The duration adequacy of antibiotic regimens is one of the key points of Antimicrobial Stewardship Programs (ASP) given the relationship between the risk of resistance and days of exposure to antimicrobials.
MethodsMonitoring activities of intravenous antibiotics longer than 7 days at Hospital Infantil Vall d’Hebron, Barcelona, by reviewing data over a 34-weeks period from weekly cross-sectional analysis, followed by recommendations to prescribers to adapt their use.
ResultsA total of 81 patients with 146 prolonged intravenous antibiotic treatments (78.8% of prescriptions were adequate) were reviewed. A total of 190 revisions were performed. 31 interventions on inappropriate prescriptions were carried out (61.3% of adherence to recommendation). Nineteen treatments were optimized (14 suspended, 5 de-escalated) reducing their duration by 8.75%.
ConclusionsActive intervention of ASP group is an effective tool to improve antibiotic optimization, reducing unnecessarily prolonged treatments, mainly on these areas with a greater range of improvement.
Adecuar la duración del tratamiento antibiótico es uno de los puntos claves de los programas de optimización del uso de antimicrobianos (PROA) dada la relación entre el riesgo de aparición de resistencias con los días de exposición a antimicrobianos.
MétodosMonitorización de tratamientos antibióticos intravenosos de >7 días de duración en el Hospital Infantil Vall d’Hebron, Barcelona, mediante cortes transversales semanales durante 24 semanas, con recomendaciones posteriores a los prescriptores para adecuar su uso.
ResultadosSe realizaron 190 revisiones de 146 tratamientos antibióticos prolongados, prescritos en 81 pacientes. El 78.7% de prescripciones fueron adecuadas. Se realizaron 31 intervenciones sobre las prescripciones inadecuadas, con 61,3% de adaptación a la recomendación. Se optimizaron 19 tratamientos (14 suspendidos, 5 desescalados) reduciendo su duración un 8,75%.
ConclusionesLa intervención activa del grupo PROA-NEN permite mejorar la adecuación antibiótica, reduciendo los tratamientos innecesariamente prolongados, especialmente en ámbitos con mayor margen de mejora.
Antimicrobial agents are well known to play an important role in morbidity and mortality rates in patients with infections. However, it has also been found that the development of resistance mechanisms has a detrimental impact on clinical outcomes, and exposure to antimicrobial agents is one of the main factors linked to this phenomenon.1
Evidence that the use of antimicrobial agents in a hospital setting could be improved has led to the development and implementation of programmes for optimising the use of antimicrobial agents (PROA) with the objective of improving clinical outcomes in infectious diseases, minimising the adverse effects associated with the use of antimicrobial agents, including resistance, and optimising the cost-effectiveness of treatments.2 The implementation of these programmes is particularly important in special populations such as the paediatric population. Thus, in 2015, the PROA-NEN paediatric team was formally established at Hospital Infantil Vall d'Hebron [Vall d'Hebron Children's Hospital] in Barcelona, Spain.
PROA programmes, comprised of a multidisciplinary team, are based on a strategy of continuous improvement, wherein the team verifies its interventions using process and outcome indicators, taking into account the different aspects of the appropriate use of antimicrobials in a global way.
At present, there is scientific evidence showing that treatment periods shorter than those traditionally established demonstrate equal effectiveness while reducing adverse effects. Although most paediatric studies focus on community-acquired infections, some studies have supported the use of briefer intravenous antibiotic treatments in seriously ill paediatric inpatients.3,4
For this reason, one PROA strategy consists of acting on treatment duration, based on evidence that the efficacy of antibiotic treatment builds up in the first few days of treatment and does not improve with a longer treatment period, while development of resistance, toxicity and expenditure increase after those first few days.5,6
The objective of this study is to identify all prolonged-duration (>7 days) intravenous antibacterial treatments, so as to avoid overly prolonged antibiotic treatments and minimise the associated risks, as a supplement to the regular active consulting done by the PROA-NEN team.
Patients and methodsThe PROA-NEN team was formed in 2015 to implement a PROA programme specific to paediatrics with institutional support. The team, whose members are listed below, consists of specialists in paediatric infectious diseases, microbiology, pharmacy, preventive medicine and all other paediatric specialisations.
A prospective, observational, single-centre, weekly cross-sectional study was conducted. For 24 weeks (between June 2018 and January 2019), prolonged-duration (>7 days) intravenous antibiotic treatments were identified by the Hospital Pharmacy Department in the software programs used electronic prescription (BO-Silicon®, Grifols, S.A., Barcelona, Spain; and Centricity®, General Electric Company, Boston, Massachusetts, United States). Multiple reviews of a single treatment were included if the treatment lasted for two or more cross-sections. Any patient admitted to the Neonatology Department was excluded from the analysis, regardless of age, due to these patients' special characteristics and the technical difficulties involved in data acquisition.
All prolonged treatments were evaluated by the PROA-NEN team, who analysed the treatment indication (type of infection, patient characteristics), suitability of the regimen, microbiological data (micro-organism, sensitivity) and conformity with current protocols at the centre, or national and international clinical practice guidelines when no recommended treatment duration was specified in internal protocols, in order to classify each treatment as suitable or unsuitable. Suitable prolonged treatment was considered to be that which was adapted to the treatment indication (empirical, targeted), the characteristics of the patient and the infection (location, severity, comorbidities, concomitant infections, etc.), the dose and route of administration, the microbiology results (in targeted treatments) and the internal protocols of the centre.
Treatments considered suitable were recorded without carrying out any sort of active intervention. In treatments deemed unsuitable, an intervention was made consisting of a non-enforceable recommendation (switching to a narrower spectrum antibiotic, changing the route of administration or suspending the treatment), either in writing or through direct contact, for the prescribing team to optimise treatment.
To assess the quality of the prescription, the proportion of adequate treatments among the long-term prescribed treatments was determined (considering a treatment as a single antibiotic during the entire prescription), globally and according to the different hospitalisation areas. To assess the impact of the audit team's actions, the weekly reviews, audit team's recommendations and acceptance rate among prescribing teams were measured. Acceptance of recommendations was calculated for both unsuitable treatments and reviews performed, since the same treatment could have been evaluated more than once, with the resulting recommendations.
Annual antibiotic use data were collected from the hospital's information systems. Clinical and microbiological data were gathered from the patients' electronic medical records. Data analysis was performed with the R statistical software environment (R version 3.5.2 [20/12/2018]©, 2015, The R Foundation for Statistical Computing, Vienna, Austria). The local Independent Ethics Committee authorised a waiver of the informed consent request, since no patient-identifying data were collected and the PROA team interventions were made as part of the team's regular clinical activity.
ResultsDuring the study period, 146 prolonged intravenous antibiotic treatments prescribed in 81 patients were reviewed. Most (115, 78.8%) prolonged treatments lasted less than 14 days (73% suitable), followed by 18 lasting 14–20 days (100% suitable), nine lasting 21–28 days (55.5% suitable) and four lasting more than 28 days (100% suitable). To put the study data in context, the percentage of prolonged (>7 days) intravenous antibiotic treatments for all of 2018 was determined. This figure was 6.8% (417/6,083).
The most commonly prescribed prolonged-duration antibiotics were piperacillin/tazobactam (38 prescriptions, 94.7% suitable), meropenem (26 prescriptions, 50% suitable), levofloxacin (9 prescriptions, 89% suitable) and ciprofloxacin (7 prescriptions, 71.4% suitable). Regarding prolonged-use antibiotics, 31 treatments (21%) were considered unsuitable. Among them, 45.1% (14/31) were a carbapenem.
A total of 190 reviews were conducted. Suitable prescription was found in 154 cases (81%), and unsuitable prescription was found in 36 cases (19%). Among the latter, interventions were made by directly contacting the prescribing team (97%) or by writing a note in the relevant medical record (3%). In 19/36 (52.7%) of these interventions, the prescriber adjusted the treatment to the recommendation: 14 unsuitable treatments were suspended and 5 were switched to narrower spectrum antibiotics.
Analysis of the response to the recommendations of the PROA-NEN team revealed that the response was positive in 61.3% of the total number of unsuitable treatments (31), while in 38.7% of cases the unsuitable treatment was not modified.
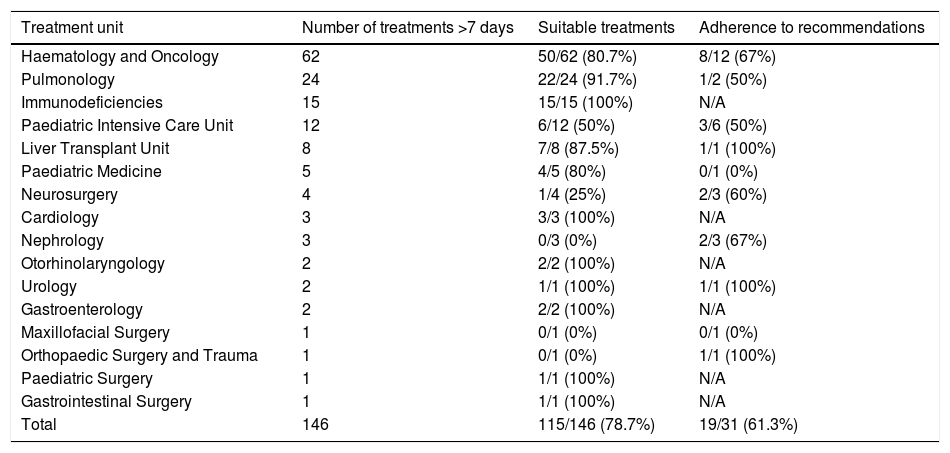
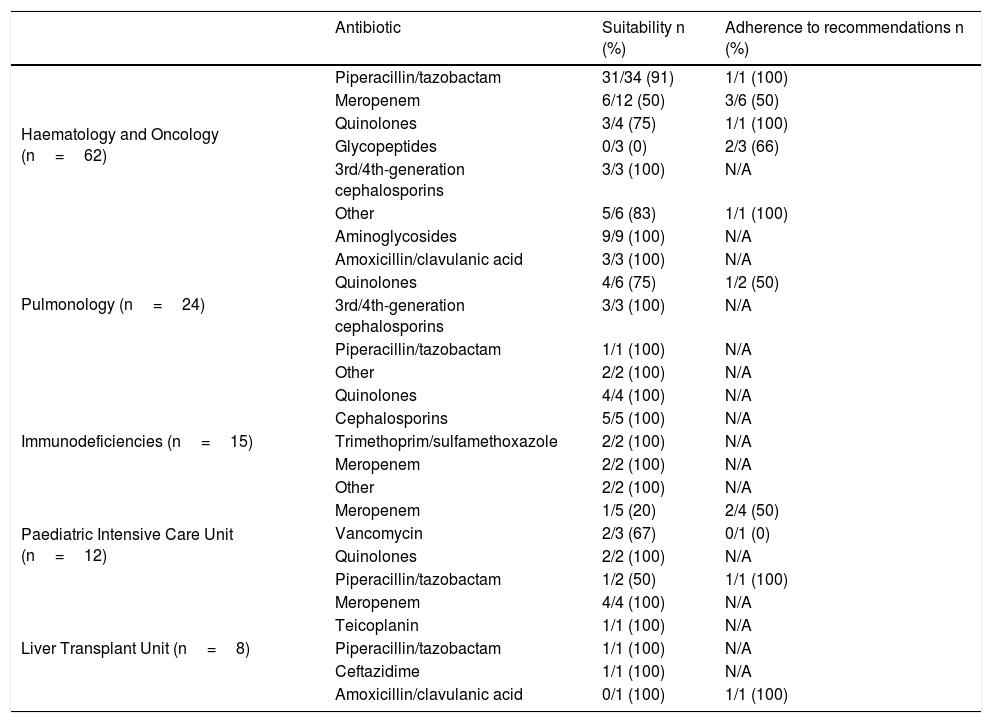
Table 1 shows the prolonged prescriptions in the different departments and their percentage of suitability. Table 2 gives a breakdown, by antibiotic, of suitability and adherence to the PROA-NEN team's recommendations, in departments with >5 prolonged treatments. The Paediatric Haematology and Oncology Department had the highest absolute number of prolonged and unsuitable treatments overall (12 treatments), though it did have a high percentage of suitability among all prolonged prescriptions (80.7%). In addition, as many as eight of the 12 (67%) prescriptions deemed unsuitable were adjusted to bring them into conformity with the recommendations of the PROA-NEN team. The units with the next-highest absolute numbers of prolonged treatments were Paediatric Pulmonology and the Primary Immunodeficiencies Unit, again with a very high percentage of suitability among all prolonged treatments (91.7% and 100%, respectively). However, the Paediatric Intensive Care Unit (PICU), another unit with a significant number of prolonged treatments, had a total of 6/12 unsuitable prolonged treatments, and just three of the six treatments considered unsuitable were subsequently adjusted according to the PROA team's recommendations.
Absolute number of prolonged treatments prescribed and percentage of treatments considered suitable and in adherence to recommendations, according to the prescribing treatment unit.
| Treatment unit | Number of treatments >7 days | Suitable treatments | Adherence to recommendations |
|---|---|---|---|
| Haematology and Oncology | 62 | 50/62 (80.7%) | 8/12 (67%) |
| Pulmonology | 24 | 22/24 (91.7%) | 1/2 (50%) |
| Immunodeficiencies | 15 | 15/15 (100%) | N/A |
| Paediatric Intensive Care Unit | 12 | 6/12 (50%) | 3/6 (50%) |
| Liver Transplant Unit | 8 | 7/8 (87.5%) | 1/1 (100%) |
| Paediatric Medicine | 5 | 4/5 (80%) | 0/1 (0%) |
| Neurosurgery | 4 | 1/4 (25%) | 2/3 (60%) |
| Cardiology | 3 | 3/3 (100%) | N/A |
| Nephrology | 3 | 0/3 (0%) | 2/3 (67%) |
| Otorhinolaryngology | 2 | 2/2 (100%) | N/A |
| Urology | 2 | 1/1 (100%) | 1/1 (100%) |
| Gastroenterology | 2 | 2/2 (100%) | N/A |
| Maxillofacial Surgery | 1 | 0/1 (0%) | 0/1 (0%) |
| Orthopaedic Surgery and Trauma | 1 | 0/1 (0%) | 1/1 (100%) |
| Paediatric Surgery | 1 | 1/1 (100%) | N/A |
| Gastrointestinal Surgery | 1 | 1/1 (100%) | N/A |
| Total | 146 | 115/146 (78.7%) | 19/31 (61.3%) |
N/A: not applicable.
Suitability and response to recommendation by type of antibiotic in units with more than 5 prolonged treatments.
| Antibiotic | Suitability n (%) | Adherence to recommendations n (%) | |
|---|---|---|---|
| Haematology and Oncology (n=62) | Piperacillin/tazobactam | 31/34 (91) | 1/1 (100) |
| Meropenem | 6/12 (50) | 3/6 (50) | |
| Quinolones | 3/4 (75) | 1/1 (100) | |
| Glycopeptides | 0/3 (0) | 2/3 (66) | |
| 3rd/4th-generation cephalosporins | 3/3 (100) | N/A | |
| Other | 5/6 (83) | 1/1 (100) | |
| Pulmonology (n=24) | Aminoglycosides | 9/9 (100) | N/A |
| Amoxicillin/clavulanic acid | 3/3 (100) | N/A | |
| Quinolones | 4/6 (75) | 1/2 (50) | |
| 3rd/4th-generation cephalosporins | 3/3 (100) | N/A | |
| Piperacillin/tazobactam | 1/1 (100) | N/A | |
| Other | 2/2 (100) | N/A | |
| Immunodeficiencies (n=15) | Quinolones | 4/4 (100) | N/A |
| Cephalosporins | 5/5 (100) | N/A | |
| Trimethoprim/sulfamethoxazole | 2/2 (100) | N/A | |
| Meropenem | 2/2 (100) | N/A | |
| Other | 2/2 (100) | N/A | |
| Paediatric Intensive Care Unit (n=12) | Meropenem | 1/5 (20) | 2/4 (50) |
| Vancomycin | 2/3 (67) | 0/1 (0) | |
| Quinolones | 2/2 (100) | N/A | |
| Piperacillin/tazobactam | 1/2 (50) | 1/1 (100) | |
| Liver Transplant Unit (n=8) | Meropenem | 4/4 (100) | N/A |
| Teicoplanin | 1/1 (100) | N/A | |
| Piperacillin/tazobactam | 1/1 (100) | N/A | |
| Ceftazidime | 1/1 (100) | N/A | |
| Amoxicillin/clavulanic acid | 0/1 (100) | 1/1 (100) |
N/A: not applicable.
At our centre, prescription of prolonged-duration (>7 days) intravenous antibiotic treatments is clinically justified and conforms to local protocols in the vast majority of cases. However, in cases of unsuitable prescription, the response on the part of prescribers to the PROA-NEN team's recommendations could be improved.
We found that, although more and more treatment recommendations with shorter antibiotic regimens are available, at a highly complex hospital such as ours, indications for prolonged treatments are relatively common. In 2018, 6.8% (417/6083) of intravenous antibiotic treatments had a duration exceeding seven days, with frequent involvement of broad-spectrum antibiotics. It must be added that 21% of prolonged-duration antibiotics were deemed unsuitable and, among these, 45% were carbapenems. This highlights the need for monitoring of prolonged treatments by PROA teams.7
Concerning the evaluation of prescription quality by departments and units, special mention should be given to areas such as the Paediatric Haematology and Oncology Department, which had a high number of prolonged treatments, but with high suitability. This is explained by the internal protocol of maintaining broad-spectrum antibiotic treatment in case of febrile neutropenia up to haematological recovery, as recommended by most paediatric guidelines to date.8 Similarly, specialised areas such as Paediatric Pulmonology and the Primary Immunodeficiencies Unit also had high figures for treatments >7 days, most of which were considered suitable, due to the complexity of the disease in both cases with regard to duration and recommendations for prolonged antibiotic treatment in exacerbations in patients with cystic fibrosis9 in the first case, and due to the particular nature of our centre, where these diseases are treated directly by the paediatric infectious diseases team, regarding its suitability. However, in other units, such as the PICU, 50% of prolonged-duration prescriptions during the study period were inappropriate. This might be explained by the difficulty in distinguishing between infectious and non-infectious systemic inflammatory response syndrome, added to the relatively low yield of cultures in paediatric patients. In any case, it is important to bear in mind that inadequate treatment is associated with a longer stay in the PICU and in the hospital ward, with the consequent associated cost and greater appearance of adverse effects, as well as resistance.10 Hence, it is crucial to develop specific measures for improving antibiotic treatment in critically ill paediatric patients.
It is difficult to compare our data with the literature since there are few available studies that have evaluated data on the prescription and suitability of prolonged antibiotic treatments in paediatrics, either overall or by area. In addition, most existing studies have focused on the A&E setting, which was not included in our study, and excluded general and critical care wards.11,12
Regarding prescribers' response to the recommendations of the PROA-NEN team, our data showed a significant margin for improvement, especially in certain units. In this regard, despite obtaining similar results to some previous publications, other studies have shown greater acceptance of interventions. This demonstrates the need for new training and prescription support strategies that increase adherence of prescribers to PROA recommendations.13–15
The limitations of our study must be taken into consideration. The patient sample was relatively small, the study period was just 34 weeks and the study design was a weekly cross-sectional design that did not include data from Neonatology due to its peculiarities. In addition, the lack of recommendations in clinical practice guidelines on highly complex specific cases and of consensus definitions (e.g. sepsis) led in some cases to the PROA-NEN team's recommendation being based more on the experience of its members than on validated protocols. Nevertheless, this was the first Spanish national study on evaluation and optimisation of prolonged intravenous antibiotic treatment duration in paediatric inpatients. It points to the usefulness of these measures and opens the door to the conduct of multi-centre studies that enable evaluation of different strategies used to optimise treatment in paediatrics, especially in hospital settings.
In conclusion, active intervention by PROA teams makes it possible to improve antibiotic suitability, greatly reducing unnecessary prolonged treatments, especially in areas with greater room for improvement. It is recommended that such interventions represent a routine activity for these teams.
Conflicts of interestThe authors declare that they have no conflicts of interest.
Pere Soler Palacín. Specialist physician. Paediatric Infectious Diseases and Immunodeficiencies Unit. Paediatrics Department. Hospital Universitari Vall d'Hebron [Vall d'Hebron University Hospital], Barcelona.
Susana Melendo Pérez. Specialist physician. Paediatric Infectious Diseases and Immunodeficiencies Unit. Paediatrics Department. Hospital Universitari Vall d'Hebron, Barcelona.
Natalia Mendoza Palomar. Specialist physician. Paediatric Infectious Diseases and Immunodeficiencies Unit. Paediatrics Department. Hospital Universitari Vall d'Hebron, Barcelona.
Aurora Fernández Polo. Specialist pharmacist. Pharmacy Department. Hospital Universitari Vall d'Hebron, Barcelona.
M. Nieves Larrosa Escartín. Specialist physician. Microbiology Department. Hospital Universitari Vall d'Hebron, Barcelona.
Carlos Rodrigo Gonzalo de Liria. Paediatrics Department. Hospital Universitari Vall d'Hebron, Barcelona.
Montse Pujol Jover. Specialist physician. Paediatric Intensive Care Unit. Hospital Universitari Vall d'Hebron, Barcelona.
Yolanda Castilla Fernández. Specialist physician. Neonatology Department. Hospital Universitari Vall d'Hebron, Barcelona.
Laura Alonso García. Specialist physician. Paediatric Haematology and Oncology Department. Hospital Universitari Vall d'Hebron, Barcelona.
Sergio López Fernández. Specialist physician. Paediatric Surgery Department. Hospital Universitari Vall d'Hebron, Barcelona.
Jesús Quintero Bernabeu. Specialist physician. Hepatology and Liver Transplant Functional Unit. Paediatrics Department. Hospital Universitari Vall d'Hebron, Barcelona.
Núria Wörner Tomasa. Specialist physician. Paediatric Emergency Unit. Paediatrics Department. Hospital Universitari Vall d'Hebron, Barcelona.
Ignacio Iglesias Serrano. Specialist physician. Paediatric Allergy, Pulmonology and Cystic Fibrosis Unit. Paediatrics Department. Hospital Universitari Vall d'Hebron, Barcelona.
José Àngel Rodrigo Pendas. Specialist physician. Preventive Medicine — Infection Control Department. Hospital Universitari Vall d'Hebron, Barcelona.
Elisa Navarro Royo. Nurse. Preventive Medicine — Infection Control Department. Hospital Universitari Vall d'Hebron, Barcelona.
Maria Estrella Barceló. Specialist physician. Medicine Area. Servei d'Atenció Primària [Primary Care Department] (SAP) Muntanya.
Please cite this article as: Melendo S, Fernández-Polo A, Castellnou Asens I, Mendoza-Palomar N, Barnés-Mayolas M, Soler-Palacín P, et al. Calidad de prescripción de la antibioterapia prolongada en pediatría. Impacto de las intervenciones programas PROA. Enferm Infecc Microbiol Clin. 2021;39:134–138.