The epidemiology of human fascioliasis is related to the geographical and environmental characteristics of the area where transmission occurs. There are gaps in the current knowledge of the distribution of fascioliasis in Spain. The objective of the study was to determine the incidence and geographic distribution of patients with fascioliasis who required hospitalization in Spain.
MethodAn observational study of the hospitalised population (166 patients) with a diagnosis of fascioliasis, according to the minimum basic data set (MBDS) for hospital discharge, between 1997 and 2014 was carried out. Most of the hospitals were Spanish National Health System hospitals.
ResultsThe incidence of hospitalised patients with fascioliasis was 2.1 (99% CI, 2.03–2.13) cases/10,000,000 inhabitants per year. The annual incidence was reduced during the 18 years of the study. The relative risk of men was 1.136 (99% CI, 0.299–0.993) with respect to women. A focal geographic aggregation of cases was observed in areas especially in northern and central Spain. The greatest incidents were found in the provinces of Segovia and Lugo. Intrahospital death occurred in 3 patients.
ConclusionThe incidence of hospitalisations with fascioliasis in Spain is low, has been reduced and has predominated in the provinces of Segovia and Lugo.
La epidemiología de la fascioliasis humana está relacionada con las características geográficas y ambientales del área donde se produce la transmisión. Existen lagunas en el conocimiento actual de la distribución de la fascioliasis en España. El objetivo del estudio fue conocer la incidencia y distribución geográfica de pacientes con fascioliasis que requirieron hospitalización en España.
MétodoSe realizó un estudio observacional de población hospitalizada con diagnóstico de fascioliasis, según el conjunto mínimo de datos al alta hospitalaria (CMBD), entre 1997 y 2014 (166 pacientes), de la mayoría de los hospitales del Sistema Nacional de Salud de España.
ResultadosLa incidencia de ingresos con fascioliasis fue de 2,1 (IC99%, 2,03-2,13) casos/10.000.000 habitantes y año. La incidencia anual se redujo durante los 18 años del estudio. El riesgo relativo de los hombres fue de 1,136 (IC99%, 0,299-0,993) respecto a las mujeres. Se observó una agregación geográfica focal de casos en determinadas áreas, especialmente del norte y centro de España. Las mayores incidencias se encontraron en las provincias de Segovia y Lugo. El fallecimiento intrahospitalario se produjo en 3 pacientes.
ConclusiónLa incidencia de hospitalizaciones con fascioliasis en España es baja, se ha reducido y ha predominado en las provincias de Segovia y Lugo.
Fascioliasis is caused by two liverworm trematodes: Fasciola hepatica which has global distribution and Fasciola gigantica which is restricted to the regions of Africa and Asia.1 Humans do not usually contribute to the parasite's life cycle; they usually become infected when they consume watercress and other raw vegetables contaminated with larvae or drink water infected with larvae.1
The distribution of disease caused by F. hepatica is uneven, with outbreaks related to the distribution of the population of freshwater snails of the genus Lymnaea, especially in areas with sheep and cattle. In Europe, ruminant fascioliasis is mainly associated with sheep, cows and goats, although there are large regional variations in prevalence.2 For example, Sánchez-Andrade et al. detected a high prevalence of bovine fascioliasis in Galicia (84.3%).3 The recognized areas of high human transmission are the highlands of South America, the Nile Valley, the Caspian Sea Basin, and East and Southeast Asia.1 Its current incidence in humans is unknown in Spain, although it has been cited together with countries with high prevalence of the disease.4 In Spain, cases have been described mostly from the north and especially from Navarra, the Basque Country and La Rioja.5–8
Fascioliasis is not a disease with mandatory reporting in Spain. Knowledge of the disease burden is therefore limited.9 In Spain, there are gaps in the information on its epidemiology and, to our knowledge, there is no study based on broad series such as that of admissions in all Spanish public hospitals over an extended period of time. The primary objective of this work has been to know the incidence and geographic location of hospital admissions with fascioliasis in Spain.
Materials and methodsAn observational study of the population treated in Spanish hospitals whose diagnosis at hospital discharge included fascioliasis was carried out during the period 1997–2014. Records of patients treated as outpatients in specialized or primary care were not analyzed.
Source of informationInformation regarding patients hospitalized for fascioliasis was collected from the Spanish minimum basic data set (MBDS) of the Health Information Institute, Ministry of Health, Consumption and Social Welfare (MSCBS). Spanish hospitals, especially public hospitals, record the MBDS upon discharge of each patient treated. The National Health System (SNS) covers 99.5% of the Spanish population and the MBDS receives reports from around 98% of Spanish public hospitals. The Spanish MSCBS sets standards for record keeping and performs periodic audits.
Until 2015, the diagnoses and procedures collected were coded following the International Classification of Diseases, in its clinical modification (ICD 9-CM), allowing the different care episodes provided by a hospital to be organized in groups related to diagnosis (GRD),10 on the basis of which the costs are valued. In Spain, the Ministry of Health periodically draws up SNS costs through a process of estimating the hospital costs of the GRDs obtained from the analytical accounting systems in a sample of hospitals.
Design and study populationThe design of the work took place after the occurrence of the data. The data for the study extracted from the Spanish MBDS included, among others, the anonymized identification of the patient, the post code of the patient's place of residence, the autonomous community, the date of admission, age, sex, length of hospital stay, hospitalization department, primary diagnosis and 13 secondary diagnoses, hospital discharge characteristics (patient destination or death), cost, severity index and mortality risk.11 Patients whose diagnoses contained the code 121.3 (fascioliasis) according to the ICD 9-CM in the MBDS, in any of the 14 diagnostic positions (primary or secondary) were considered “hospitalization with fascioliasis”. Hospitalization was considered to be due to the disease when the diagnosis of fascioliasis appeared as the main diagnosis. Re-admission records were excluded for the incidence calculation. As this is a passive follow-up of the population (search of a pre-established registry in an IT system), the eligibility criteria were applied when the data was extracted from the ministry's MBDS.
Statistical analysisTo calculate the incidence, the patient's first admission with the diagnosis was taken into account. The incidence rate for admissions with fascioliasis in Spain was calculated from the population register of the National Statistics Institute (INE).12 The ages for some calculations were distributed by the age groups children (0–14 years), 15–64 years, and elderly (>64 years). For the calculation of patient mortality rates, only the last admission was considered.
The presence of incongruous or lost data in each variable was analyzed to exclude these from the analysis. Statistical analysis was performed using SPSS version 23 and Excel. The comparison between the values of two qualitative variables was performed using the chi-squared test. For all hypothesis tests, a significance level of less than 0.01 was accepted. Quantitative variables that did not fit a normal distribution (age, length of stay and cost) were described with the median, interquartile range (IQR) and range.
Ethical aspectsIn addition to being anonymized, the data were processed with absolute confidentiality according to Spanish legislation and the regulations of the MSCBS.
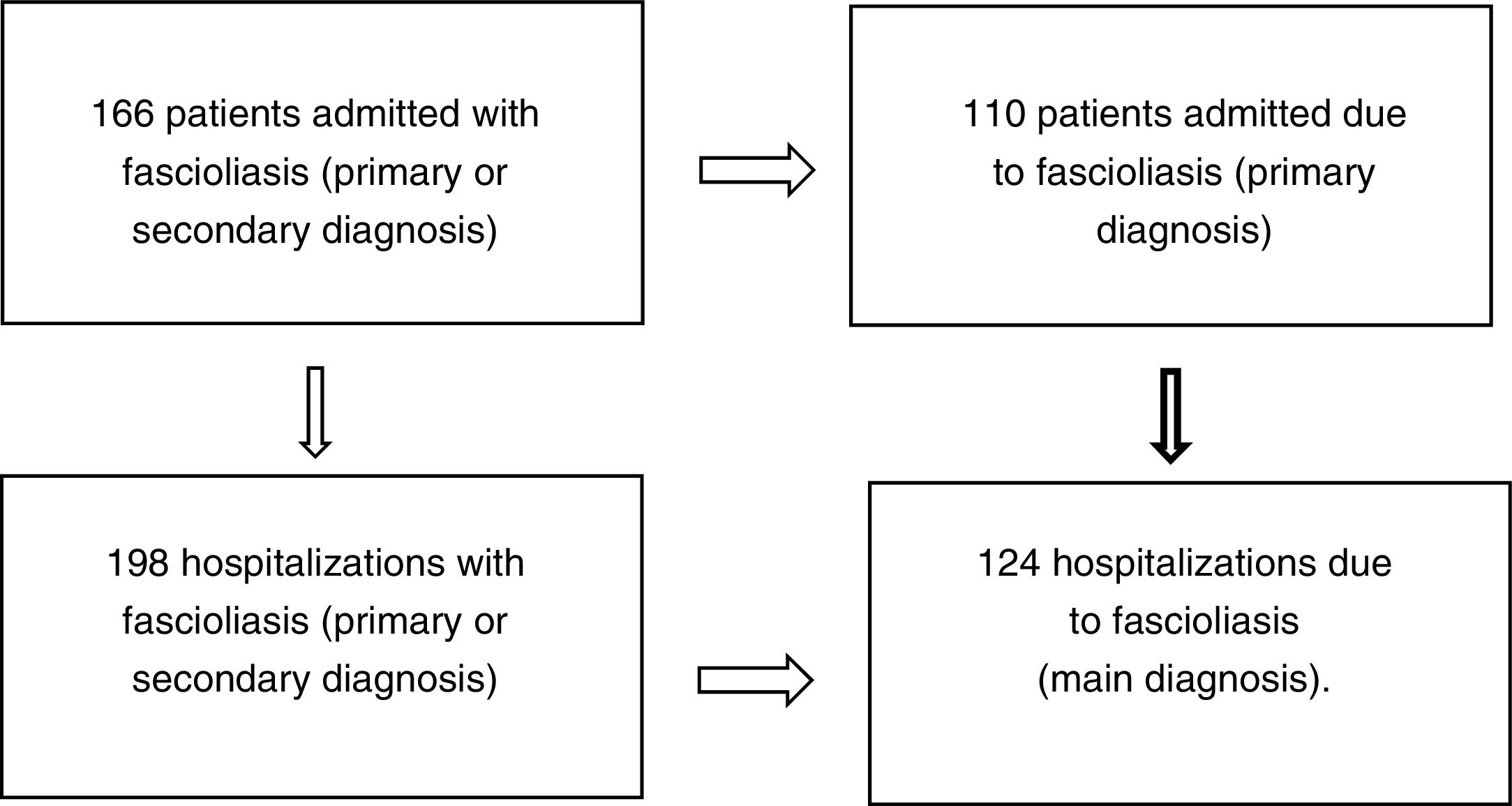
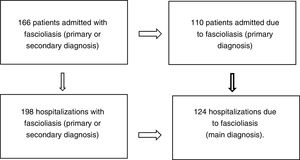
ResultsOne hundred and sixty-six patients met the inclusion and exclusion criteria (110 admitted for fascioliasis as the main diagnosis) with 198 admissions (of which included 124 had fascioliasis as the main diagnosis) (Fig. 1).
The mean was 52 years (IQR 38–67 years) and range from 4 to 89 years. Four point two percent (7 cases, with a mean of 7 years and interquartile range of 6) corresponded to children, 57.8% (96 cases, with a mean of 45 years and RIQ of 17 years) to patients between 15 and 64 years of age and 38.0% (63 cases, with a median of 72 years and RIQ of 12) to those over 64 years. Some 52.4% (87 cases) were men.
There was no significant difference between the percentages of cases diagnosed in the different seasons of the year (p=0.637), although in spring and winter (44 and 46 new cases, respectively) more cases were diagnosed than in summer and autumn (41 and 35 new cases, respectively).
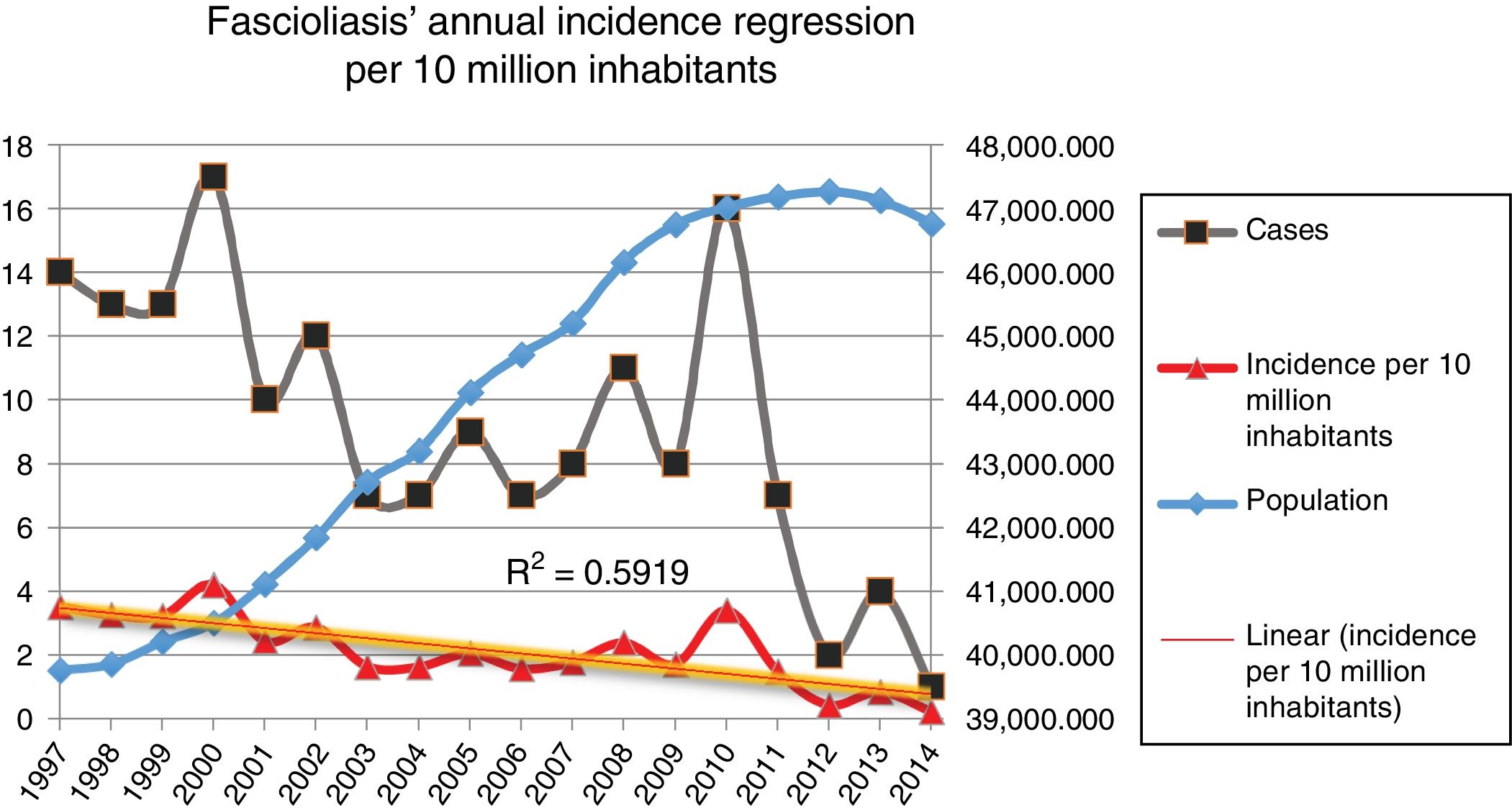
Incidence of fascioliasis in SpainThe average incidence of patients admitted with fascioliasis was 2.09 (99% CI, 2.03–2.13) cases per 10,000,000 inhabitants per year. The incidence in men and women was 2.25 and 1.98 per 10,000,000 inhabitants per year, respectively (p=0.3305), with a relative risk (RR) in men of 1.136 (99% CI, 0.299–0.993) with respect to women. The incidence per 10,000,000 inhabitants per year, in paediatric patients (0–14 years) was 0.61 cases; between 15 and 64 years of age it was 2.05 cases; and in those over 64 years of age 3.43 cases.
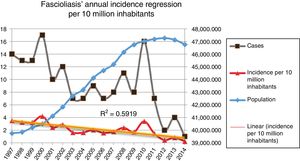
Fig. 2 shows the downward trend line of the annual incidence from 1997 to 2014. There were significant differences (p=0.0001, RR 1.82) between the incidence of fascioliasis in the periods 1997–2005 (2.5/1,000,000 inhabitants) and 2006–2014 (1.4/1,000,000 inhabitants).
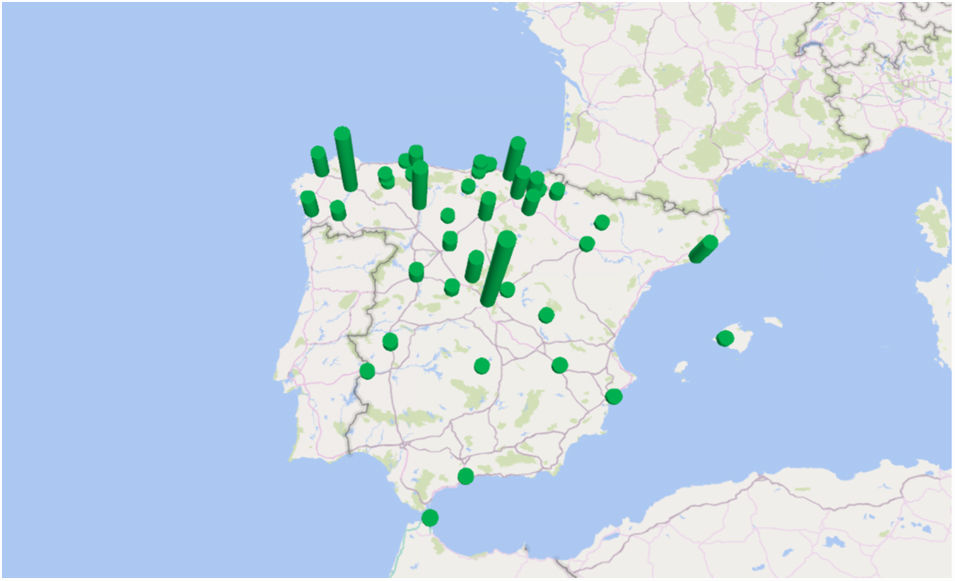
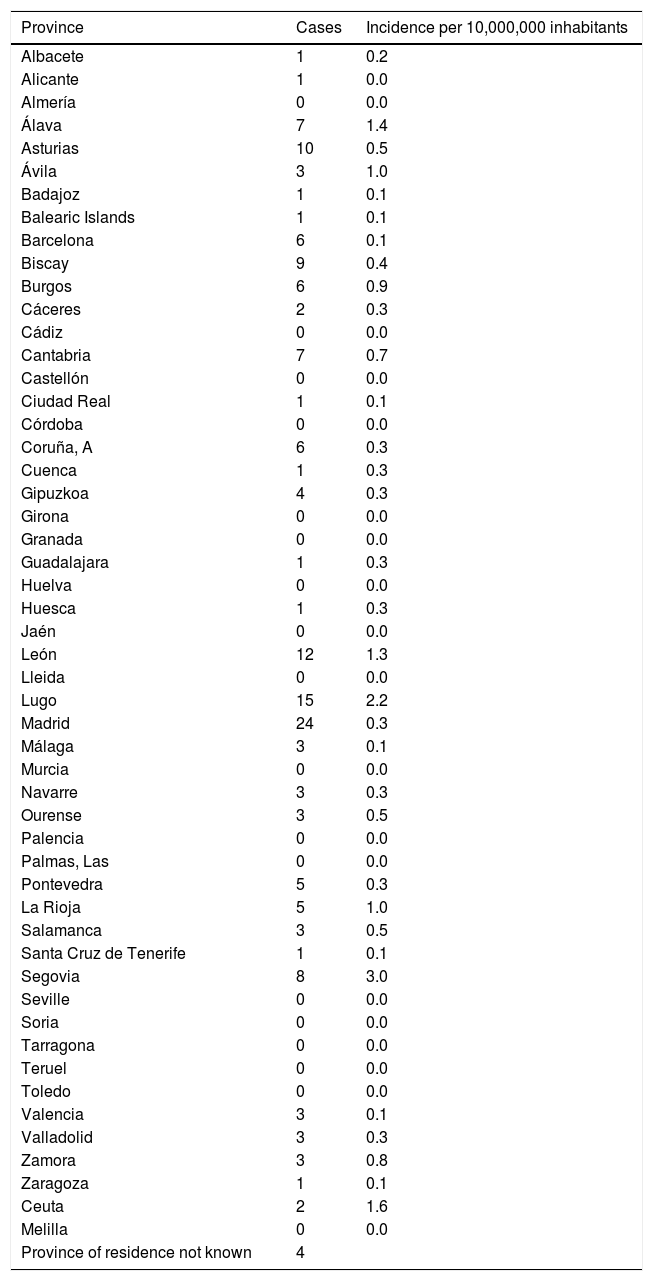
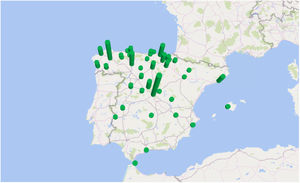
The incidence by autonomous communities was heterogeneous and a geographical aggregation of cases in certain provinces, especially in northern and central Spain, can be seen on the map (Fig. 3). The greatest incidences were observed in the provinces of Segovia and Lugo; the city of Ceuta and the provinces of Alava and León followed in incidence (Table 1). In certain years, some cases coincided in the same post code of residence. In the years 2000 and 2010, in which there were peaks of incidence, a third of the year's cases coincided in groups of 2, in 3 post codes of residence.
Number of cases and incidence of fascioliasis by province.
| Province | Cases | Incidence per 10,000,000 inhabitants |
|---|---|---|
| Albacete | 1 | 0.2 |
| Alicante | 1 | 0.0 |
| Almería | 0 | 0.0 |
| Álava | 7 | 1.4 |
| Asturias | 10 | 0.5 |
| Ávila | 3 | 1.0 |
| Badajoz | 1 | 0.1 |
| Balearic Islands | 1 | 0.1 |
| Barcelona | 6 | 0.1 |
| Biscay | 9 | 0.4 |
| Burgos | 6 | 0.9 |
| Cáceres | 2 | 0.3 |
| Cádiz | 0 | 0.0 |
| Cantabria | 7 | 0.7 |
| Castellón | 0 | 0.0 |
| Ciudad Real | 1 | 0.1 |
| Córdoba | 0 | 0.0 |
| Coruña, A | 6 | 0.3 |
| Cuenca | 1 | 0.3 |
| Gipuzkoa | 4 | 0.3 |
| Girona | 0 | 0.0 |
| Granada | 0 | 0.0 |
| Guadalajara | 1 | 0.3 |
| Huelva | 0 | 0.0 |
| Huesca | 1 | 0.3 |
| Jaén | 0 | 0.0 |
| León | 12 | 1.3 |
| Lleida | 0 | 0.0 |
| Lugo | 15 | 2.2 |
| Madrid | 24 | 0.3 |
| Málaga | 3 | 0.1 |
| Murcia | 0 | 0.0 |
| Navarre | 3 | 0.3 |
| Ourense | 3 | 0.5 |
| Palencia | 0 | 0.0 |
| Palmas, Las | 0 | 0.0 |
| Pontevedra | 5 | 0.3 |
| La Rioja | 5 | 1.0 |
| Salamanca | 3 | 0.5 |
| Santa Cruz de Tenerife | 1 | 0.1 |
| Segovia | 8 | 3.0 |
| Seville | 0 | 0.0 |
| Soria | 0 | 0.0 |
| Tarragona | 0 | 0.0 |
| Teruel | 0 | 0.0 |
| Toledo | 0 | 0.0 |
| Valencia | 3 | 0.1 |
| Valladolid | 3 | 0.3 |
| Zamora | 3 | 0.8 |
| Zaragoza | 1 | 0.1 |
| Ceuta | 2 | 1.6 |
| Melilla | 0 | 0.0 |
| Province of residence not known | 4 |
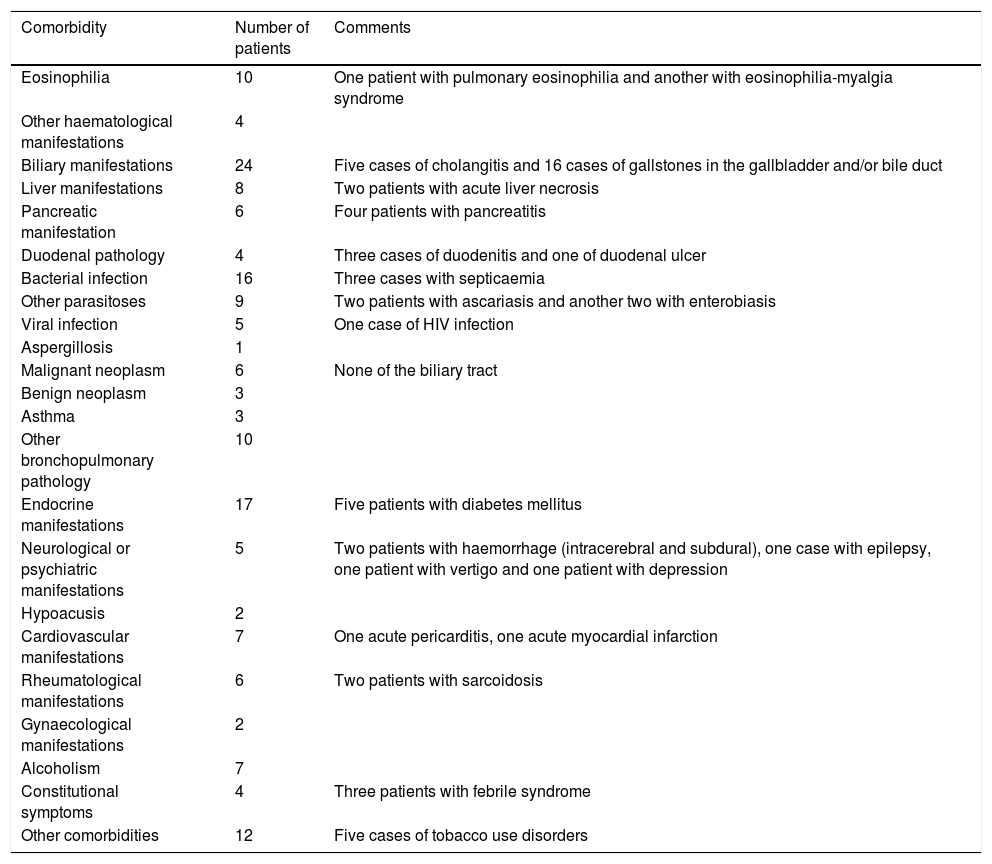
The summary of the clinical manifestations that appeared as specified diagnoses at hospital discharge, both in patients with primary or secondary diagnoses of fascioliasis, are shown in Table 2. Eosinophilia was specified in ten patients (including a case with pulmonary eosinophilia and another with eosinophilia-myalgia syndrome), biliary manifestations in 24 (including 5 cases of cholangitis), liver manifestations in eight (including 2 cases of acute liver necrosis) and pancreatic manifestations in six (including 4 cases of pancreatitis).
Clinical manifestations of patients with fascioliasis, collected in other diagnoses at discharge.
| Comorbidity | Number of patients | Comments |
|---|---|---|
| Eosinophilia | 10 | One patient with pulmonary eosinophilia and another with eosinophilia-myalgia syndrome |
| Other haematological manifestations | 4 | |
| Biliary manifestations | 24 | Five cases of cholangitis and 16 cases of gallstones in the gallbladder and/or bile duct |
| Liver manifestations | 8 | Two patients with acute liver necrosis |
| Pancreatic manifestation | 6 | Four patients with pancreatitis |
| Duodenal pathology | 4 | Three cases of duodenitis and one of duodenal ulcer |
| Bacterial infection | 16 | Three cases with septicaemia |
| Other parasitoses | 9 | Two patients with ascariasis and another two with enterobiasis |
| Viral infection | 5 | One case of HIV infection |
| Aspergillosis | 1 | |
| Malignant neoplasm | 6 | None of the biliary tract |
| Benign neoplasm | 3 | |
| Asthma | 3 | |
| Other bronchopulmonary pathology | 10 | |
| Endocrine manifestations | 17 | Five patients with diabetes mellitus |
| Neurological or psychiatric manifestations | 5 | Two patients with haemorrhage (intracerebral and subdural), one case with epilepsy, one patient with vertigo and one patient with depression |
| Hypoacusis | 2 | |
| Cardiovascular manifestations | 7 | One acute pericarditis, one acute myocardial infarction |
| Rheumatological manifestations | 6 | Two patients with sarcoidosis |
| Gynaecological manifestations | 2 | |
| Alcoholism | 7 | |
| Constitutional symptoms | 4 | Three patients with febrile syndrome |
| Other comorbidities | 12 | Five cases of tobacco use disorders |
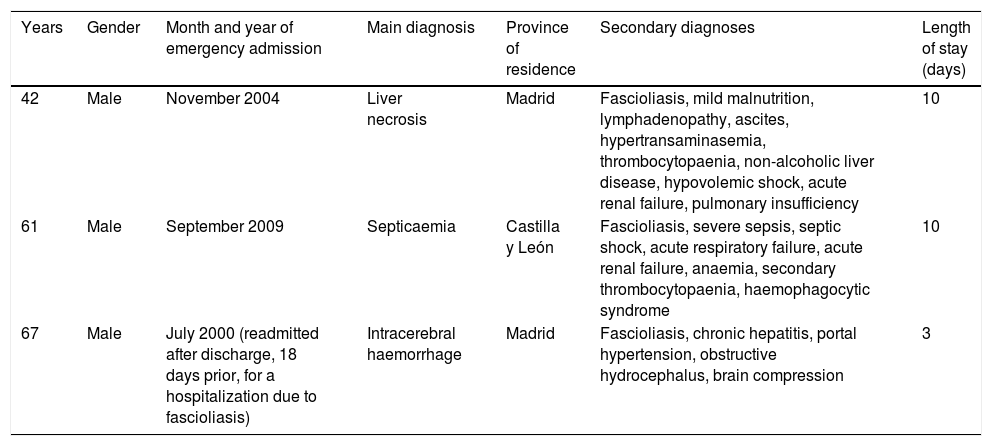
Most hospitalizations had a low or moderate severity level and mortality risk (index 1 or 2); only three admissions had an extreme level (index 4). Three patients died, with a main diagnoses of acute or subacute hepatic necrosis, septicaemia and intracerebral haemorrhage (Table 3). The three patients were men; in two it was the first admission with fascioliasis and in one of them it was a re-admission after a hospitalization for fascioliasis as the main diagnosis (Table 3). Mortality was associated with extreme levels of severity and mortality risk.
Deaths with fascioliasis during hospital admission.
| Years | Gender | Month and year of emergency admission | Main diagnosis | Province of residence | Secondary diagnoses | Length of stay (days) |
|---|---|---|---|---|---|---|
| 42 | Male | November 2004 | Liver necrosis | Madrid | Fascioliasis, mild malnutrition, lymphadenopathy, ascites, hypertransaminasemia, thrombocytopaenia, non-alcoholic liver disease, hypovolemic shock, acute renal failure, pulmonary insufficiency | 10 |
| 61 | Male | September 2009 | Septicaemia | Castilla y León | Fascioliasis, severe sepsis, septic shock, acute respiratory failure, acute renal failure, anaemia, secondary thrombocytopaenia, haemophagocytic syndrome | 10 |
| 67 | Male | July 2000 (readmitted after discharge, 18 days prior, for a hospitalization due to fascioliasis) | Intracerebral haemorrhage | Madrid | Fascioliasis, chronic hepatitis, portal hypertension, obstructive hydrocephalus, brain compression | 3 |
Some 70.2% of hospitalizations with the disease were emergency hospitalisations.
The five hospital departments with the highest number of admissions were, in order of frequency, Internal Medicine (19.2%), Digestive Medicine (8.6%), Paediatrics (3%), Infectious Diseases (2.5%) and General and Gastrointestinal Surgery (2%).
Some 62.6% of the hospitalizations were caused by fascioliasis (main diagnosis). In the 124 admissions due to fascioliasis, the mean length of stay was 13 days, the median length of stay was 10 days, (IQR 4–19) standard deviation 11.9 days, minimum 1 day and maximum 78 days. Eighteen of the 124 hospitalizations (14.5%) were readmitted to the same centre in the same year and within 30 days following a previous discharge.
The median cost of patients admitted for the disease was €1945.60, IQR €1185.07 (minimum €1227.71, maximum €8390.82). The sum of the costs of all admissions of patients with the disease (main or secondary diagnosis of fascioliasis) was €603,068.
DiscussionThis study offers estimates of the epidemiological characteristics of admissions with fascioliasis based on the population of Spain and shows that its distribution is heterogeneous, predominating in northern and central Spain, and that there is a downward trend in its incidence. Although studies of certain Spanish geographical areas have been carried out, to our knowledge, this is the first work that analyses the epidemiology of fascioliasis at the national level.
However, the estimates in this study should be taken with caution. The incidence figure for hospitalizations with fascioliasis underestimates the overall frequency of the disease since it does not include cases that have not required hospital admission (as may occur in chronic manifestations without complications) or patients not studied due to mild clinical manifestations.13,14 Important limitations of the study are the retrospective nature of the MBDS and the variability that may exist in the diagnostic criteria and in the coding by hospitals, in addition to the fact that the MBDS may contain coding errors. Moreover, Europe is the continent where the most imported cases have been reported,15 but the MBDS does not report the origin of patients or previous trips; in areas where the immigration rate is high, such as in Ceuta, cases are likely to be imported. The MBDS data do not allow all patients’ clinical manifestations (e.g., the rate of eosinophilia), the time of evolution of the disease, the treatments applied or the effectiveness thereof to be detected in a sensitive way. Nor do they allow us to know the diagnostic criteria or diagnostic techniques used or whether the case is native or imported. Nevertheless, although the diagnosis of fascioliasis poses difficulties,16 the analysis of hospitalizations with fascioliasis does allow adjusted data on the distribution of the disease in the population admitted to Spanish hospitals to be obtained.
Fascioliasis, where it occurs sporadically and there is no specific risk group, affects people of all age groups.1 The epidemiological pattern of fascioliasis is varied,17 although the infection, in developed countries, generally has a hypoendmic pattern with low and stable prevalence levels in a defined population. The geographical distribution of outbreaks is related to the distribution of intermediate populations of freshwater snails, as well as physical geography and climatic conditions.1,17,18
In the past, it was considered that human fascioliasis in Spain was mainly distributed throughout the autonomous communities of the Basque Country, Castilla y León, Cantabria, Navarra and La Rioja.6,17 In our study of patients admitted with a diagnosis between 1997 and 2014 we have detected an extension of the geographical aggregation of cases, especially in certain areas of northern and central Spain. The greatest incidences were observed in the provinces of Segovia and Lugo; the city of Ceuta and the provinces of Alava and León followed in incidence. In certain years, some cases coincided in the same post code of residence, which suggests the presence of small epidemic outbreaks.
The annual incidence of fascioliasis in hospital admissions has decreased over the 18 years of the study. The incidence reduction was observed by Cilla et al. in Guipúzcoa and related to the change in eating habits,7 a fact that could have happened in the rest of Spain and, along with information campaigns, would explain the downward trend in diagnoses.
The incidence of the disease in the different Spanish regions is very low compared to other geographical areas with a very high prevalence of parasitization such as the Andean highlands of Bolivia, Peru or Chile.18–20
The risk of suffering fascioliasis in the Spanish male population slightly exceeded the female population, a predominance already documented in the Guipúzcoa series.21
Due to the special liver tropism of F. hepatica, abdominal pain and hepatomegaly, in addition to constitutional symptoms, are among the most common manifestations of fascioliasis in its acute stage.8,13 The adult worm is located in the bile ducts and usually causes abnormalities in liver function tests and eosinophilia.22,23 The eosinophilia detected by the MBDS as a diagnosis significantly underestimates the rate of this analytical manifestation. In the chronic stage, inflammation and intermittent obstruction of the bile ducts cause biliary colic and cholangitis as predominant manifestations and images in the bile duct corresponding to adult worms can be detected.8,13,22,24,25 The diagnosis of cholangitis was included in the MBDS in 6 patients, and in 16 patients lithiasis was detected in the gallbladder or bile ducts. Long-term complications are gallstones, sclerosing cholangitis and biliary cirrhosis.26 In a meta-analysis on liver parasites (opisthorchiasis, clonorchiasis and hepatic fascioliasis), the disease was significantly associated with cholangitis, cholecystitis, cholelithiasis, hepatocellular carcinoma and cholangiocarcinoma26; in this observational study, no cases of hepatocellular carcinoma or cholangiocarcinoma, tumours that are related to opisthorchiasis and clonorchiasis, have been detected.26 Four cases were associated with pancreatitis, although fascioliasis is considered an uncommon cause of pancreatitis in Spain.27 Eosinophilia is the most common laboratory abnormality8; one of the cases in the series was diagnosed with pulmonary eosinophilia, a rare and atypical manifestation of fascioliasis.28 The clinical spectrum of fascioliasis is variable and patients may present with extrahepatic abnormalities, such as pulmonary infiltrates, pleuropericarditis, meningitis, lymphadenopathy or retroperitoneal implantation.1,8,29 Recently it has been indicated that Spain has second highest number of cases in the world of neurological manifestations associated with fascioliasis, including possible indirect pathogenic mechanisms.30 In some cases, neurological manifestations coinciding with fascioliasis were detected, but in a retrospective study such as this, the cause-effect relationship cannot be established.
Mortality is very low and few patients reached extreme rates of severity and mortality risk during their hospital stay.12 Deaths cannot be attributed to fascioliasis, although in some cases they may be indirectly related.
This work, from a perspective of the population treated in Spanish hospitals, contributes to the characterization of hospitalizations with fascioliasis and shows a low incidence, a decrease in hospital diagnoses during the period studied and a higher prevalence in the provinces of Lugo and Segovia.
Conflicts of interestNone of the authors have any conflicts of interest.
To the Institute of Health Information of the Ministry of Health, Consumption and Social Welfare, the source of the study's primary data.
Please cite this article as: Guerrero-Espejo A, Bernad-Anso A. Incidencia y distribución geográfica de pacientes hospitalizados con fascioliasis en España. Enferm Infecc Microbiol Clin. 2020;38:257–262.