Hepatitis E virus (HEV) infection is one of the main causes of acute hepatitis in both developed and developing countries. This infectious disease has a high prevalence and incidence in Europe. HEV infection has a greater clinical impact in vulnerable populations, such as immunosuppressed patients, pregnant women and patients with underlying liver disease. Therefore, the Study Group for Viral Hepatitis (Grupo de Estudio de Hepatitis Víricas, GEHEP) of the Spanish Society of Infectious Diseases and Clinical Microbiology (Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica, SEIMC) believed it very important to prepare a consensus document to help in decision-making regarding diagnosis, clinical and therapeutic management, and prevention of HEV infection.
La infección por el virus de la hepatitis E (VHE) es una de las principales causas de hepatitis aguda tanto en países desarrollados como en vías de desarrollo, situándose como una enfermedad infecciosa de alta prevalencia e incidencia en Europa. La infección por el VHE tiene mayor impacto clínico en poblaciones especialmente vulnerables, como pacientes inmunodeprimidos, mujeres embarazadas y pacientes con hepatopatía base. Por todo ello, desde el Grupo de Estudio de las Hepatitis Víricas (GeHEP) de la Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) se considera de gran relevancia la elaboración de un Documento de Consenso que sirva de ayuda en la toma de decisiones sobre el diagnóstico, manejo clínico-terapéutico y la prevención de la infección por el VHE.
Hepatitis E virus (HEV) infection is one of the main causes of acute hepatitis in both developed and developing countries. This infectious disease has a high prevalence and incidence in Europe.1 HEV infection has a greater clinical impact in vulnerable populations, such as immunosuppressed patients, pregnant women, and patients with underlying liver disease.2,3 Thus, the World Health Organisation (WHO) ranks it as one of the leading causes of death due to acute hepatitis of viral origin worldwide.4 However, national and international recommendations for the screening, diagnosis, and treatment of HEV have not been developed (EASL guidelines has been reported after submission of the present document),5 which makes it difficult to manage patients. This, combined with the fact that HEV infection is not a notifiable disease in most countries, allows us to speculate that its incidence and clinical impact may be higher than expected. The European Food Safety Authority (EFSA) has indicated that HEV infection is a major public health problem in Europe because the infection is transmitted efficiently by the consumption of contaminated animal foods (mainly pork and game meat), and there are no protocols or specific plans for prevention in animal production or in food production chains.6 Finally, there are no recommendations for the screening of this disease in blood, tissue, or organ donors, which may cause this route to be an important source of disease transmission.7,8
By these reasons, the Grupo de Estudio de Hepatitis Víricas (GEHEP) of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC) considered it very important to prepare a Consensus Document to help in decision making about the diagnosis, clinical, therapeutic management, and prevention of HEV infection. The panel of experts for the preparation of this document is composed of HEV infection experts in the areas of clinical management, diagnostic microbiology, food technology, and veterinary medicine. The recommendations in these guides are based on scientific evidence. The strength of the recommendation and grading of the evidence that supports it are based on a modification of the criteria of the Infectious Diseases Society of America.9 According to these criteria, each recommendation must always be offered (A), in general (B), or optionally (C), and must be based on data obtained from one or more randomised clinical trials with clinical or laboratory results (I), one or more nonrandomised trials or observational cohort studies (II), or the opinion of experts (III).
Who should be screened for the hepatitis E virus?Screening in patients with acute or chronic hepatitisRecommendations- •
All patients with acute hepatitis should be screened for HEV infection (AII).
- •
All patients with acute liver failure (ALF) should be screened for HEV infection (AII).
- •
In patients with known or recently diagnosed chronic liver disease with decompensation and/or data suggestive of acute liver inflammation HEV should be screened (AII).
- •
HEV screening should be included in the diagnostic of chronic hepatitis (AII).
- •
In patients with unexplained liver disease HEV screening should performed (AII).
- •
In patients with suspected drug-induced hepatitis, HEV screening should be performed (BII).
- •
HEV screening should be performed on all organ donors, living or deceased (AII).
- •
Studies on the prevalence of HEV infection in blood donations should be conducted across the different areas of blood bank influence to adapt HEV screening strategies to the prevalence of HEV infection in each area (AII).
- •
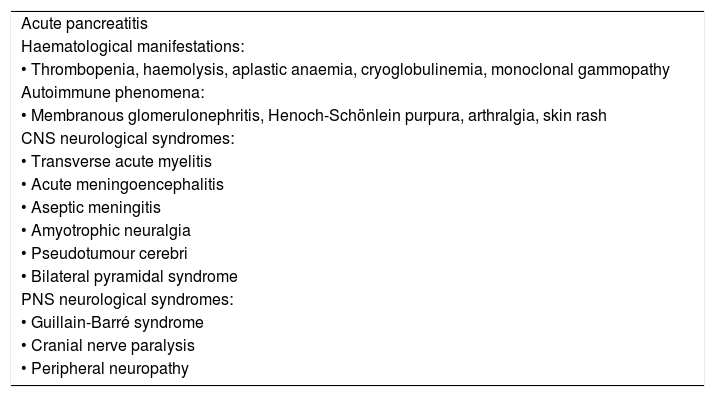
In patients with any of the extrahepatic clinical manifestations that have been associated with HEV infection (Table 1), screening for HEV infection should performed, even in the absence of liver abnormalities (BII).
Table 1.Extrahepatic manifestations of HEV infection.
Acute pancreatitis Haematological manifestations: • Thrombopenia, haemolysis, aplastic anaemia, cryoglobulinemia, monoclonal gammopathy Autoimmune phenomena: • Membranous glomerulonephritis, Henoch-Schönlein purpura, arthralgia, skin rash CNS neurological syndromes: • Transverse acute myelitis • Acute meningoencephalitis • Aseptic meningitis • Amyotrophic neuralgia • Pseudotumour cerebri • Bilateral pyramidal syndrome PNS neurological syndromes: • Guillain-Barré syndrome • Cranial nerve paralysis • Peripheral neuropathy
- •
The study of the close contacts of a case with documented HEV infection is not recommended, except in a case where they share a source of infection with the case (AII).
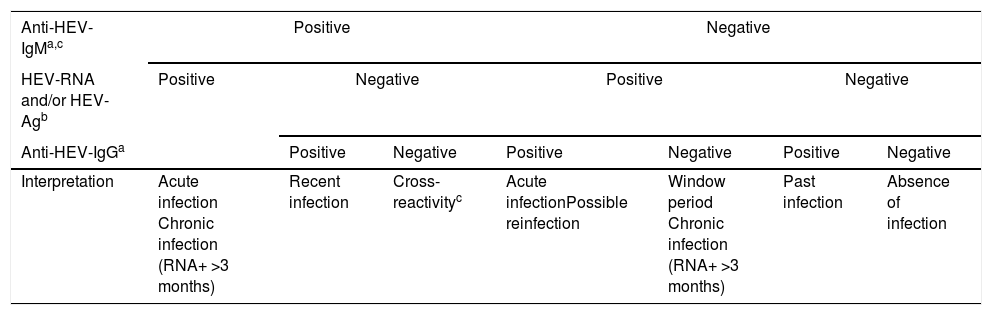
The virological markers for the diagnosis of HEV infection comprise components of the viral structure, such as nucleic acid (HEV-RNA) and the viral antigen (HEV-Ag), and products of the host immune response, such as Anti-HEV specific antibodies of classes IgA, IgG, and IgM. These virological markers are the basis not only for diagnosis but also for monitoring treatment, and they permit the characterisation of the natural history of hepatitis E in its different phases: acute, recent, resolved or past, and chronic. The principal markers of acute HEV infection are HEV-RNA, HEV-Ag, Anti-HEV-IgM, and -IgA; increasing titres or low affinity of Anti-HEV-IgG antibodies are also indicative of acute HEV infection. However, these markers appear at different times, persist for different periods, and differ in their meaning in the clinical diagnosis. Therefore, regardless of whether the high titres or low affinity of Anti-HEV-IgG-specific antibodies can be considered suggestive of acute HEV infection, their mere presence should not be considered a diagnostic criterion. Consequently, any diagnostic algorithm of HEV infection (Table 2) should be based mainly on the presence of Anti-HEV-IgM-specific antibodies and/or the presence of HEV-Ag and HEV-RNA infectivity markers. The diagnosis of chronic HEV infection is usually based almost exclusively on the presence of HEV-RNA in the blood or other body fluids for more than 3 months, whereas that of resolved infection is characterised by the isolated presence of Anti-HEV-IgG antibodies.
Diagnostic algorithm for HEV infection.
| Anti-HEV-IgMa,c | Positive | Negative | |||||
|---|---|---|---|---|---|---|---|
| HEV-RNA and/or HEV-Agb | Positive | Negative | Positive | Negative | |||
| Anti-HEV-IgGa | Positive | Negative | Positive | Negative | Positive | Negative | |
| Interpretation | Acute infection Chronic infection (RNA+ >3 months) | Recent infection | Cross-reactivityc | Acute infectionPossible reinfection | Window period Chronic infection (RNA+ >3 months) | Past infection | Absence of infection |
- •
The diagnosis of acute HEV infection in immunocompetent individuals is based on the presence of Anti-HEV-IgM and/or HEVRNA (AII).
- •
In laboratories that do not have adequate technology for molecular diagnosis, despite its lower sensitivity, it's recommended the detection of HEV-Ag as alternative, as a direct method for the diagnosis of HEV infection (AIII).
- •
Although it lacks diagnostic utility, the characterisation of HEV at the genotype and subtype levels through direct or new generation sequencing and subsequent phylogenetic analysis may be important for studies of the molecular epidemiology of HEV infection (AII).
- •
Quality control systems based on WHO standards should be established, and reagents available for this purpose for the standardisation of the different results obtained in molecular and serological techniques should be determined (BII).
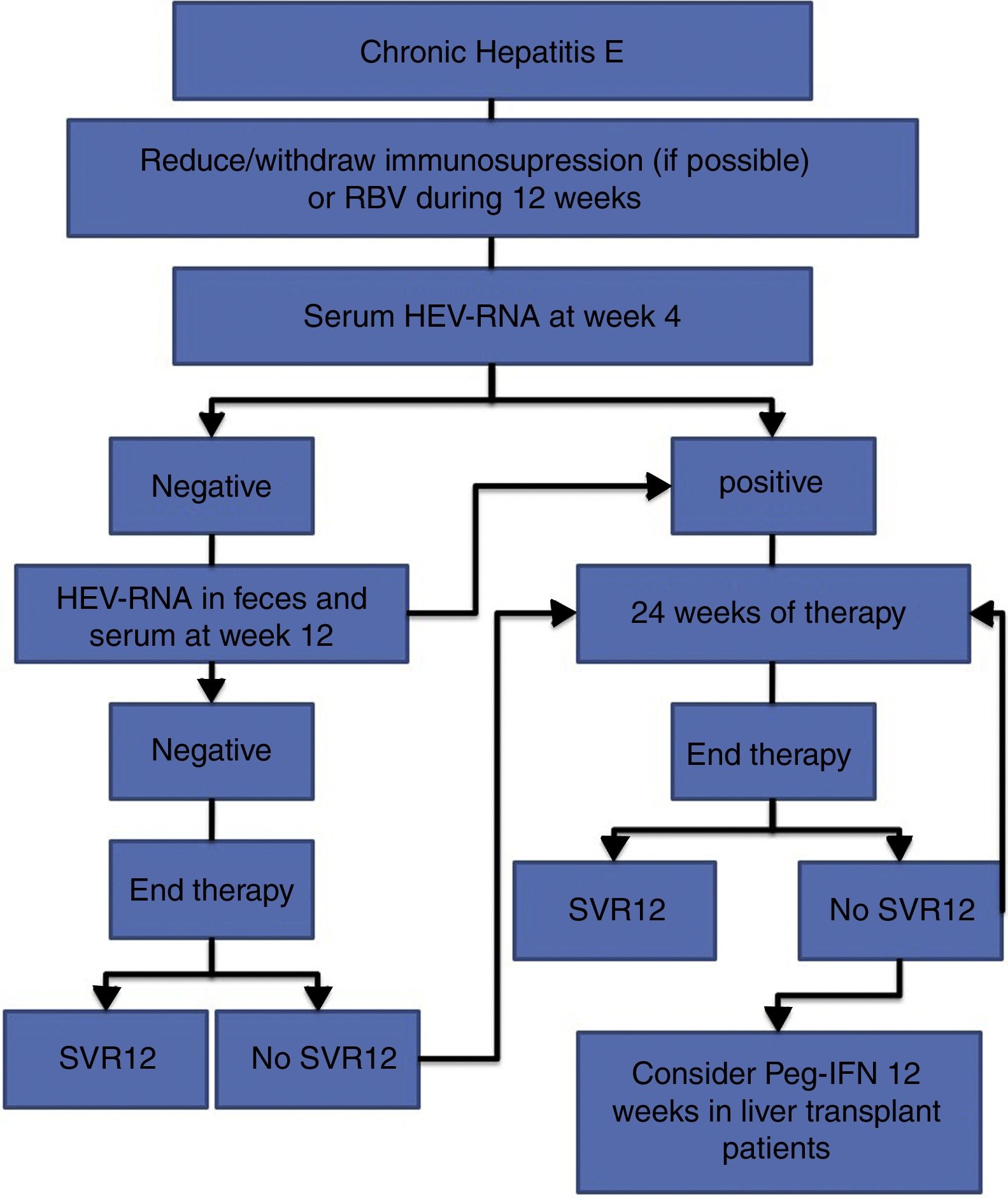
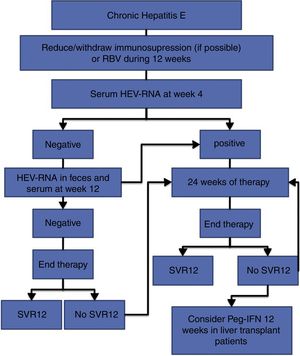
The treatment of HEV infection varies depending on the immunological situation of the patient and the clinical presentation of the infection (acute vs chronic). If indicated, the treatment objective is to eradicate HEV, which is determined by the achievement of a sustained viral response (SVR), defined as the absence of HEV-RNA at 12 weeks after the end of treatment. For this, the absence of HEV-RNA in both the serum and the faeces should be confirmed. Fig. 1 shows the proposed algorithm for treatment of chronic HEV infection.
Treatment of acute hepatitis ERecommendations- •
Antiviral treatment for acute hepatitis E should be considered in patients with liver cirrhosis or immunosuppression from any cause (BII). If treatment is indicated, this will consist of RBV adjusted for weight (1000mg if <75kg or 1200mg if >75kg) for 3 months (AII).
- •
In patients with pharmacological immunosuppression and chronic hepatitis due to HEV, immunosuppressive therapy should be reduced or discontinued if the clinical situation permits. The persistence of HEV-RNA in the blood and faeces should be reevaluated at 12 weeks (AII).
- •
In the case of persistent HEV-RNA after the reduction of immunosuppression or in those cases in which this measure is not feasible, antiviral treatment should be initiated (AII).
- •
In immunosuppressed patients of non-pharmacological causes, such as HIV-infected individuals, antiviral treatment should be considered from the beginning (AII).
- •
The antiviral treatment will consist of the administration of RBV 600mg per day for 12 weeks (AII).
- •
At 4 weeks, the presence of HEV-RNA in the serum should be evaluated, and treatment should be prolonged to 24 weeks if the viral load is positive (BII).
- •
If the viral load at week 4 is negative, the presence of HEV-RNA in the faeces and serum should be evaluated after 12 weeks of treatment, and treatment can be suspended if HEV clearance has occurred (BII).
- •
In the case of viral persistence, treatment should be continued until completing 24 weeks (BII).
- •
In all cases, the presence of HEV-RNA in the faeces and serum should be evaluated 12 weeks after completing treatment (AII).
- •
In the absence of SVR after a previous 12-week treatment, retreatment with RBV should be considered for 24 weeks (BIII).
- •
In those patients with an absence of SVR after treatment with RBV for 24 weeks, there are no current alternative treatment options that can be recommended, except for pegylated interferon in the specific scenario of liver transplantation (CIII).
- •
Given the suspicion of extrahepatic manifestations related to HEV infection, antiviral treatment with RBV can be considered following the same guidelines as for chronic hepatitis E (CIII).
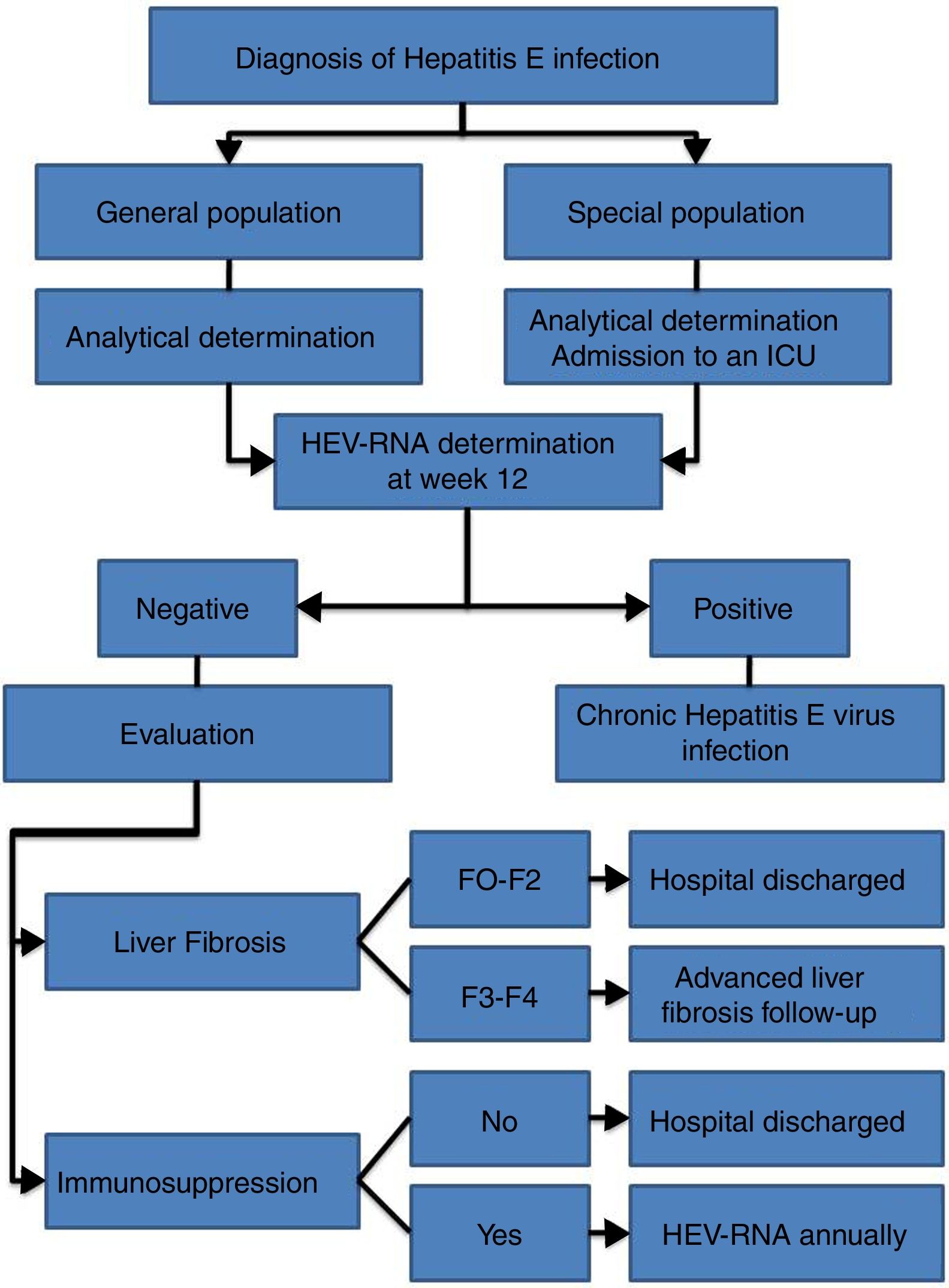
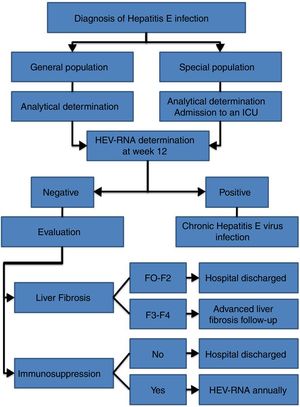
HEV infection, which depends in large part on the characteristics of the host and the virus itself, can evolve in an acute or chronic manner as well as present a wide range of clinical manifestations, ranging from asymptomatic or subclinical to fulminant hepatitis, with or without extrahepatic manifestations. Thus, an individualised clinical follow up is required for each patient (Fig. 2).
Follow-up of acute infectionRecommendations- •
Follow up is recommended for immunocompetent patients with acute HEV infection over 12 weeks to determine HEV-RNA status at the end of this period (AII).
- •
It is recommended that patients with acute HEV infection with clinical evidence of liver failure be admitted to an intensive care unit to start HEV treatment (AIII).
- •
Weekly liver function tests and close foetal monitoring are recommended for pregnant women with acute HEV infection under gynaecological advice (AII).
- •
Formula-feeding is recommended for women with acute or chronic HEV infection (CIII).
- •
It is recommended that patients with chronic HEV infection and SVR with persistent immunosuppression receive biannual HEV-RNA treatment during the first year (AII).
- •
The annual determination of HEV-RNA in the blood is recommended for immunocompromised patients in whom, after reaching SVR, the risk of exposure to HEV is maintained (professional...), given the risk of reinfection (BII).
- •
Biannual ultrasound tests are recommended indefinitely for hepatocarcinoma screening amongst patients with chronic HEV infection and stage F3 or F4 liver fibrosis, even after reaching SVR (CIII).
- •
Treatment and follow up of HEV infection is recommended for cases of extrahepatic manifestations following the same recommendations cited for acute or chronic infection, depending on the case (CIII).
The risk of acquiring HEV infection varies depending on the geographical location and the genotypes circulating in each region. Similar to all other communicable diseases, the simplest measures for preventing them are based on avoiding contact with sources of infection. In Europe, the main HEV exposure routes come from the intake of pork products, insufficiently cooked game animals (particularly the livers), and blood transfusions and their derivatives [4, 26, 122]. In travellers to developing countries in Asia, Africa, and Latin America and in the inhabitants of these countries, infections are also produced by this zoonotic route, although the main source is contaminated water and the products contaminated by it, causing large epidemic outbreaks. Considering these facts, it is essential to conduct information and awareness campaigns in the general population and amongst health workers, emphasising the acquisition pathways. Advice should be offered to the general population and, particularly in our setting, to immunosuppressed people and people who have chronic liver disease, because of the high risk of the infection becoming chronic or of having an accelerated or serious course of infection.
General measures in the general population and the immunosuppressed populationRecommendations- •
The prevention of HEV infection should be based on offering information aimed at avoiding contact with sources of infection (AII).
- •
On trips to developing countries, to avoid contact with HEV, general hygienic practices should be adopted, such as washing the hands with clean or sanitised water before handling food. Do not drink water or consume ice of unknown purity. The consumption of fruit not peeled by oneself and of raw foods in general should be avoided (AII).
- •
In addition to adopting the basic hygienic measures recommended for the general population, people at high risk of developing a severe course of infection or the chronification of it (cirrhotic and transplanted) must be specifically informed of the risk involved in eating pork products and undercooked game animals, including sausages, and to avoid the consumption of these products (AII).
- •
People at high risk of developing a severe course of infection or the chronification of it should subject food to cooking-heating at a temperature≥70°C for a minimum of 30min (AI).
- •
Given the serious and even fatal course that pregnant women may suffer, women travelling to areas of high endemicity of HEV genotypes 1 and 2 should receive information and should adhere to hygienic standards to avoid contact with HEV (AII).
- •
Patients suffering from acute or chronic HEV infection should use barrier measures in their sexual relations (AIII).
- •
Formula-feeding should be recommended in women with acute or chronic HEV infection (CIII).
- •
There is a recombinant vaccine marketed in China (Hecolin®) that has been shown to be safe and effective in healthy people over 16 and under 65 years of age (AI).
- •
Routine vaccination is not recommended in children under 16 years old, people over 65 years old, patients with chronic liver disease, patients on solid organ transplant lists, pregnant women, or travellers (CIII).
- •
Vaccination should be considered individually in people who plan to travel to an area where an epidemic is occurring (e.g., aid workers and health workers) (CIII).
There has been not funding from private institutions for the preparation of this document. The members of the Consensus Document panel have agreed to participate voluntarily and issue a declaration of conflicts of interest that can be found in the full document report (available at SEIMC web site).