vs 28% in the RC (p=0.001). 58.3% in CAT had an IC Charlson>2 vs 38.8% in RC (p=0.053).
The median hospital admission duration was 63 days (RIQ 5.3–61.50). Four patients do not have a previous hospital admission because they were admitted to OPAT program directly from consultation (3 of them with leishmaniasis).
In 54.2% of patients in CAT, central catheters (CC) were used. If we exclude the treatment administered by middle lines, CC were used more frequently in CAT than in the RC (54.2% vs 21.7%, p=0.0001) probably because many patients already had a permanent vascular access for chemotherapy. In 41.7% of patients in AF an electronic pump was used.
Six patients presented complications in the vascular access: 25% in CAT vs 3 2.3% in RC (p=0.45). These complications were: 1 chemical phlebitis that disappears after catheter removal; 3 losses of vascular access that were resolved at home; 2 suspicions of bacteraemia not confirmed in which the catheter was removed. The 4 peripheral catheters were replaced at home (more than one vascular access replacement was required in two cases) and two patients with central catheters required to go to the hospital for its removal but did not require admission. In no case it was necessary to suspend the antifungal treatment.
Of the 6 cases with vascular access complications in CAT: 4 were due to free fall and 2 were due to a pump. Vascular access problems occurred in 2 of 8 patients with pump (33.3%) and in 4 of 14 (66.7%) due to free fall but there was not significant difference (p=0.506).
128 (10.4%) episodes of the 1225 attended in our OPAT program, re-entered in an unscheduled way. The variables associated to re-entry in multivariate analysis for these episodes were: presence of heart failure (OR 1.89, 95% CI 1.29–2.78), neoplastic disease (OR 1.56, 95% CI 1.05–2.29) and chronic liver disease (OR 1.90, 95% CI 1.10–3.29), but not the treatment modality.13
Although statistical significance of complication rates and readmissions differences was not reached, we believe that it is more due to the small sample size than to the actual absence of these differences.
The mean duration of antifungal treatment in OPAT was 15 days in CAT vs 10.39 days in RC (p=0.001).
OPAT treatment was successful in 21 patients (87.5%) of CAT. The rest of the patients (12.5%) re-entered for any reason vs 10% in RC (p=0.686). The patients who were readmitted were: a patient with acute myelomonocytic leukemia, HIV infection and invasive aspergillosis (Aspergillus ustus) whose antifungal treatment failed due to toxicity of the treatment which was suspended; a diabetic patient with rhinocerebral mucormycosis (Rhizopus oryzae) who also presented an adverse reaction with LAmB that required suspension of the treatment and a patient with gastrointestinal neoplasia and fungal peritonitis (Candida tropicalis) who was readmitted due to exacerbation of his basal pathology dehydration and aggravation, dying after 48h. One (4%) of the patients in CAT suffered exitus during treatment vs 1.2% in RC (p=0.121).
One patient treated with LAmB presented nausea and vomiting as adverse reactions to treatment.
DiscussionNo significant differences in efficacy and safety were found between antifungal therapy and the antibiotic cohort, nevertheless there were more patients with neoplasia and co-morbidity in CAT.
In our cohort, less than 2.18% of the patients were treated with antifungal, this is similar to other cohorts. In the study by Mirón-Rubio et al., just 1.23% of a cohort of 5088 patients were treated with antifungal.14 In our study the most common diagnosis was candidiasis (45.83%) and the most isolated microorganism was Candida albicans. The reason why these infections were treated via IV when an oral treatment was available was its complexity: infections in immunocompromised patients, disseminated candidiasis with endophthalmitis, post-surgery candidiasis with insufficient focus control.
The most commonly used antifungal was LAmB, which was used more frequently than in other studies which can be explained, in part, by the inclusion of leishmaniasis in our cohort. One of the innovations in the field of antifungal treatment by OPAT has been the administration of LAmB every 72h. This decision was based on its pharmacokinetic characteristics. LAmB has a long terminal half-life in plasma (152h). This contribution prevents patients from having to go to the hospital daily (in those hospital-based OPAT modalities) thus benefiting their quality of life.15–20 Patients who started LAmB treatment every 72h had previously received induction treatment during their hospital admission until their clinical stabilization.
The availability of antifungal treatment by OPAT has allowed antifungal infections as serious as mucormycosis or aspergillosis, that previously received treatment exclusively inpatient, can be treated on an outpatient basis.
Although in the CAT the duration of treatment is longer, the number of patients with neoplasia is greater and the IC Charlson is higher than in RC, it does not entail a greater risk of complications or re-admissions.
Despite the seriousness of the infections and the comorbidity of the patients, the percentage of success was high, although lower than that obtained in OPAT studies that only use antibiotics.1 This success could be explained by the adequate selection of patients and by the follow-up was carried out by infectious diseases physicians.
Although our study presents a limited number of patients and we cannot obtain representative results, in our opinion like a review published recently,8 other studies21,22 and the updates guidelines23 OPAT is useful and safe for patients treated with antifungal, but, it is very important to select them, so patients susceptible to receive antifungal treatment by OPAT must meet the following criteria, whenever possible:
- 1.
Confirmation of the diagnosis with microbiological isolation if possible.
- 2.
A control of the focus (whenever possible). In candidemia: withdrawal from the central catheter, negative blood cultures and echocardiography without findings suggestive of endocarditis.
- 3.
Initial antifungal treatment in inpatient regimen (10–14 days).
- 4.
Hemodynamic stability.
- 5.
Close follow-up by infectious diseases physician. As the study of Shah et al. proved infectious disease consultation during OPAT is associated with large and significant reductions in the rate of emergency department admission and hospital admission, as well as lower total healthcare spending.24
Our OPAT program emphasizes close monitoring of patients by skilled nurses with extensive training in infectious diseases management. Close communication with an infectious disease physician, allows optimizing OPAT programs without the need for a routine medical visit. Our nursing team can consult physicians, if necessary, by phone or videoconference from the patient's home. This model has been recently published by other authors.25
In selected patients, clinically stable and guided by infectious disease physician, antifungal treatment can be administered in OPAT programs.
Due to the limited number of patients and the real-life prevalence of this subgroup of patients, it is desirable to perform additional studies.
FundingThis work was supported by the Plan Nacional de I+D+i 2013, 2016 and Instituto de Salud Carlos III, Subdirección General de Redes y Centros de Investigación Cooperativa, Ministerio de Economía, Industria y Competitividad, Spanish Network for Research in Infectious Diseases (RD16/0016/0001 and RD16/0016/0009) co financed by European Development Regional Fund “A way to achieve Europe, Operative program Intelligent Growth 2014–2020.
Conflict of interestLuis Eduardo López-Cortés has served as scientific advisor for Novartis, speaker for MSD, Pfizer, ViiV, and Angelini, and has served as a trainer for MSD.
We highly appreciate the collaboration offered from the OPAT “DOMUS” team, particularly to P. Retamar, M. Ramón, E. Delgado, J.A. Pazos-Casado, P. Gil, M. Gutierrez and J.L. Pérez-Blanco, and the medical and nursing staff of the Clinical Units of Infectious Diseases, Microbiology, Preventive Medicine, and Pharmacy of University Hospitals Virgen del Rocio for their valuable collaboration in this study.