Ribavirin is a molecule with antiviral activity against different viruses. In clinical practice, it has made its niche almost exclusively for the treatment of the hepatitis C virus. However, there are other diseases in which it could be of benefit and it has the advantage of being suitable for oral, intravenous and inhaled administration. We conducted a review of the indications of the main drug agencies (Spanish, European and American) and other possible indications, mainly haemorrhagic fevers and coronavirus.
La ribavirina es una molécula con actividad antiviral sobre diferentes virus. Ha encontrado su hueco en la práctica clínica de forma casi exclusiva para el tratamiento del virus de la hepatitis C, pero existen otras enfermedades que podrían beneficiarse de su empleo. Su disponibilidad para administración por vía oral, por vía intravenosa e inhalada es una característica beneficiosa. En este trabajo se realiza una revisión de las indicaciones en las principales agencias del medicamento (española, europea y americana), así como de otras posibles indicaciones, principalmente sobre fiebres hemorrágicas y coronavirus.
Ribavirin is a molecule which was synthesised for the first time in 1972. It is also known by the name of Virazole or 1-f8-d-ribofuranosyl-1,2,4-triazole-3-carboxamide. It is a pyrimidine nucleoside which was designed as an in vitro broad-spectrum agent, and the first description was published in the journal Science.1 The action on both DNA (herpes virus 1 and 2, murine cytomegalovirus, vaccine virus, adenovirus type 3) and RNA viruses (rabies-related viruses, virus responsible for myxoma, parainfluenza virus types 1 and 3, influenza A and B, rhinovirus 1A, 13 and 56, Coxsackie B, poliovirus type 2, vesicular stomatitis virus and Semliki Forest virus) were detailed in this work. Subsequently, its activity on other viral diseases was determined, and it found its place in clinical practice almost exclusively for the treatment of hepatitis C virus (HCV).
In this paper, a review of the references in the PubMed database (up to 31 December 2017) was carried out, crossing (in English) the keyword “ribavirin” with those corresponding to the following viruses or diseases: adenovirus, hantavirus, syncytial respiratory virus, Lassa fever, Crimean-Congo haemorrhagic fever, Argentinian haemorrhagic fever and coronavirus. Articles written in English and in Spanish were reviewed, preferentially selecting the meta-analyses for each disease; in the event that they did not exist, phase I, II, III and IV clinical trials were reviewed. Finally, if they were not found in the previous searches, a review of the cases or case series published was performed. In some cases, references to other works were obtained from the articles reviewed.
Mechanism of actionDifferent mechanisms of action have been proposed for this molecule, and the most likely scenario is that it works in different ways in different viruses.2 The first mechanism that has been suggested is the interference in the de novo synthesis of the guanosine derivatives (in particular guanosine triphosphate [GTP]). Inosine monophosphate dehydrogenase catalyses the formation of xanthine monophosphate from inosine monophosphate to subsequently continue the synthesis pathway of the guanosine derivatives. Ribavirin monophosphate is capable of competitively and potently inhibiting this enzyme, reducing the reserves of these nucleotides (or nucleosides) to half in the treated cells. Antiviral activity parallel to the depletion of GTP reserves has been demonstrated in studies with the yellow fever virus and paramyxovirus, such as the respiratory syncytial virus (RSV). Secondly, an immunomodulatory activity has been suggested, indicating that ribavirin may increase the activity of T lymphocytes, and, in particular, Th lymphocytes. This hypothesis was based on the observation that during the treatment with ribavirin in patients infected with HCV, it could reduce cytolysis without modifying the circulating viral load. Therefore, it would not have an exclusively antiviral effect. The observation that the L-enantiomer of ribavirin (levovirin) does not present direct antiviral activity, being capable of also producing an induction of the Th1 response in the murine model, supported this hypothesis. However, it has not been possible to recognise the immunomodulatory effect in vivo, and, even in mathematical models designed for this, it is concluded that the effect is based on direct antiviral activity. Another mechanism that has been proposed is that of direct inhibition of the polymerase. The main intracellular metabolite of ribavirin is ribavirin triphosphate (RTP). In this way, it could be a competitive inhibitor of other similar nitrogenous bases, such as adenosine triphosphate (ATP) or GTP. This effect has been observed in studies conducted with the influenza virus, reovirus and vesicular stomatitis virus. Some RNA viruses contain a structure with 7-methylguanosine, which is essential for the stability of RNA and which is synthesised in three different catalytic processes. In other viruses, the translation process is measured by a molecule known as eukaryotic translation initiation factor 4E (eIF4E). The ribavirin molecule could interact with the enzymes responsible for capping (RNA cap synthesis) or bind to eIF4E, preventing the start of translation. This mechanism could be responsible for the antiviral activity observed in some viruses (Lassa fever, SARS). Lastly, a mechanism of mutagenesis has been proposed. Given the structural similarity, the incorporation of ribavirin in the RNA chain would be possible and would produce mutant viruses during replication. However, this would not only alter the “original” chain, replacing GTP with RTP, but also the binding to the complementary bases cytidine and thymidine (or uracil) has the same efficacy. This is due to the spatial arrangement and the flexibility in the rotation of a carboxamide group. The mutagenic effect of ribavirin has been demonstrated in studies with poliovirus, hand-foot-and-mouth disease, West Nile virus and hantavirus.
Indications in the summary of product characteristicsAs we have mentioned previously, the clinical use of ribavirin is almost exclusively limited to the treatment of HCV infection. However, when a review is performed of the indications in the main medicines agencies (Spanish Agency of Medicines and Medical Devices, European Medicines Agency and U.S. Food and Drug Administration [FDA]), other less well-known approved indications can be found.
- •
The drug Virazole, in the form of vials for respiratory inhalation, can be found on the website of the Spanish Agency of Medicines and Medical Devices.3 There is no mention of the specific indication, stating that the drug is not currently marketed in Spain and that there are supply problems. It was used in aerosols in children with severe lower respiratory tract infection caused by RSV. Treatment consisted of nebulisation for 12–18h for a period of three to seven days.
- •
On the European Medicines Agency's website,4 two applications of ribavirin which have been investigated, apart from the indication for HCV, are also found. These are for adenovirus infections and hantavirus infections. In both cases, it was decided to withdraw its consideration in these indications.
- •
Finally, the approval of Virazole for nebulisation is indicated on the FDA's website,5 although the conditions are not specified.
We will now review the clinical evidence of the effectiveness of ribavirin for treating infections caused by RSV, adenovirus and hantavirus.
Respiratory syncytial virusThere is a meta-analysis carried out by the Cochrane Database of Systematic Reviews6 in relation to the use of ribavirin in lower respiratory tract infections in children and adolescents, with two subsequent updates and one withdrawn in 2010. The latest review is therefore the one published in 2007. In this review, after analysing 12 clinical trials, the conclusion is reached that ribavirin is not effective at reducing mortality or in the development of respiratory deterioration; however, a reduction in the hospital stay and in the number of days of mechanical ventilation (approximately two days in both cases) is observed.
Another different aspect of the use of ribavirin in transplant recipients was the treatment of respiratory infections caused by RSV. Shah and Chemaly7 carried out a review of the literature regarding the works performed on adult transplant recipients with RSV infection and published between 1980 and 2010. They evaluated 26 studies, both prospective and retrospective (randomised and observational clinical trials), in which the main variable was mortality attributed to RSV or disease progression. The use of ribavirin in any form (aerosolised, oral or intravenous) appeared in these works. The overall conclusion is that the use of ribavirin (whether or not it was associated with immunoglobulins) reduced progression to disease of the lower respiratory tracts and reduced mortality. Inhaled ribavirin was useful, but it was especially more effective when associated with immunoglobulin, although its diffusion in the consolidated parenchyma could be reduced. The cost was the main drawback of this therapy (approximately $50,000 per patient).
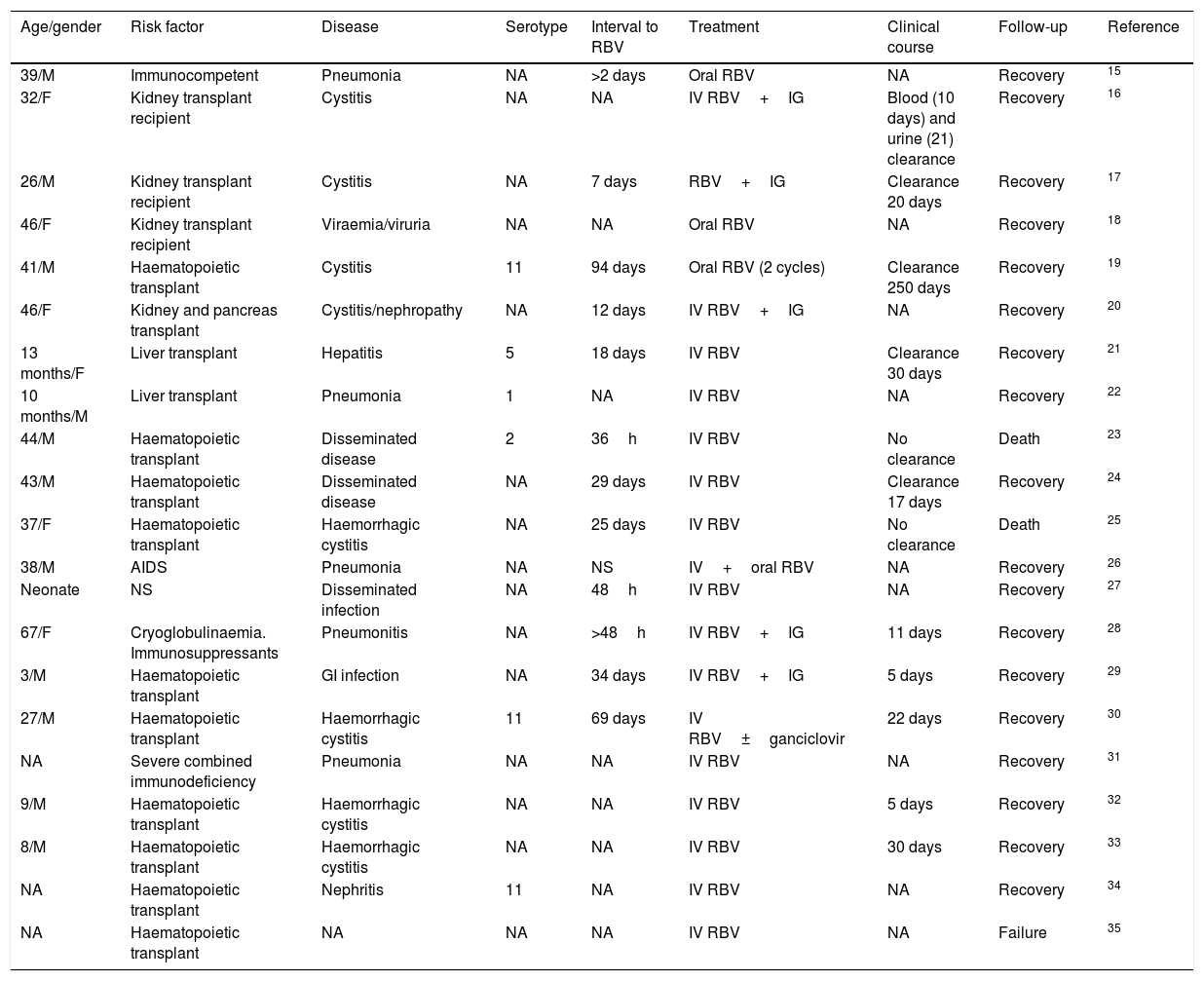
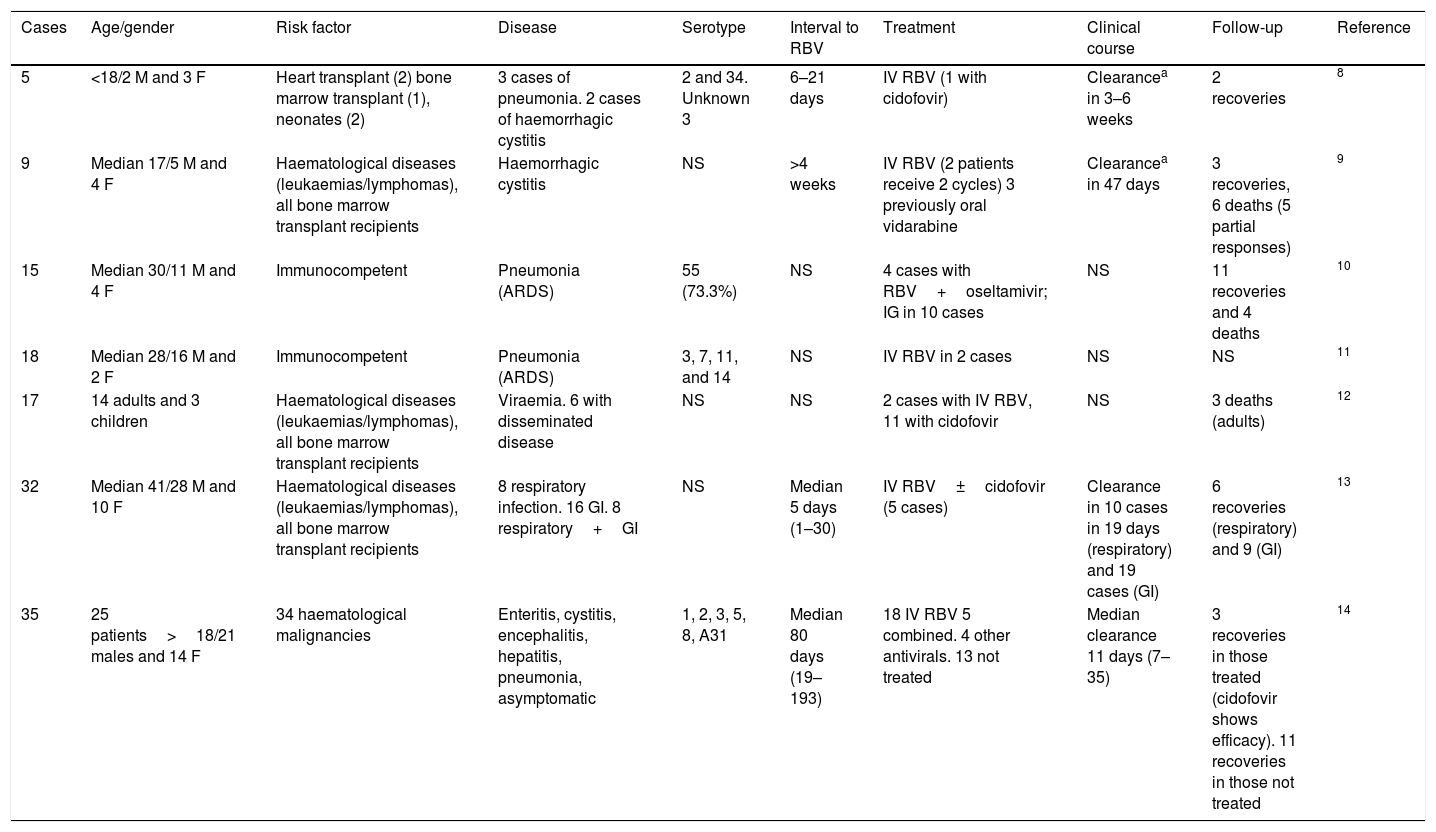
AdenovirusFirstly, it must be pointed out that, when the published literature was reviewed (109 articles in PubMed), there were no meta-analyses or clinical trials. The scientific evidence is available from case series and isolated cases. There are seven published case series8–14 and 21 isolated cases,15–35 which accounts in total for published experience on less than 150 cases (Tables 1 and 2). As can be observed, there is heterogeneity both regarding the virus treated (serotypes) and in the characteristics of the patients (age, gender, risk factors) and in the syndromes produced (pneumonia, cystitis, digestive disease, focal/disseminated disease), disease progression time, combination with other drugs, etc. Therefore, a conclusion cannot be obtained and, for this reason, its use has not been approved to date.
Notifications of isolated cases of disease due to adenovirus treated with ribavirin.
| Age/gender | Risk factor | Disease | Serotype | Interval to RBV | Treatment | Clinical course | Follow-up | Reference |
|---|---|---|---|---|---|---|---|---|
| 39/M | Immunocompetent | Pneumonia | NA | >2 days | Oral RBV | NA | Recovery | 15 |
| 32/F | Kidney transplant recipient | Cystitis | NA | NA | IV RBV+IG | Blood (10 days) and urine (21) clearance | Recovery | 16 |
| 26/M | Kidney transplant recipient | Cystitis | NA | 7 days | RBV+IG | Clearance 20 days | Recovery | 17 |
| 46/F | Kidney transplant recipient | Viraemia/viruria | NA | NA | Oral RBV | NA | Recovery | 18 |
| 41/M | Haematopoietic transplant | Cystitis | 11 | 94 days | Oral RBV (2 cycles) | Clearance 250 days | Recovery | 19 |
| 46/F | Kidney and pancreas transplant | Cystitis/nephropathy | NA | 12 days | IV RBV+IG | NA | Recovery | 20 |
| 13 months/F | Liver transplant | Hepatitis | 5 | 18 days | IV RBV | Clearance 30 days | Recovery | 21 |
| 10 months/M | Liver transplant | Pneumonia | 1 | NA | IV RBV | NA | Recovery | 22 |
| 44/M | Haematopoietic transplant | Disseminated disease | 2 | 36h | IV RBV | No clearance | Death | 23 |
| 43/M | Haematopoietic transplant | Disseminated disease | NA | 29 days | IV RBV | Clearance 17 days | Recovery | 24 |
| 37/F | Haematopoietic transplant | Haemorrhagic cystitis | NA | 25 days | IV RBV | No clearance | Death | 25 |
| 38/M | AIDS | Pneumonia | NA | NS | IV+oral RBV | NA | Recovery | 26 |
| Neonate | NS | Disseminated infection | NA | 48h | IV RBV | NA | Recovery | 27 |
| 67/F | Cryoglobulinaemia. Immunosuppressants | Pneumonitis | NA | >48h | IV RBV+IG | 11 days | Recovery | 28 |
| 3/M | Haematopoietic transplant | GI infection | NA | 34 days | IV RBV+IG | 5 days | Recovery | 29 |
| 27/M | Haematopoietic transplant | Haemorrhagic cystitis | 11 | 69 days | IV RBV±ganciclovir | 22 days | Recovery | 30 |
| NA | Severe combined immunodeficiency | Pneumonia | NA | NA | IV RBV | NA | Recovery | 31 |
| 9/M | Haematopoietic transplant | Haemorrhagic cystitis | NA | NA | IV RBV | 5 days | Recovery | 32 |
| 8/M | Haematopoietic transplant | Haemorrhagic cystitis | NA | NA | IV RBV | 30 days | Recovery | 33 |
| NA | Haematopoietic transplant | Nephritis | 11 | NA | IV RBV | NA | Recovery | 34 |
| NA | Haematopoietic transplant | NA | NA | NA | IV RBV | NA | Failure | 35 |
GI: gastrointestinal; IG: immunoglobulin; IV: intravenous; F: female; M: male; NA: not available; NS: not specified; RBV: ribavirin.
Case series of disease due to adenovirus treated with ribavirin.
| Cases | Age/gender | Risk factor | Disease | Serotype | Interval to RBV | Treatment | Clinical course | Follow-up | Reference |
|---|---|---|---|---|---|---|---|---|---|
| 5 | <18/2 M and 3 F | Heart transplant (2) bone marrow transplant (1), neonates (2) | 3 cases of pneumonia. 2 cases of haemorrhagic cystitis | 2 and 34. Unknown 3 | 6–21 days | IV RBV (1 with cidofovir) | Clearancea in 3–6 weeks | 2 recoveries | 8 |
| 9 | Median 17/5 M and 4 F | Haematological diseases (leukaemias/lymphomas), all bone marrow transplant recipients | Haemorrhagic cystitis | NS | >4 weeks | IV RBV (2 patients receive 2 cycles) 3 previously oral vidarabine | Clearancea in 47 days | 3 recoveries, 6 deaths (5 partial responses) | 9 |
| 15 | Median 30/11 M and 4 F | Immunocompetent | Pneumonia (ARDS) | 55 (73.3%) | NS | 4 cases with RBV+oseltamivir; IG in 10 cases | NS | 11 recoveries and 4 deaths | 10 |
| 18 | Median 28/16 M and 2 F | Immunocompetent | Pneumonia (ARDS) | 3, 7, 11, and 14 | NS | IV RBV in 2 cases | NS | NS | 11 |
| 17 | 14 adults and 3 children | Haematological diseases (leukaemias/lymphomas), all bone marrow transplant recipients | Viraemia. 6 with disseminated disease | NS | NS | 2 cases with IV RBV, 11 with cidofovir | NS | 3 deaths (adults) | 12 |
| 32 | Median 41/28 M and 10 F | Haematological diseases (leukaemias/lymphomas), all bone marrow transplant recipients | 8 respiratory infection. 16 GI. 8 respiratory+GI | NS | Median 5 days (1–30) | IV RBV±cidofovir (5 cases) | Clearance in 10 cases in 19 days (respiratory) and 19 cases (GI) | 6 recoveries (respiratory) and 9 (GI) | 13 |
| 35 | 25 patients>18/21 males and 14 F | 34 haematological malignancies | Enteritis, cystitis, encephalitis, hepatitis, pneumonia, asymptomatic | 1, 2, 3, 5, 8, A31 | Median 80 days (19–193) | 18 IV RBV 5 combined. 4 other antivirals. 13 not treated | Median clearance 11 days (7–35) | 3 recoveries in those treated (cidofovir shows efficacy). 11 recoveries in those not treated | 14 |
GI: gastrointestinal; IG: immunoglobulin; IV: intravenous; F: female; M: male; NS: not specified; RBV: ribavirin.
Case series of disease due to adenovirus treated with ribavirin.
Regarding the use of ribavirin, in diseases caused by hantavirus, there is a meta-analysis published in 2014 by Moreli et al.36 which is worthy of an in-depth review. It is a systematic review in which 518 studies are identified; after applying the exclusion criteria, only seven studies can be analysed, which accounts for a total of 506 patients.
Two groups (haemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome), in addition to two subgroups (studies conducted in animals [4] or in humans [3]) are distinguished. Overall mortality was 35%. In the hantavirus pulmonary syndrome group, a randomised study and three prospective cohorts were identified. In the animal studies, a reduction in mortality was observed in the treatment group versus placebo, but not in the studies in humans, with a mortality rate in both groups of 40%. In the haemorrhagic fever with renal syndrome group, three studies were included. A reduction in mortality was observed when ribavirin was used compared to placebo (2.5% versus 8.5% mortality) with a relative risk (RR) of 0.56 (0.42–0.76) in both the animal and human subgroups.
With the existing data in this meta-analysis, it could be suggested that Old World hantavirus infections (haemorrhagic fever with renal syndrome) could benefit from the use of ribavirin, whereas it would not be useful in hantavirus pulmonary syndrome. The authors state that they have not been able to consider the time from diagnosis to the administration of ribavirin: hantavirus pulmonary syndrome has a mortality rate of 50% and may indicate a more rapid progression of the disease than in haemorrhagic fever with renal syndrome, which has a mortality rate of 15%. This would be a key factor, indicating the greater need to start therapy at an early stage.
Another bias may have been the restriction of works selected according to language: only works published in English, Spanish or Portuguese were selected. Given that China has the most cases of hantavirus with haemorrhagic fever (in the period from 1950 to 2007, a total of 1,557,622 cases of haemorrhagic fever with renal syndrome were reported, with a clear tendency to reduce the incidence), it is possible that a priori information published in Chinese has been eliminated which would modify the results obtained.
Lastly, it must be taken into account that the list of hantaviruses is extensive (23, including the Hantaan, Seoul, Puumala and Dobrava viruses for haemorrhagic fever with renal syndrome and the Sin Nombre and Andes viruses in hantavirus pulmonary syndrome). The heterogeneity between viruses (not only between syndromes) may determine the results. This is what may be indicated by a recent study conducted to evaluate the efficacy and safety of ribavirin in the treatment of haemorrhagic fever with renal syndrome caused by the Puumala virus, which does not benefit from the use of ribavirin.37 It was an open, randomised study, on 73 patients, of whom 36 received standard support therapy and 37 the same therapy in addition to intravenous ribavirin. The study was not able to demonstrate efficacy in clinical aspects or in viral kinetics in the treatment, with important side effects (mainly anaemia, hyperbilirubinaemia, sinus bradycardia and skin rash).
Other indications (aside from the summary of product characteristics)Lassa feverWhen a review is performed of the treatment of Lassa fever with ribavirin, 108 publications are obtained. However, when the clinical trials and meta-analyses are evaluated, only one reference in relation to the effectiveness of the treatment is found.38 It is a study conducted in Sierra Leone in 1986 by the Ministry of Health in collaboration with the Centers for Disease Control and Prevention (CDC), which started in 1977, and in which the administration of convalescent plasma, oral ribavirin and intravenous ribavirin was evaluated. The study is rather complex. However, despite this fact, it can be concluded that treatment with intravenous or oral ribavirin is recommended at any time of the disease (with it being more effective in the first six days of the disease). Initially, a phase I case-control study of the clinical and diagnostic aspects of Lassa fever in untreated patients was designed (30 clinical and laboratory variables were collected which were analysed subsequently), and, once these variables were collected, a randomised trial was conducted with 70 patients (39 with oral ribavirin and 31 with plasma; pregnant women were included without being randomised in the latter group), without knowing at this time the results of the analysis of the variables collected. In all cases, treatment was started in the first 24h. Subsequently, elevation of transaminases and viraemia greater than 1000copies/ml were identified as poor prognosis variables.
At a later stage, after the variables indicating poor prognosis had been determined, a phase II study was developed, selecting the patients with one of the poor prognosis variables (elevation of transaminases upon diagnosis; viraemia was not available). Two groups of patients (29 patients in the intravenous ribavirin group and 33 in the intravenous ribavirin plus plasma group) were randomised. Pregnant women received only two plasma units. Overall, treatment with ribavirin reduced mortality. This included the analysis of the patients included both in the phase I study and in the phase II study. In patients with viraemia >1000copies/ml and treated with ribavirin, mortality was lower than in those who were not receiving treatment (mortality with intravenous ribavirin of 32% versus 76% without therapy (p=0.00015); mortality with oral ribavirin of 30% versus 76% without therapy (p=0.008). The same occurred if the viraemia was <1000copies/ml (mortality with intravenous ribavirin of 9% versus 28% without therapy (p=0.02); mortality with oral ribavirin of 7% versus 28% without therapy (p=0.01).
There is a second study, conducted by Fisher-Hoch et al.,39 which appears in PubMed as a clinical trial regarding the use of ribavirin in Lassa fever. Although it is true that it refers to its use in a clinical trial, what it describes is the onset of an adverse effect which is associated with recovery (“shivering/chills”) and what seems to be related to the speed of administration of the drug (<1min). These side effects disappeared after correcting the speed of infusion. Some studies have been conducted to evaluate ribavirin as post-exposure prophylaxis, but they have not provided conclusive results.40,41
Crimean-Congo haemorrhagic feverThere is only one randomised clinical trial which evaluates the use of ribavirin for Crimean-Congo haemorrhagic fever, conducted between 2004 and 2007 with 136 patients.42 In a baseline form, there were no differences in gender, age, incubation period (around five days), clinical or analytical characteristics. The administration of ribavirin was done before confirming the diagnosis, meaning that the time to administration of it was around four days. No differences were found in terms of mortality, analytical recovery or length of hospital stay. Subsequently, two meta-analyses evaluated the usefulness of ribavirin for Crimean-Congo haemorrhagic fever. The first is from 201043 and reviews 21 works. The conclusion that the authors draw is that ribavirin in the clinical trial was not superior to not using ribavirin, but in the joint evaluation of all the results, mortality was reduced (RR: 0.56; 95% CI: 0.35–0.90; 955 participants). What was not found was a difference in duration of the hospital admission. The second meta-analysis is from 2011,44 and also includes the only randomised clinical trial, but only seven more works, which are observational with control group, are introduced. No benefit of ribavirin with the aggregated data is found either. Both meta-analyses underline the risk of bias in all the works, whether it be selection, external validity or attrition bias.
South American haemorrhagic feversRibavirin has not managed to demonstrate its usefulness in the reduction of mortality due to the Junin virus (Argentine haemorrhagic fever) in two very small clinical trials.45,46 Nevertheless, in the first study, blocked viral replication and a delay in the time to death was observed, compared with historical controls. Clearance of viraemia was even obtained in patients who died (in this phase of the disease the virus is detected in up to 80% of cases). Given that there is a treatment which has proven to be effective if administered in the first seven days of the disease (convalescent serum), studies which used ribavirin were conducted in advanced stages of the disease, which would justify the poor outcomes. It is probably not possible to conduct new studies in this regard, since there is also a vaccine which has proven to be effective at reducing the incidence of the disease.47
There are practically no reported cases of the Machupo virus treated with ribavirin (Bolivian haemorrhagic fever): only two isolated cases in which intravenous ribavirin was administered and they recovered.48 The same applies to cases of Sabia virus. In 1995, one case of Sabia virus was treated with intravenous ribavirin, managing to cure the patient.49
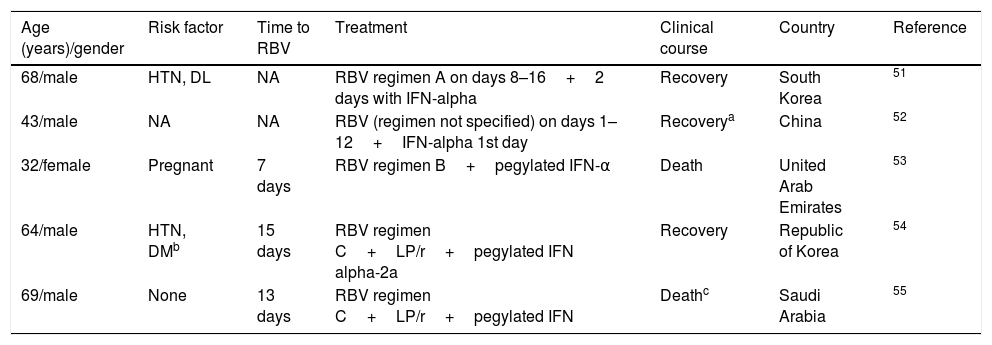
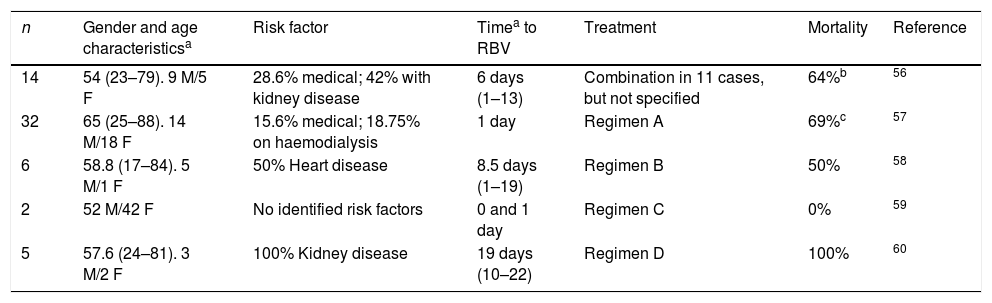
Respiratory viruses: coronavirusOne retrospective study was conducted in 44 patients with coronavirus50 to compare the combination of interferon and ribavirin versus placebo, observing an improvement in survival at 14 (70% vs 29%) and 28 days (30% vs 17%) in favour of the combination of drugs, although the difference at 28 days was not statistically significant (probably due to the small sample size). The mean treatment initiation time with ribavirin was three days. In addition, isolated cases51–55 and case series,56–60 which reflect variable degrees of therapeutic success (Tables 3 and 4), have been reported.
Cases of disease due to MERS-CoV treated with ribavirin.
| Age (years)/gender | Risk factor | Time to RBV | Treatment | Clinical course | Country | Reference |
|---|---|---|---|---|---|---|
| 68/male | HTN, DL | NA | RBV regimen A on days 8–16+2 days with IFN-alpha | Recovery | South Korea | 51 |
| 43/male | NA | NA | RBV (regimen not specified) on days 1–12+IFN-alpha 1st day | Recoverya | China | 52 |
| 32/female | Pregnant | 7 days | RBV regimen B+pegylated IFN-α | Death | United Arab Emirates | 53 |
| 64/male | HTN, DMb | 15 days | RBV regimen C+LP/r+pegylated IFN alpha-2a | Recovery | Republic of Korea | 54 |
| 69/male | None | 13 days | RBV regimen C+LP/r+pegylated IFN | Deathc | Saudi Arabia | 55 |
DL: dyslipidaemia; DM: diabetes mellitus; HTN: hypertension; IFN: interferon; LP/r: lopinavir/ritonavir; NA: not available or unknown; RBV: ribavirin.
He also had other comorbidities, such as splenectomy and pancreatectomy, and Mycobacterium avium-intracellulare infection.
Death due to colon adenocarcinoma.
Cases of disease due to MERS-CoV treated with interferon and ribavirin.
Regimen A. 2000mg as loading dose, 600mg/8h 3 days, and 400mg/8h for 4 days.
Regimen B. Loading dose (400mg 0–600mg) and subsequently 1200mg/12h.
The doses of lopinavir/ritonavir were 400/100mg/12h, administered orally.
Regimen C. Loading dose 2000mg and then 1200mg/8h orally.
Case series of disease due to MERS-CoV treated with ribavirin (+ IFN).
| n | Gender and age characteristicsa | Risk factor | Timea to RBV | Treatment | Mortality | Reference |
|---|---|---|---|---|---|---|
| 14 | 54 (23–79). 9 M/5 F | 28.6% medical; 42% with kidney disease | 6 days (1–13) | Combination in 11 cases, but not specified | 64%b | 56 |
| 32 | 65 (25–88). 14 M/18 F | 15.6% medical; 18.75% on haemodialysis | 1 day | Regimen A | 69%c | 57 |
| 6 | 58.8 (17–84). 5 M/1 F | 50% Heart disease | 8.5 days (1–19) | Regimen B | 50% | 58 |
| 2 | 52 M/42 F | No identified risk factors | 0 and 1 day | Regimen C | 0% | 59 |
| 5 | 57.6 (24–81). 3 M/2 F | 100% Kidney disease | 19 days (10–22) | Regimen D | 100% | 60 |
F: female; M: male.
Regimen A. Ribavirin (2000mg loading dose, followed by 600mg/12h)+IFN alpha-2a (180μg/week for 2 weeks) or IFN beta-1a (44mg 3 times/week).
Regimen B. Ribavirin (2000mg loading dose, then 1200mg/8h for 4 days), followed by 600mg/8h for 4–6 days (adjusted to kidney function)+IFN alpha-2b (180μg/week for 2 weeks).
Regimen C. Ribavirin (2000mg loading dose, then 1200mg/8h for 4 days)+pegylated IFN alpha-2b 180μg/week for 2 weeks).
Regimen D. Ribavirin (2000mg loading dose, then 800mg/8h through nasogastric tube)+IFN alpha-2b 100mg/week for 2 weeks.
New applications of ribavirin are currently being investigated. Four studies to investigate its usefulness in influenza, three on arbovirus, three on hepatitis B, three on hantavirus, two on paramyxovirus, two on Coxsackie virus and one on coronavirus are registered.
ConclusionRibavirin is a broad-spectrum antiviral that is currently looking for a new role in the treatment of various infections. Its availability for administration via the oral, intravenous or inhaled route is a beneficial characteristic. There is evidence of benefit in RSV infection and Lassa fever, with its use being proposed in certain situations of disease due to hantavirus, Crimean-Congo haemorrhagic fever and coronavirus, such as Middle East respiratory syndrome coronavirus (MERS-CoV). The availability of ribavirin in oral formulation currently exists in Spain, while the inhaled and intravenous forms must be requested through the Spanish Agency of Medicines and Medical Devices.
Please cite this article as: Ramírez-Olivencia G, Estébanez M, Membrillo FJ, Ybarra MC. Uso de ribavirina en virus distintos de la hepatitis C. Una revisión de la evidencia. Enferm Infecc Microbiol Clin. 2019;37:602–608.