Enterovirus (EV) infections are the most frequent infections in the neonatal period and in many cases lead to hospital admission of the newborn (NB).
The aim of this study was to determine the incidence of EV in the etiology of neonatal meningitis and to define the clinical characteristics of newborns with EV meningitis.
Material and methodRetrospective observational cohort study. Including 91 NBs with meningitis and gestational age greater than 34 weeks gestational age (GA) attended in our center over a period of 16 years.
ResultsThe percentage of NBs with EV meningitis was higher than that of NBs with bacterial meningitis (BM) and accounted for 78% (n=71). Half of the NBs with EV infection had a history of epidemic environment among their caregivers. Fever was present in 96% of cases as a clinical sign and, in general, sensory disturbances represented the main neurological alterations. Antibiotics (ATB) were given to 71.4% of patients with EV infection.
Detection of EV in CSF samples showed a high sensitivity for the diagnosis of EV meningitis. The most frequently implicated EV types were echovirus 11, coxsackievirus B5, echovirus 18, 25 and 7.
ConclusionsThe results of this series show that enterovirus infection is a common cause of neonatal meningitis. These data underline the importance of rapid EV testing of infants with suspected meningitis. This allows early diagnosis and reduces antibiotic treatment, hospitalization time and related costs.
Las infecciones por enterovirus (EV) constituyen las infecciones más frecuentes en el periodo neonatal y provocan en muchos casos el ingreso hospitalario del recién nacido (RN).
El objetivo del estudio es conocer la incidencia de los EV en la etiología de las meningitis neonatales y definir qué características clínicas presentan los RN con meningitis por EV.
Material y métodoEstudio observacional retrospectivo de cohortes. Incluye 91 RN con meningitis y edad gestacional mayor de 34 semanas (SG) atendidos en nuestro centro durante un periodo de 16 años.
ResultadosEl porcentaje de RN con meningitis por EV fue superior al de RN con meningitis bacteriana y representó el 78% (n=71). La mitad de los RN con infección por EV presentó antecedentes de ambiente epidémico entre sus cuidadores. La fiebre apareció en el 96% de los casos como signo clínico y, en general, las alteraciones del sensorio representaron las principales alteraciones neurológicas. Un 71,4% de los pacientes con infección por EV recibió antibióticos.
La detección de EV en muestras de LCR mostró una elevada sensibilidad para el diagnóstico de meningitis por EV. Los tipos de EV más frecuentemente implicados fueron echovirus 11, coxsackievirus B5, echovirus 18, 25 y 7.
ConclusionesLos resultados de esta serie muestran que la infección por enterovirus es una causa común de meningitis neonatal. Estos datos subrayan la importancia de realizar pruebas de detección rápida de EV en lactantes con sospecha de meningitis. Ello permite obtener un diagnóstico precoz y reducir el tratamiento antibiótico, el tiempo de hospitalización y los costes relacionados.
Neonatal infections are one of the most prevalent diseases and, alongside prematurity and neonatal asphyxia, represent the main causes of death in newborn babies1. The incidence of bacterial meningitis (BM) has not changed in recent years and is estimated at between 0.16 and 0.45 cases/1000 newborns2. In addition to bacteria as the responsible pathogens, various studies have shown the importance of viruses as causal agents in neonatal meningitis, although the actual incidence is unknown3,4.
Enterovirus (EV) infections are common in the general population and are estimated to be responsible for 1000 million human infections per year3–6. Newborn babies are particularly vulnerable to infection by EV, with an estimated incidence of seven cases per 1000 newborns5,6. Although EV can cause a wide range of clinical manifestations, only 21% of infected neonates have clinical symptoms5. Of the newborns who do develop symptoms, approximately 26% have non-specific febrile symptoms, 47% aseptic meningitis, and the remaining 30% severe forms with liver, myocardial and brain involvement6,7.
Meningitis is the most common clinical manifestation of EV infection during the neonatal period. EV meningitis cannot be differentiated from BM by clinical symptoms alone. For this reason, most newborns with EV meningitis are admitted to hospital and treated with broad-spectrum antibiotic therapy until more data are available to exclude a bacterial origin. The course of meningeal disease is usually mild, with a low mortality rate and few long-term sequelae. The diagnosis is established after ruling out a bacterial origin and confirming the presence of EV in the cerebrospinal fluid (CSF). If EV is not detected in the CSF, diagnosis can be made based on the presence of pleocytosis and the detection of the virus in another biological sample6,8.
The aim of this study was to determine the incidence of enteroviruses as pathogens in neonatal meningitis and identify the epidemiological, clinical and developmental characteristics of newborns with this disease.
Material and methodsThis was a retrospective observational study carried out on newborns admitted to our level 3B centre from January 2002 to December 2017.
PatientsAs inclusion criteria, newborns with a gestational age greater than 34 weeks and a postnatal age less than 30 days of life were admitted to the study.
The definition of meningitis included newborns who had neurological symptoms and CSF abnormalities, indications of meningeal inflammation such as pleocytosis, increased protein and/or low glucose levels, combined with the isolation or detection of EV by virus culture or real-time polymerase chain reaction (RT-PCR) in the CSF or in another biological sample when there were coexisting CSF abnormalities.
Symptoms of neurological involvement included irritability, difficulty waking up or staying awake, refusing food, increased or decreased reflexes typical of newborns, changes in muscle tone in the form of hypotonia or hypertonia, focal neurological signs and seizures.
We examined maternal disease during pregnancy in the newborns analysed, such as diabetes, hypertensive disease of pregnancy, loss of foetal well-being or perinatal asphyxia, and maternal chorioamnionitis. The need for maternal antibiotic therapy at the time of delivery was also considered.
We also looked for any clinical symptoms consistent with an infectious process, or in other words, an epidemic environment in the mothers and other family members at the time of admission of the newborn. These data were obtained from the parents' history in their medical records.
DiagnosisIn order to isolate EV in nasopharyngeal aspirate and stool samples, cell culture was used in three cell lines (MRC-5, A549, HEp2 and RD) according to the WHO polio laboratory manual9. For the detection of EV in the CSF, RT-PCR assay (Cepheid®Xpert EV) was performed. All the EV isolated in the culture and all those detected in the CSF for which we had an original sample were characterised.
Genotyping of the EV was carried out using serum neutralisation and sequencing techniques. The former were applied to patients who had the infection before 2009, while the latter were performed in cases after that date. The serum neutralisation technique uses mixtures of specific monoclonal antibodies for different types of known EV, in combination with a conjugate that detects the binding of these antibodies with the viral antigen. The sequencing technique is performed from the amplification of the 3′ region of the VP1 gene of the EV capsid, using conventional nested RT-PCR techniques specific for the different EV species, with subsequent sequencing and BLAST analysis of the sequences obtained.
Statistical processingThe normality of the variables was analysed using the Kolmogorov–Smirnov test. In the descriptive analysis, tables were obtained from the results, showing as indicators of central tendency and dispersion the mean and the 95% confidence interval in the quantitative variables and the number and percentages for the qualitative variables. In the bivariate analysis, statistical comparisons were made using Student’s t test of independent data for quantitative variables, and the Chi-square test or Fisher’s exact test for categorical or qualitative variables. We set a type I error to 5%, therefore, alpha=0.05, two-tailed test.
Clinical Research and Ethics CommitteeThe study was approved by the Research Ethics Committee of the Instituto de Investigación de Sant Pau [Sant Pau Research Institute], with code IIBSP-ENT-2020-152-21/084. Throughout the research process, the principles enshrined in the Declaration of Helsinki were observed. This study received no specific funding from public, private or non-profit organisations.
ResultsWe analysed 332 newborns admitted to our centre with suspected diagnosis of neonatal sepsis. All the newborns showed clinical abnormalities and laboratory parameters suggestive of systemic inflammatory response syndrome. The aetiology was not identified in 32.8% of all the newborns assessed (n=109). In 108 newborns, a bacterium was found to be responsible for the infection and in 115 (34.6%), the origin of the infection was EV.
Of the cases studied, 91 newborns had meningitis at the time of admission and in 78% of the cases (n=71), the aetiology of the meningitis was EV. The incidence of meningitis in the newborns with proven infection was 40%. In our series, the most common pathogens in the aetiology of neonatal meningitis were EV, which represented 78% of the total number of microorganisms detected (71 newborns). EV were followed by S. agalactiae with 11 cases (12%) and Listeria monocytogenes with four cases (4.4%). It should be mentioned that no bacterial growth was isolated in the CSF of any of the newborns with EV meningitis and EV was not detected in the newborns with BM.
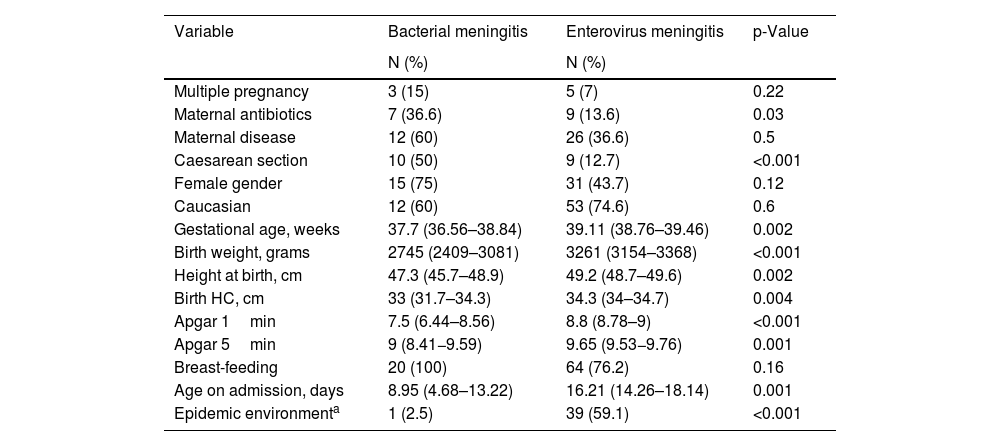
The perinatal characteristics of the newborns with EV infection are summarised in Table 1. The mothers of newborns with EV meningitis had little illness during pregnancy, and most of these infants were healthy term infants born by vaginal delivery.
Perinatal characteristics of newborns affected by bacterial meningitis and by EV: number of newborns and percentage for qualitative variables, and mean and confidence interval for quantitative variables.
| Variable | Bacterial meningitis | Enterovirus meningitis | p-Value |
|---|---|---|---|
| N (%) | N (%) | ||
| Multiple pregnancy | 3 (15) | 5 (7) | 0.22 |
| Maternal antibiotics | 7 (36.6) | 9 (13.6) | 0.03 |
| Maternal disease | 12 (60) | 26 (36.6) | 0.5 |
| Caesarean section | 10 (50) | 9 (12.7) | <0.001 |
| Female gender | 15 (75) | 31 (43.7) | 0.12 |
| Caucasian | 12 (60) | 53 (74.6) | 0.6 |
| Gestational age, weeks | 37.7 (36.56–38.84) | 39.11 (38.76–39.46) | 0.002 |
| Birth weight, grams | 2745 (2409–3081) | 3261 (3154–3368) | <0.001 |
| Height at birth, cm | 47.3 (45.7–48.9) | 49.2 (48.7–49.6) | 0.002 |
| Birth HC, cm | 33 (31.7–34.3) | 34.3 (34–34.7) | 0.004 |
| Apgar 1min | 7.5 (6.44–8.56) | 8.8 (8.78–9) | <0.001 |
| Apgar 5min | 9 (8.41−9.59) | 9.65 (9.53−9.76) | 0.001 |
| Breast-feeding | 20 (100) | 64 (76.2) | 0.16 |
| Age on admission, days | 8.95 (4.68–13.22) | 16.21 (14.26–18.14) | 0.001 |
| Epidemic environmenta | 1 (2.5) | 39 (59.1) | <0.001 |
Apgar 1min: vitality scale of the newborn after one minute of life; Apgar 5min: vitality scale of the newborn after 5min of life; HC; head circumference.
With regard to the characteristics of newborns with EV meningitis, we found that these newborns became infected from the second week of life, from eight to 24 days, and that 76% of them were being breastfed. In 59.1% of the cases, we found a history of mild infectious process among the members of the newborn’s family, mainly in the mother and siblings.
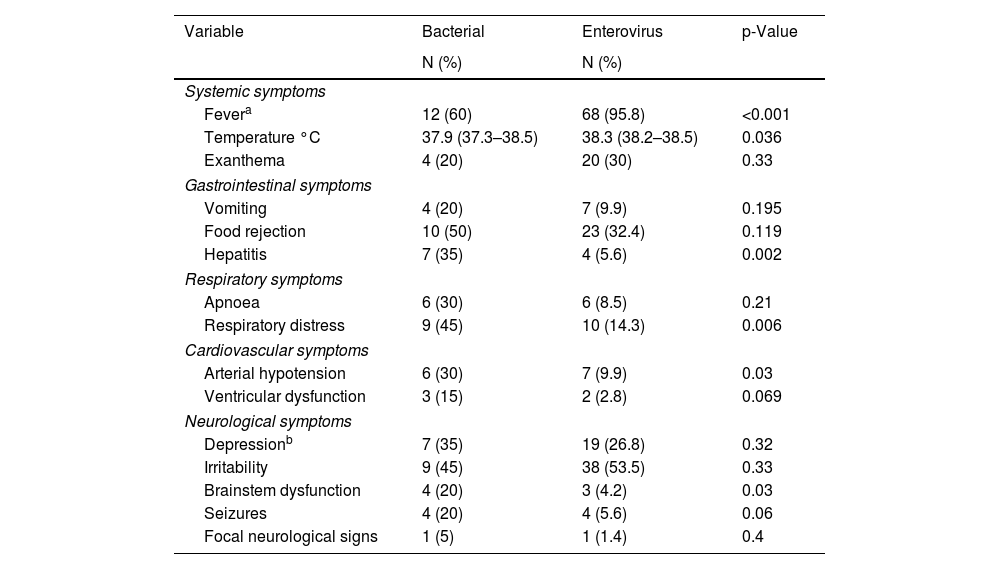
Analysing the clinical symptoms and signs of the EV infection, we found that fever occurred in 95.8% of the newborns and skin rash in 20% (Table 2), both of which were significantly higher than in newborns with BM. We looked for any association during the infection with cardiovascular, respiratory, gastrointestinal or neurological organ dysfunction. Clinical signs of organ dysfunction occurred with a significantly higher incidence in newborns with BM. Respiratory distress associated with the neurological symptoms occurred in 10 newborns with EV meningitis (14.3%) and in 45% of those with BM (p=0.006). Arterial hypotension was also more common in newborns with bacterial infection (30%), occurring in only 9.9% of newborns with EV (p=0.03). Of the newborns with EV infection, 32% had gastrointestinal problems in the form of vomiting or refusing food. Only four newborns with EV developed hepatitis (5.6%), significantly less than the 30% of patients with BM who developed hepatitis (p=0.002). Sensory abnormalities were the most common neurological symptoms. However, seizures and brain stem dysfunction were uncommon in newborns with EV meningitis (5.6% and 1.4%, respectively).
Organic dysfunction in newborns with bacterial and EV meningitis: number of newborns and percentage.
| Variable | Bacterial | Enterovirus | p-Value |
|---|---|---|---|
| N (%) | N (%) | ||
| Systemic symptoms | |||
| Fevera | 12 (60) | 68 (95.8) | <0.001 |
| Temperature °C | 37.9 (37.3–38.5) | 38.3 (38.2–38.5) | 0.036 |
| Exanthema | 4 (20) | 20 (30) | 0.33 |
| Gastrointestinal symptoms | |||
| Vomiting | 4 (20) | 7 (9.9) | 0.195 |
| Food rejection | 10 (50) | 23 (32.4) | 0.119 |
| Hepatitis | 7 (35) | 4 (5.6) | 0.002 |
| Respiratory symptoms | |||
| Apnoea | 6 (30) | 6 (8.5) | 0.21 |
| Respiratory distress | 9 (45) | 10 (14.3) | 0.006 |
| Cardiovascular symptoms | |||
| Arterial hypotension | 6 (30) | 7 (9.9) | 0.03 |
| Ventricular dysfunction | 3 (15) | 2 (2.8) | 0.069 |
| Neurological symptoms | |||
| Depressionb | 7 (35) | 19 (26.8) | 0.32 |
| Irritability | 9 (45) | 38 (53.5) | 0.33 |
| Brainstem dysfunction | 4 (20) | 3 (4.2) | 0.03 |
| Seizures | 4 (20) | 4 (5.6) | 0.06 |
| Focal neurological signs | 1 (5) | 1 (1.4) | 0.4 |
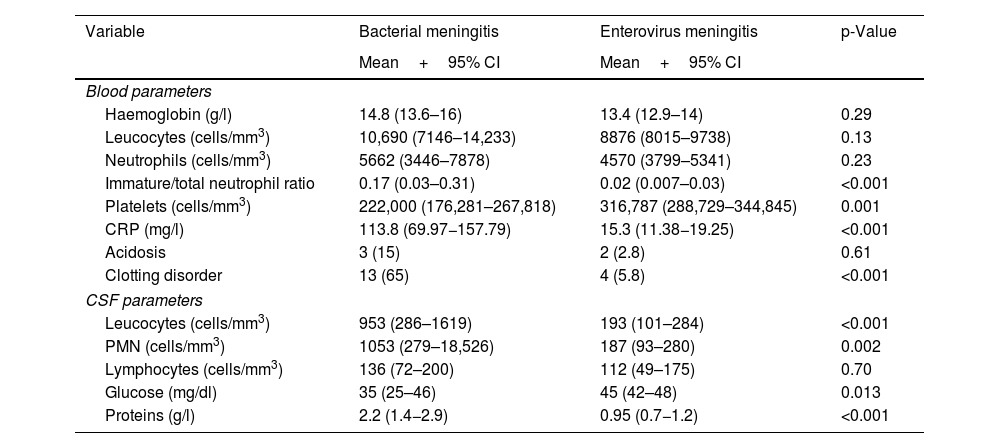
In general, the newborns with EV meningitis did not show abnormal values in haematological parameters, transaminases or C-reactive protein, unlike BM patients, in whom leucocytosis and increased acute-phase reactants were common (Table 3). In the analysis of the CSF samples, pleocytosis was seen in 50.8% of the cases (32 newborns) with a predominance of polymorphonuclear cells and normal protein and glucose concentrations (Table 3). There were marked differences in CSF characteristics between the two groups of newborns with meningitis, and differences were also found in cell count and protein concentration, which were significantly higher in newborns with BM (p<0.001).
Blood parameters and CSF characteristics in newborns with bacterial and EV meningitis: number of newborns and percentage for qualitative variables, and mean and confidence interval for quantitative variables.
| Variable | Bacterial meningitis | Enterovirus meningitis | p-Value |
|---|---|---|---|
| Mean+95% CI | Mean+95% CI | ||
| Blood parameters | |||
| Haemoglobin (g/l) | 14.8 (13.6–16) | 13.4 (12.9–14) | 0.29 |
| Leucocytes (cells/mm3) | 10,690 (7146–14,233) | 8876 (8015–9738) | 0.13 |
| Neutrophils (cells/mm3) | 5662 (3446–7878) | 4570 (3799–5341) | 0.23 |
| Immature/total neutrophil ratio | 0.17 (0.03–0.31) | 0.02 (0.007–0.03) | <0.001 |
| Platelets (cells/mm3) | 222,000 (176,281–267,818) | 316,787 (288,729–344,845) | 0.001 |
| CRP (mg/l) | 113.8 (69.97−157.79) | 15.3 (11.38−19.25) | <0.001 |
| Acidosis | 3 (15) | 2 (2.8) | 0.61 |
| Clotting disorder | 13 (65) | 4 (5.8) | <0.001 |
| CSF parameters | |||
| Leucocytes (cells/mm3) | 953 (286–1619) | 193 (101–284) | <0.001 |
| PMN (cells/mm3) | 1053 (279–18,526) | 187 (93–280) | 0.002 |
| Lymphocytes (cells/mm3) | 136 (72–200) | 112 (49–175) | 0.70 |
| Glucose (mg/dl) | 35 (25–46) | 45 (42–48) | 0.013 |
| Proteins (g/l) | 2.2 (1.4−2.9) | 0.95 (0.7−1.2) | <0.001 |
Haematological parameters and normal values in the newborn. Haemoglobin (Hb) 13–15g/l; leucocytes vary over the course of the first month from 5000 cells/mm3 as the lower limit of normal to 30,000 during the first 15 days of life to 15,000 cells/mm3 after the second week. Neutrophils, normal values between 2000 and 15,000 cells/mm3 depending on days of life. Platelets between 150,000 and 400,000 cells/mm3.
CSF parameters and normal values in the newborn. CSF leucocytes up to 20 cells/mm3 in the first two weeks of life and subsequently <10 cells/mm3. Glucose 50% of the value in blood. CSF proteins <1.5g/l in the first week of life and <1g/l thereafter.
PMN: polymorphonuclear cells.
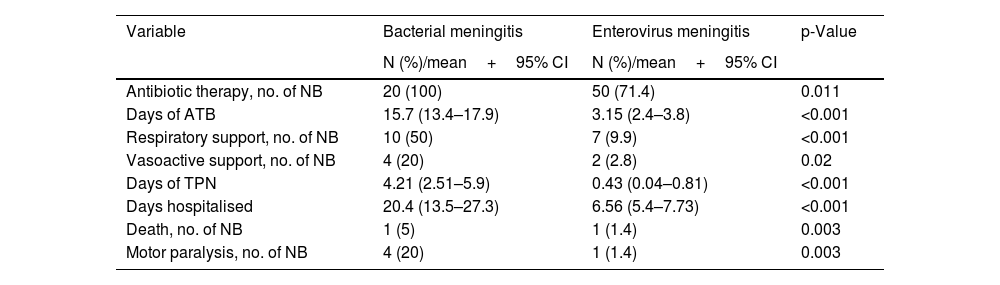
Of the newborns with EV meningitis, 51.4% received combination antibiotic therapy with cefotaxime and ampicillin. The mean length of antibiotic therapy was 3.1 days (95% CI: 2.4–3.8). Respiratory support was required by 9.9% of the newborns and 2.8% required inotropes due to haemodynamic instability (Table 4). The need for intensive care was significantly higher in newborns with BM (Table 4).
Treatment received by the newborns with bacterial and EV meningitis: number of newborns and percentage for qualitative variables, and mean and confidence interval for quantitative variables.
| Variable | Bacterial meningitis | Enterovirus meningitis | p-Value |
|---|---|---|---|
| N (%)/mean+95% CI | N (%)/mean+95% CI | ||
| Antibiotic therapy, no. of NB | 20 (100) | 50 (71.4) | 0.011 |
| Days of ATB | 15.7 (13.4–17.9) | 3.15 (2.4–3.8) | <0.001 |
| Respiratory support, no. of NB | 10 (50) | 7 (9.9) | <0.001 |
| Vasoactive support, no. of NB | 4 (20) | 2 (2.8) | 0.02 |
| Days of TPN | 4.21 (2.51–5.9) | 0.43 (0.04–0.81) | <0.001 |
| Days hospitalised | 20.4 (13.5–27.3) | 6.56 (5.4–7.73) | <0.001 |
| Death, no. of NB | 1 (5) | 1 (1.4) | 0.003 |
| Motor paralysis, no. of NB | 4 (20) | 1 (1.4) | 0.003 |
ATB: antibiotic therapy; IVIG: intravenous immunoglobulins; NB: newborns; TPN: total parenteral nutrition.
Newborns with EV meningitis were hospitalised for a mean of 6.56 days (95% CI: 5.4–7.73), while those with bacterial infection remained hospitalised three times as long (20.4 days, p<0.001). Five newborns with EV meningitis (7%) had severe forms, although in these patients meningitis was not their main condition. Of the newborns with severe disease, three cases developed hepatitis, one case myocarditis, and another patient had liver and myocardial involvement combined.
In our study, 95.8% of the newborns with EV meningitis recovered without sequelae. One newborn died and another developed a permanent motor deficit.
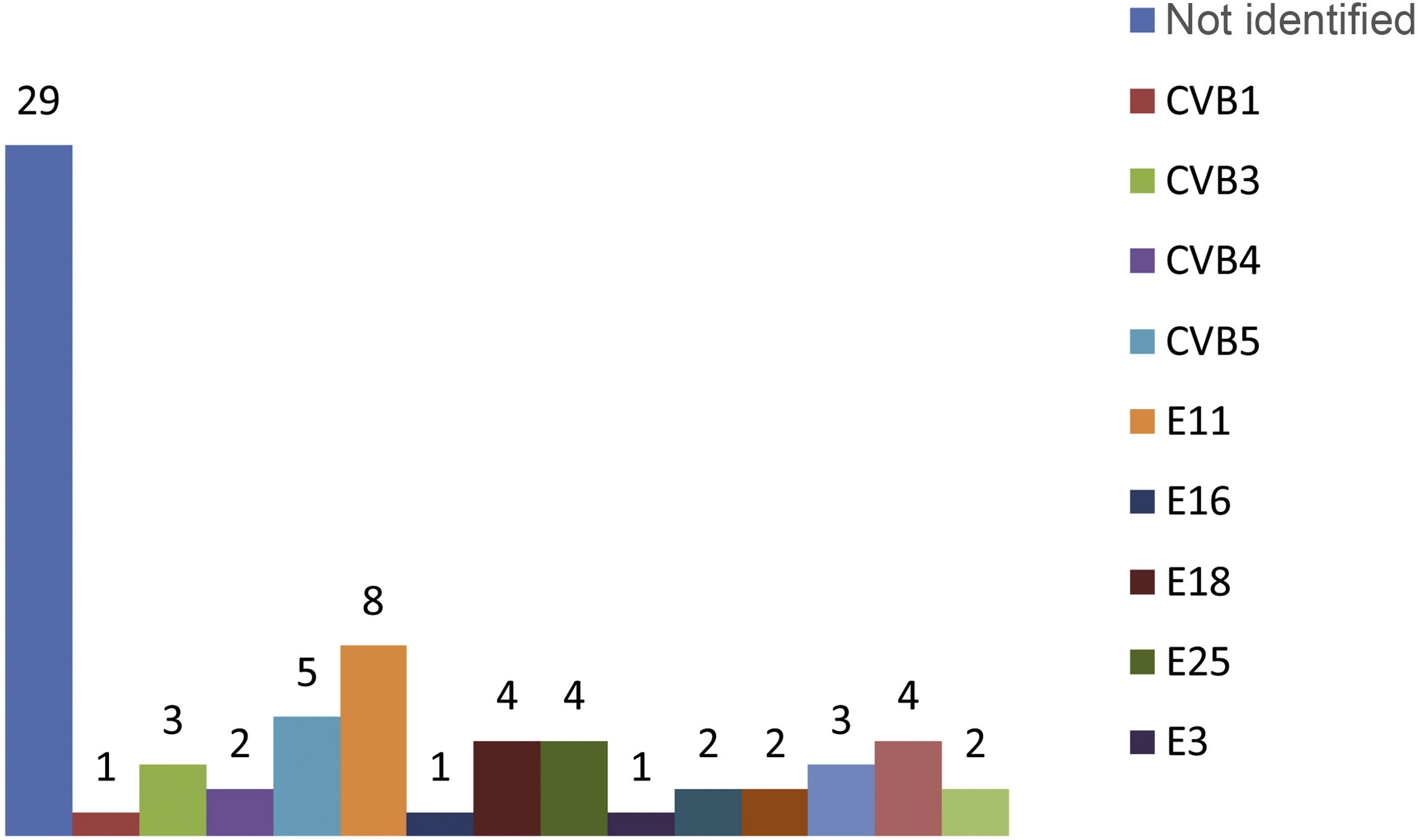
In the microbiological study, viral culture was used in the nasopharyngeal aspirate and stool samples to isolate the EV, and in the CSF, EV was detected by RT-PCR. The nasopharyngeal aspirate sample was positive in 52 patients (73%), while in 43 patients (61%) the virus grew in the stool culture. Detection of EV in CSF by RT-PCR was positive in 91% of cases (n=64). None of the patients with EV meningitis showed an associated bacterial infection in blood, CSF or urine. It was possible to identify the EV genotypes in 42 of the newborns with meningitis caused by this pathogen. The most common types of EV isolated were echovirus (E) 11 in eight newborns, coxsackievirus (CV) B5 in five and E 18, E 25 and E 7, each of them in four patients (Fig. 1).
The genotypes isolated in the newborns who developed more severe forms were echovirus 11 in the patient with severe necrotising hepatitis, coxsackievirus B4 in the patient who developed severe myocarditis, and echovirus 30 in a patient with hepatitis but no clotting disorder. In another two patients, the EV could not be characterised.
DiscussionNewborns are particularly vulnerable to EV infection. The clinical expression of neonatal disease is broad and ranges from asymptomatic cases to severe systemic disease with liver necrosis, myocarditis and meningoencephalitis5,6.
Infants with EV meningitis are healthy newborns without disease, and the history commonly reveals colds or gastroenteritis in other family members. The data from our study are consistent with the literature, where other authors assert that the source of the viral infection is in the newborn’s family environment6–8.
In general, EV meningitis is most common after the second week of life10,11. The initial clinical signs of neonatal meningitis are refusing food, vomiting and neurological manifestations such as sensory disturbances and seizures, and these clinical manifestations are common in bacterial and EV meningitis10,11.
The involvement of other organs during meningeal infection is more frequently associated with bacterial infection10. Organ dysfunction occurred in our study in BM newborns with a significantly higher incidence than in newborns with EV meningitis, and was only found in newborns with severe EV infection.
The respiratory problems described during EV infection in the neonatal period occur in 2% of cases and tend to be associated with severe conditions with liver and cardiac involvement11. In our study, respiratory symptoms occurred twice as often as those reported in the literature. However, respiratory problems are common during the course of meningeal infections of bacterial origin10.
Neurological involvement was mostly mild in our patients. In general, the neurological abnormalities in newborns during the course of the disease are mild and manifest clinically with fluctuations in the level of consciousness, alternating phases of drowsiness and irritability. The development of more severe neurological symptoms is not common, as these occur in the context of meningoencephalitis associated with liver and myocardial disease6,11,12.
The most useful biochemical parameter for distinguishing bacterial or viral aetiology in meningitis seems to be C-reactive protein (CRP), and published studies have shown its value in assessing children with fever and the risk of invasive bacterial infection13,14. In our study, CRP values in the newborns with EV meningitis were normal.
EV meningitis generally develops with a moderate increase in cells in the CSF, which does not exceed 1000 cells/mm33,8,15. However, in the neonatal period, leucocytosis is only identified in half of cases15–17. If they have pleocytosis, up to 30% of newborns show a predominance of polymorphonuclear cells at the onset of the disease11,16,17. The data published in the literature, and those from our study, show that a lack of CSF abnormalities is common in EV meningitis; this could be explained by CSF analysis being performed at a very early stage, in view of the age of the patient, a possible bacterial origin, and the potential severity of the condition.
Newborns who suffer from EV meningitis usually have good outcomes, with complete neurological recovery. Neurodevelopmental sequelae have been more commonly associated with EV meningoencephalitis18, and there is no evidence of such sequelae in meningitis without EV encephalitis. The patients who develop neurological deficits are those with meningitis that coexisted with other more severe manifestations of the infection12,15. Various studies have reported that up to 20% of newborns with uncomplicated EV meningitis develop neurological deficits during follow-up. However, these findings are not confirmed by other studies and no differences have been found in the neurological development of these children when compared with their healthy siblings19,20.
Most of the children with EV meningitis did not need intensive care. There is no effective specific treatment for severe neonatal EV infection, although these forms could benefit from the administration of high-dose immunoglobulins, which would potentially provide sufficient concentrations of antibodies to counteract the viraemia21.
The viruses enter through the oropharyngeal mucosa, and from there they start replicating before spreading through the blood and gastrointestinal tract, where they replicate actively for several weeks. It is evident, therefore, why respiratory and gastrointestinal samples contain the highest concentration of virus and, consequently, are highly sensitive for isolation and detection of EV. The virus can be detected for several weeks in stool by molecular techniques, but detection does not necessarily mean an acute infection5,11. Identifying the virus in sterile fluids is indicative of acute infection in any age group, but in newborns this is also true when detected in the stool and respiratory secretions, particularly within the first two weeks of life. The technique of reference for the isolation of EV, according to the WHO polio laboratory manual9, is viral culture. However, recovery of EV from CSF culture is low, and CSF RT-PCR techniques have advantages in both time and sensitivity. For this reason, CSF RT-PCR is the standard method of choice for detecting EV in these samples due to its greater speed and sensitivity22. Plasma RT-PCR is recommended in the study of infants under one month of age with suspected invasive bacterial disease to exclude EV infection23,24.
Testing for detection of EV in the CSF was performed in all the newborns. In our study, as described in several articles23–26, the CSF was the most sensitive sample in detecting EV infection. It should be remembered, however, that our study is a retrospective review of patients with EV meningitis, and the results therefore need to be interpreted with caution. It should also be noted that no EV was detected in the CSF samples in any of the newborns with bacterial meningitis.
The main EV genotypes isolated in our patients were E11, CVB5, E18, E25, E7 and CVB3, and these genotypes represented 40% of all the EV identified during the study period. Various published studies show that the predominance of some genotypes over others varies in relation to the geographical location and the epidemic genotype variation11. The most common types isolated in our study may not therefore be in line with other studies carried out in other countries.
Different publications suggest an association between the progression of the disease to severe forms and certain types of EV5,6,15. However, not all authors agree with that statement27. In our study, we did not find any such association and it is possible that the types associated with greater severity, mainly E11 and coxsackievirus type B, were the most common EV to be isolated in newborns.
Most newborns receiving hospital care for EV meningitis are treated with antibiotics while waiting to see how the disease progresses and for the results of the microbiological tests; this means antibiotic therapy can last for several days after admission. It is known that the administration of broad-spectrum antibiotics is associated with complications in newborns, and with intestinal colonisation by multi-resistant bacteria. Most of the publications show that 85%–100% of infants with viral meningitis are treated with antibiotic therapy26,28. These figures could be reduced by assessing the clinical and epidemiological data and by performing rapid microbiological tests to confirm or rule out the diagnosis. CSF RT-PCR for EV has high sensitivity and a positive predictive value of 100%25,26. The results of these techniques are available within a few hours and reduce antibiotic therapy in 50% of treated infants29,30.
ConclusionsThe findings of this study show that EV infection is a common cause of meningitis in newborns (78% of the cases in our series). These data highlight the importance of rapid EV detection in neonates with suspected meningitis to obtain an early diagnosis. This would help reduce the use of antibiotic therapy, thus reducing hospitalisation time and the associated healthcare costs.
FundingThis article received no funding.
Conflicts of interestThe authors declare that they have no conflicts of interest.