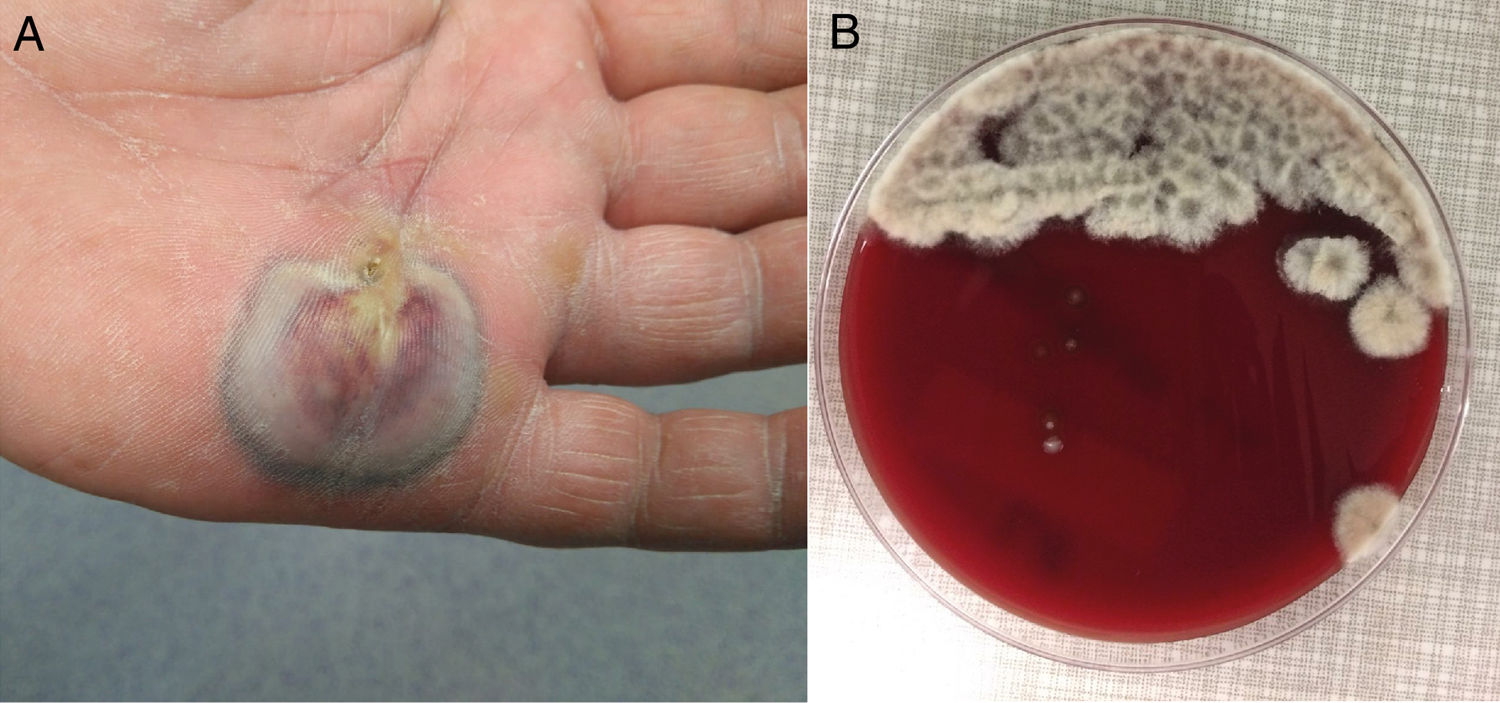
A 63-year-old male with intermittent claudication, obesity, dyslipidaemia and hypertension undergoing routine treatment came with a lesion on his palm which had been there for 4–5 days and pruritus, without fever. On examination, there was an abscess with central darkening and blackish edges measuring 6cm×2cm, with cellulitis on the back of his hand and lymphangitis up to the forearm (Fig. 1A). The patient did not remember a puncture or recent trauma, although he did work on his tomato garden. The analysis revealed a CRP of 62mg/l and leukocytes 11.96thousand/mcl (69.4% neutrophils). After surgical drainage, the purulent material was sent to microbiology and anatomical pathology. Macroscopically, plant material (seeds) was observed without grains. Empirical treatment with cefazolin and gentamicin IV was initiated.
Clinical courseThe sample was cultured in colistin-nalidixic (CNA) agar, chocolate agar, MacConkey agar and thioglycollate agar. After 48h a greyish white cotton fungus grew massively in CNA agar and chocolate agar (Fig. 1B) the staining of which with lactophenol cotton blue made us suspect the pathogen (Fig. 2A). The treatment was changed to voriconazole 400mg/12h IV (after loading dose) for 10 days, followed by 200mg/12h orally for 13 days with determination of plasma levels at 1.5μg/ml and periodic cures of the lesion. 1,3 Beta-d-glucan (BDG) serum was determined in a supplementary and serial manner, obtaining values of 20.54pg/ml and 9.05pg/ml, both negative.
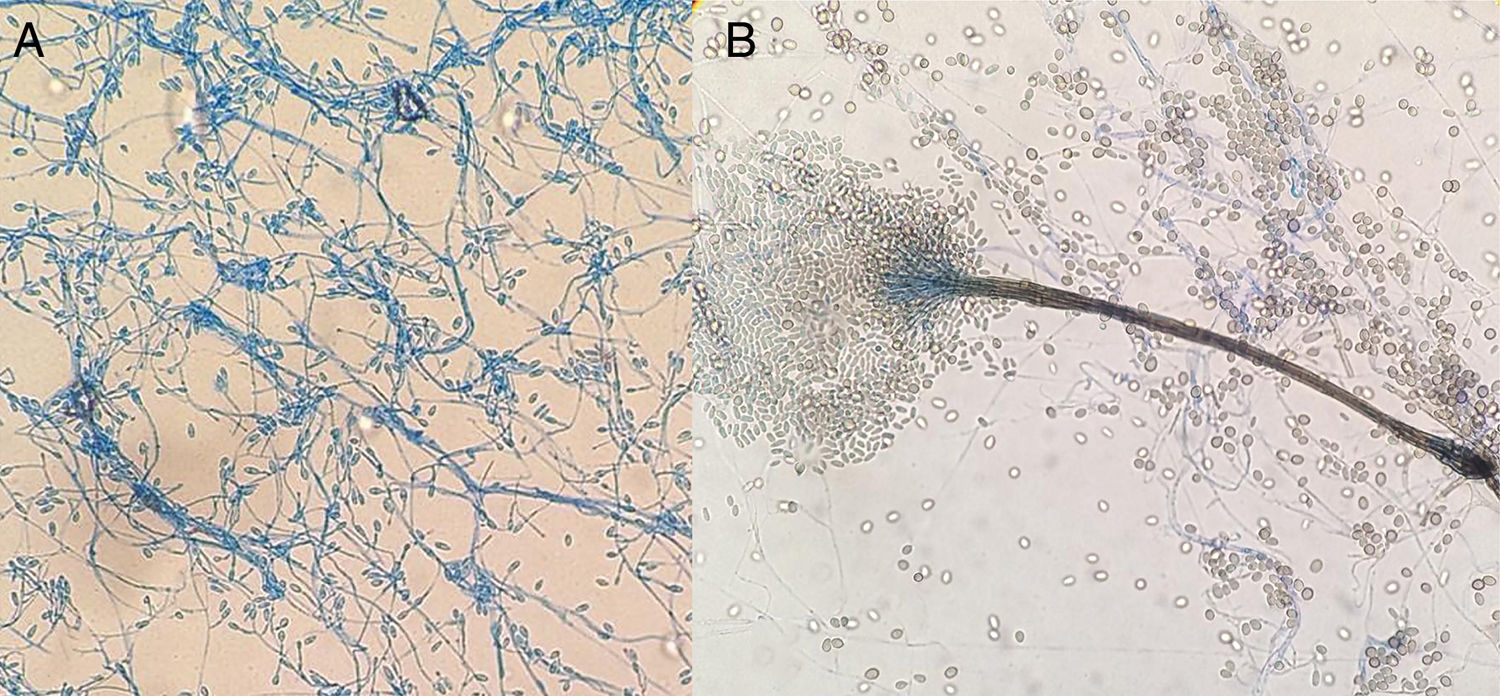
(A) Lateral conidiogenous cells or smooth-walled subhyaline terminals with production of subhyaline smooth-walled conidia measuring 5–14μm×3–5μm. (B) Graphium like: elongated and compact structure formed by stems of dark olive colour and subhyaline, cylindrical or claviform conidia with truncated base measuring 5–13μm×2–4μm. The colour in the image can only be seen in the electronic version of the article.
For confirmation of the pathogen, chloramphenicol was subcultured in Sabouraud agar without/with cycloheximide and half a dextrose potato, incubating at 35°C, 28°C and 25°C. Phenotypically, the growth with cycloheximide, the formation of sinnemas (Graphium-like) should be noted (Fig. 2B) and the absence of diffusive yellow pigment in potatoes at 25°C. After 23 days and after clinical resolution (Fig. 3), it was decided to suspend the treatment without recurrences until the present time.
CommentsThe images provided pose a differential mycological diagnosis between Scedosporium and Lomentospora; both Ascomycetes, the first with worldwide distribution and the second more restricted to Australia, Spain and California.1,2 The hyaline and dematiaceous form of the same genus Scedosporium were considered respectively, but since 20143 the previously named Scedosporium prolificans (Scedosporium inflatum) has belonged to the genus Lomentospora. This was ruled out in our case, as it did not present blackish colonies (Fig. 1B) nor did it have short conidiogenous cells with swollen base (Fig. 2A) and due to the fact that it tolerated cycloheximide. This improved the prognosis, given the multiresistance and virulence of Lomentospora prolificans.
The distinction between species of the genus Scedosporium is complex. More than ten have been described; notably: Scedosporium dehoogii (S. dehoogii), Scedosporium aurantiacum (S. aurantiacum), Scedosporium minutisporum (S. minutisporum) (Pseudallescheria minutispora), Scedosporium desertorum (Petriellidium desertorum), Scedosporium cereisporum and the complex Scedosporium apiospermum (S. apiospermum), which in turn includes another five species – S. apiospermum sensu stricto (Pseudallescheria apiosperma), Scedosporium boydii (S. boydii) (Pseudallescheria boydii), Scedosporium ellipsoideum (Pseudallescheria ellipsoidea), Scedosporium angustum (Pseudallescheria angusta) and Scedosporium fusoideum (Pseudallescheria fusoidea).4 In Europe,1,5 the five most clinically relevant for humans are: S. apiospermum, S. boydii, S. aurantiacum, S. dehoogii and S. minutisporum. All of those except S. dehoogii can form sinnemas (Fig. 2B), however, the last two are less frequent. The absence of diffusive pigment ruled out S. aurantiacum, with the identification being done by molecular biology which confirmed the presence of S. apiospermum sensu stricto.
This species, the most common in Europe,1 is ubiquitous; it isolates itself in soil, sewage and environments contaminated by human activity.4 Although their conidia are rarely isolated in the air,4 they can colonise the ear canal and respiratory tract of patients with chronic lung conditions and in 10%6 of cases with cystic fibrosis (due to the use of antibiotics and corticosteroids), which can trigger bronchitis or allergic pulmonary mycosis in them4 or act as a point of entry to deep infections. Studies conducted in the homes of subjects with cystic fibrosis demonstrated the presence of such mould1 in flowerpots on 50–89% of occasions, leading to their presence in clinical units with these pathologies being banned.
In addition, S. apiospermum sensu stricto is an opportunist in immunocompetent individuals4 generating localised infections: otitis or keratitis, endophthalmitis, mycetoma, arthritis after traumatic inoculation, as well as deep infections in immunosuppressed individuals.4
Our patient, when giving his history again, told us about the previous presence of a fistula at the point of entry of the seeds, facilitating subcutaneous infection. The absence of sclerotic bodies and grains ruled out chromoblastomycosis and mycetoma, respectively, with the clinical picture being classified as a subcutaneous hyalohyphomycosis.
Scedosporium spp. is resistant to 5-fluorcytosine, amphotericin B, first-generation triazoles (fluconazole and itraconazole) and even to isavuconazole and with a decreased sensitivity to echinocandins.4 European guidelines7 recommend voriconazole and surgical treatment, as well as determination of trough serum levels five days after starting oral treatment to ensure optimal serum concentrations of the antifungal,8 thereby achieving clinical cure.
Unlike other skin abscesses due to Scedosporium,9,10 in our case, serum 1,3 BDG was negative, perhaps because it is a different species, less extensive and recurring in an immunocompetent individual. Although it is true that Scedosporium spp. releases low levels of BDG, said biomarker can be a diagnostic tool in deep mycoses.
We would like to thank Doctor Ana Alaustrey-Izquierdo, Instituto de Salud Carlos III (Carlos III Health Institute), Majadahonda (Madrid).
Please cite this article as: García-Gutiérrez CA, Chan Moi Fat V, Puerta-Mateo A, Cuétara MS. Absceso en mano de etiología inusual. Enferm Infecc Microbiol Clin. 2019;2020:33–35.