Scarce information is available on the resources to deal with the Sexually Transmitted Infections (STIs), both in the clinic and in the laboratory. The objective is to describe and know the reality of the clinics and laboratories that treat these infections in Spain.
MethodsCross-sectional observational study with data collection through a survey aimed at the members of the GEITS Group.
ResultsResponses were obtained from 24 centers (response rate 38.1%) belonging to 10 Autonomous Communities. Regarding STI consultations, 38% require that the patient present a health card to provide assistance, and 31.8% only provide it by referral from another doctor. The 52.4% perform diagnostic methods in the care center. Regarding laboratories, 18.2% do not offer immediate response diagnostic tests, although 100% have PCR against Neisseria gonorrhoeae and Chlamydia trachomatis, 47.8% against Mycoplasma genitalium and 65% detect lymphogranuloma venereum genotypes. All laboratories continue to perform culture and gonococcal sensitivity techniques, and 20% perform molecular methods for detection of MG antimicrobial resistance.
ConclusionThere is great variability in the provision of human and material resources both in the clinics and in the laboratories that attend STIs. In a significant number of centers there are limitations for patient access. Although laboratories have molecular biology technologies, not all of them offer immediate response tests. All laboratories detect N. gonorrhoeae infection by PCR and also by culture, which allows sensitivity testing in all centers.
Hay escasa información sobre los recursos disponibles tanto en las consultas como en el laboratorio para hacer frente a las infecciones de transmisión sexual (ITS). El objetivo es describir y conocer la realidad de las consultas y laboratorios que atienden las ITS en España.
MétodosEstudio observacional transversal con obtención de datos mediante una encuesta dirigida a los miembros del Grupo de ITS de la SEIMC (GEITS).
ResultadosSe obtuvieron respuestas de 24 centros (tasa de respuesta, 38,1%) pertenecientes a 10 comunidades autónomas. Respecto a las consultas de ITS, el 38% precisan que el paciente presente tarjeta sanitaria para proporcionar asistencia, y un 31,8% solo la prestan mediante derivación de otro médico. El 52,4% realizan métodos diagnósticos en la propia consulta. El 18,2% de los laboratorios no ofrecen pruebas diagnósticas de respuesta inmediata, aunque el 100% disponen de PCR frente a Neisseria gonorrhoeae y Chlamydia trachomatis, el 47,8% frente a Mycoplasma genitalium y el 65% detectan genotipos del linfogranuloma venéreo. El 20% realizan técnicas de detección molecular de resistencias antimicrobianas. Todos los laboratorios realizan cultivo y técnicas de sensibilidad a gonococo.
ConclusionesExiste una gran variabilidad en las dotaciones de medios humanos y materiales en las consultas y en los laboratorios que atienden ITS. En un número importante de centros existen limitaciones para el acceso de los pacientes. Todos los laboratorios disponen de técnicas de biología molecular y detectan la infección de N. gonorrhoeae mediante PCR y cultivo, lo que permite la realización de pruebas de sensibilidad en todos los centros.
The incidence of sexually transmitted infections (STIs) has risen significantly since the turn of the century. If not diagnosed and treated early, STIs can have significant clinical repercussions, including pelvic inflammatory disease and infertility. Some have also been shown to be predisposing factors to HIV transmission. This means that STIs constitute an enormous public health problem worldwide. To address this problem, the World Health Organization (WHO) has established a global health strategy to reduce STIs1.
One of the aims of the Grupo de Estudio de Infecciones de Transmisión Sexual [Sexually Transmitted Infections Study Group] (GEITS) of the Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica [Spanish Society of Infectious Diseases and Clinical Microbiology] (SEIMC) is to promote cooperation and coordination among its members involved and interested in the diagnosis and clinical and epidemiological management of STIs. To achieve these aims, it is vital to understand actual clinical practice in Spain's healthcare centres — both clinics and microbiological laboratories. In this light, the GEITS Board of Directors has developed a survey of diagnostic resources to be filled in by its members.
The objectives of this study were to understand the resources available in both clinics and laboratories involved in the management of STIs in Spain, as well as to collect information about their needs. Once the survey information had been collected, the GEITS sought to establish unified diagnostic algorithms and promote coordination with STI referral centres, with the ultimate goal of designing coordinated strategies in the fight against STIs.
MethodsA descriptive, observational and cross-sectional study was designed. Data were collected through a questionnaire on diagnostic resources at both STI clinics and laboratories that was distributed to SEIMC and GEITS members. Members were invited to participate in an email explaining the project, its objectives, the method and the instructions for filling in the survey. Access to an online questionnaire was provided through a link. Information technology support was provided by the technical secretariat of the SEIMC.
The questionnaire, designed and approved by the GEITS Board of Directors, consisted of 104 questions: four to identify the respondent; 23 concerning clinic resources and 77 pertaining to laboratory resources. Of the laboratory questions, seven were general questions, 14 concerned PCR techniques, 25 were about cultures, 10 were on syphilis, seven on hepatitis, five on HIV, six on herpes and three on quality control.
Each site filled in a single survey. The response period ran from 16 April 2018 to 11 May 2018 (25 days).
ResultsResponses receivedIn total, 124 GEITS members from 63 different sites were invited to take part. Twenty-four sites (response rate 38.1%) from 10 different autonomous communities completed the survey. The distribution of respondent sites by autonomous community was as follows: five sites in the Basque Country, four sites in Andalusia, three sites in the Valencian Community, three sites in Madrid, two sites in Asturias, two sites in the Canary Islands, two sites in Catalonia, one site in Castile and León, one site in Galicia, and one site in Navarre.
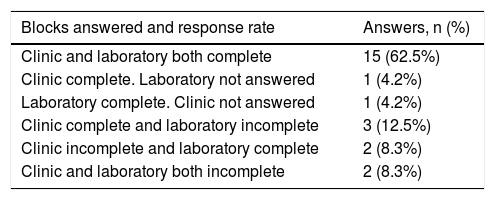
Responses were arranged in two large blocks (clinic and laboratory), and each respondent answered the questions either in a single block, or in both blocks, depending on their profile, as shown in Table 1. As the questions in each block were highly specific, in some cases participants answered the questions in one block but did not answer the questions in the other block, or only partially answered them, meaning that not all sites answered all questions (Table 1). For this reason, the percentages obtained for each question were calculated as a proportion of all answers received for that question and not of all participating sites.
Answers for each block (clinic/laboratory) and response rates.
| Blocks answered and response rate | Answers, n (%) |
|---|---|
| Clinic and laboratory both complete | 15 (62.5%) |
| Clinic complete. Laboratory not answered | 1 (4.2%) |
| Laboratory complete. Clinic not answered | 1 (4.2%) |
| Clinic complete and laboratory incomplete | 3 (12.5%) |
| Clinic incomplete and laboratory complete | 2 (8.3%) |
| Clinic and laboratory both incomplete | 2 (8.3%) |
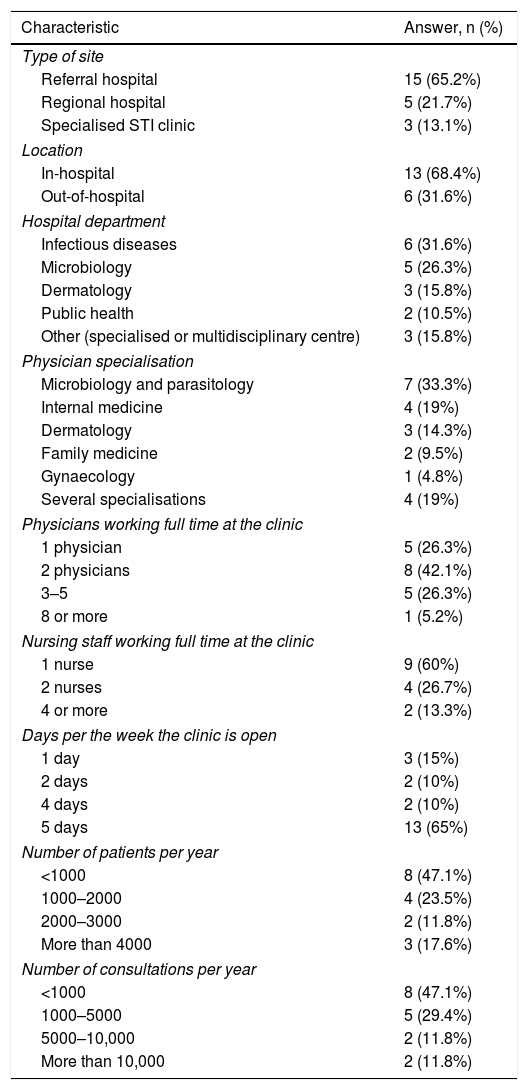
The main characteristics of the clinics are detailed in Table 2.
Characteristics of STI clinics.
| Characteristic | Answer, n (%) |
|---|---|
| Type of site | |
| Referral hospital | 15 (65.2%) |
| Regional hospital | 5 (21.7%) |
| Specialised STI clinic | 3 (13.1%) |
| Location | |
| In-hospital | 13 (68.4%) |
| Out-of-hospital | 6 (31.6%) |
| Hospital department | |
| Infectious diseases | 6 (31.6%) |
| Microbiology | 5 (26.3%) |
| Dermatology | 3 (15.8%) |
| Public health | 2 (10.5%) |
| Other (specialised or multidisciplinary centre) | 3 (15.8%) |
| Physician specialisation | |
| Microbiology and parasitology | 7 (33.3%) |
| Internal medicine | 4 (19%) |
| Dermatology | 3 (14.3%) |
| Family medicine | 2 (9.5%) |
| Gynaecology | 1 (4.8%) |
| Several specialisations | 4 (19%) |
| Physicians working full time at the clinic | |
| 1 physician | 5 (26.3%) |
| 2 physicians | 8 (42.1%) |
| 3–5 | 5 (26.3%) |
| 8 or more | 1 (5.2%) |
| Nursing staff working full time at the clinic | |
| 1 nurse | 9 (60%) |
| 2 nurses | 4 (26.7%) |
| 4 or more | 2 (13.3%) |
| Days per the week the clinic is open | |
| 1 day | 3 (15%) |
| 2 days | 2 (10%) |
| 4 days | 2 (10%) |
| 5 days | 13 (65%) |
| Number of patients per year | |
| <1000 | 8 (47.1%) |
| 1000–2000 | 4 (23.5%) |
| 2000–3000 | 2 (11.8%) |
| More than 4000 | 3 (17.6%) |
| Number of consultations per year | |
| <1000 | 8 (47.1%) |
| 1000–5000 | 5 (29.4%) |
| 5000–10,000 | 2 (11.8%) |
| More than 10,000 | 2 (11.8%) |
Most STI clinics — 15 of them — were in a referral hospital (65.2%), whereas five (21.7%) were in a regional hospital and three (13.1%) were specialised STI clinics. In total, 31.6% of STI clinics were under the remit of the Infectious Diseases Department and 68.4% were in the hospital itself. The clinic opening hours for users were as follows: at 13 sites (65%), the clinic was open from Monday to Friday; at two sites, the clinic was open four days a week; at another two sites, it was open two days a week; and at three sites, it was only open one day a week. Activity was measured in terms of numbers of appointments per year and numbers of patients seen.
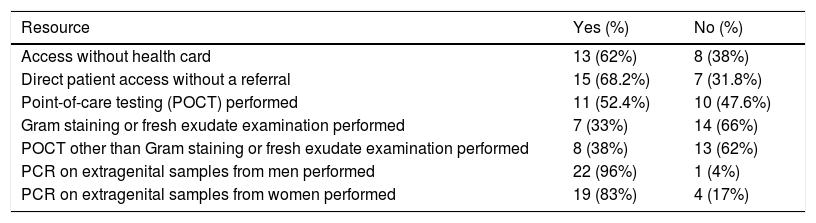
Table 3 summarises the ease of access to the diagnostic resources available in the clinics. A health card was not required at 62% of sites, and patients had direct access to 68.2% of clinics. Seven clinics (33%) had a microscope and 11 (52.4%) performed point-of-care testing (POCT).
Clinic access and diagnostic resources.
| Resource | Yes (%) | No (%) |
|---|---|---|
| Access without health card | 13 (62%) | 8 (38%) |
| Direct patient access without a referral | 15 (68.2%) | 7 (31.8%) |
| Point-of-care testing (POCT) performed | 11 (52.4%) | 10 (47.6%) |
| Gram staining or fresh exudate examination performed | 7 (33%) | 14 (66%) |
| POCT other than Gram staining or fresh exudate examination performed | 8 (38%) | 13 (62%) |
| PCR on extragenital samples from men performed | 22 (96%) | 1 (4%) |
| PCR on extragenital samples from women performed | 19 (83%) | 4 (17%) |
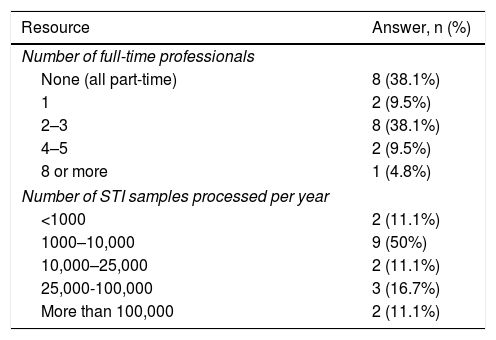
All the laboratories that filled in the survey did not deal exclusively with STI diagnosis. This was reflected in the numbers of workers dedicated to STI diagnosis on a full-time basis: eight labs had staff dedicated to STIs on a part-time basis, while 13 had varying numbers of staff dedicated exclusively to STIs, as shown in Table 4. Table 4 also details the number of STI samples processed each year, which is an indicator of the workload of the different laboratories.
Laboratories: human resources and samples processed.
| Resource | Answer, n (%) |
|---|---|
| Number of full-time professionals | |
| None (all part-time) | 8 (38.1%) |
| 1 | 2 (9.5%) |
| 2–3 | 8 (38.1%) |
| 4–5 | 2 (9.5%) |
| 8 or more | 1 (4.8%) |
| Number of STI samples processed per year | |
| <1000 | 2 (11.1%) |
| 1000–10,000 | 9 (50%) |
| 10,000–25,000 | 2 (11.1%) |
| 25,000-100,000 | 3 (16.7%) |
| More than 100,000 | 2 (11.1%) |
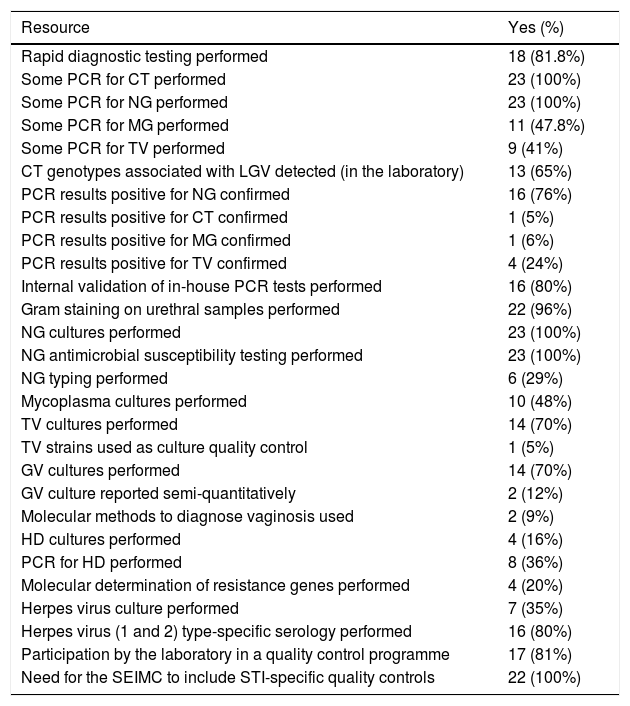
Table 5 shows the results for the diagnostic resources of the laboratories. First, they were asked about their capacity to conduct and compile reports on rapid diagnostic tests, which were available at 18 sites (81.8%). Most were Gram staining (seven sites), dark-field microscopy for syphilis (seven sites) and different PCR tests (11 sites).
Laboratory diagnostic resources.
| Resource | Yes (%) |
|---|---|
| Rapid diagnostic testing performed | 18 (81.8%) |
| Some PCR for CT performed | 23 (100%) |
| Some PCR for NG performed | 23 (100%) |
| Some PCR for MG performed | 11 (47.8%) |
| Some PCR for TV performed | 9 (41%) |
| CT genotypes associated with LGV detected (in the laboratory) | 13 (65%) |
| PCR results positive for NG confirmed | 16 (76%) |
| PCR results positive for CT confirmed | 1 (5%) |
| PCR results positive for MG confirmed | 1 (6%) |
| PCR results positive for TV confirmed | 4 (24%) |
| Internal validation of in-house PCR tests performed | 16 (80%) |
| Gram staining on urethral samples performed | 22 (96%) |
| NG cultures performed | 23 (100%) |
| NG antimicrobial susceptibility testing performed | 23 (100%) |
| NG typing performed | 6 (29%) |
| Mycoplasma cultures performed | 10 (48%) |
| TV cultures performed | 14 (70%) |
| TV strains used as culture quality control | 1 (5%) |
| GV cultures performed | 14 (70%) |
| GV culture reported semi-quantitatively | 2 (12%) |
| Molecular methods to diagnose vaginosis used | 2 (9%) |
| HD cultures performed | 4 (16%) |
| PCR for HD performed | 8 (36%) |
| Molecular determination of resistance genes performed | 4 (20%) |
| Herpes virus culture performed | 7 (35%) |
| Herpes virus (1 and 2) type-specific serology performed | 16 (80%) |
| Participation by the laboratory in a quality control programme | 17 (81%) |
| Need for the SEIMC to include STI-specific quality controls | 22 (100%) |
CT: Chlamydia trachomatis; GV: Gardnerella vaginalis; HD: Haemophilus ducreyi; LGV: Lymphogranuloma venereum; MG: Mycoplasma genitalium; NG: Neisseria gonorrhoeae; TV: Trichomonas vaginalis.
To evaluate access to molecular technology, respondents were asked if their laboratory was equipped with any PCR technique; all 23 (100%) confirmed this to be their case. The pathogens most commonly detected using PCR techniques were Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) at 23 sites (100%), followed by Mycoplasma genitalium (MG) at 11 (47.8%). Specific detection of genotypes L1-L3 associated with lymphogranuloma venereum (LGV) was conducted in house for 13 participating laboratories (65%) and at a third-party site for seven other laboratories (35%).
NG culture and antimicrobial susceptibility testing were performed at all laboratories, with the E-test used in 73.9% of cases. To interpret sensitivity, 11 labs (50%) used the cut-off values defined by EUCAST and nine (41%) applied those established by the CLSI. Two used both criteria depending on the antimicrobial being tested.
Antimicrobial resistance molecular markers in MG were detected using molecular biology techniques at four sites (two using an in-house technique and two using a commercial technique), with detection of mutations in the 23S rRNA gene (macrolides at three labs), parC and gyrA (fluoroquinolones, at two sites).
Treponema pallidum (TP) was detected by PCR at seven labs and by dark-field microscopy at five. For syphilis serology, 16 sites (80%) performed a reverse algorithm, while four labs followed the classic model of initiating serological screening with a non-treponemal test confirmed with a treponemal test.
Of the 20 laboratories that answered the questions concerning the diagnostic resources used to diagnose the herpes simplex (HS) virus, seven (35%) performed a culture, 16 (80%) performed PCR and 16 (80%) used type-specific serological testing.
Responses to questions on hepatitis B and C virus detection indicated that most laboratories were capable of performing chemiluminescence assays, although at one site hepatitis was diagnosed by the Clinical Analysis Laboratory. Hepatitis C in particular was confirmed by positive serology at 16 (80%) of the laboratories that answered the question and by immunoblotting at 13 (81.3%). One-step diagnostic testing (antibodies and viral load in a single sample) was not evaluated in this study. For HIV diagnosis, 10 laboratories offered rapid testing. Positive results were confirmed in house in 89.5% of laboratories; the rest relied on a reference centre.
In terms of quality control, 17 of the 21 laboratories that responded (81%) performed some form of external quality control; most adopted the SEIMC External Quality Control Programme (88%). All respondents recognised the need for the SEIMC External Quality Control Programme to include quality controls for other STI pathogens in addition to those for HIV, hepatitis and syphilis.
DiscussionThis study was a GEITS initiative that aimed to ascertain the resources available to specialised STI clinics and laboratories in Spain, based on the premise that the various professionals who devote a significant amount of their professional time to these infections belong to the GEITS. However, this must be acknowledged to be a limitation of the study, as it prevented us from distributing the questionnaire to other professionals involved in STI diagnosis who are not GEITS members.
The responses received gave the study an appropriate level of representation, given that both clinics and laboratories distributed across much of Spain took part. Moreover, the survey involved not only large specialist centres, but also smaller clinics and laboratories that had access to fewer resources but were nonetheless involved in the fight against STIs. This study serves to highlight the diverse and heterogeneous practices adopted in Spain.
To the best of our knowledge, few studies of this kind have been conducted in other countries. The European Centre for Disease Prevention and Control (ECDC) conducted a survey in 2010, but it was only distributed to the referral centres of the different European countries2. A study more similar to ours was published in Ireland3 in 2018. Aimed solely at laboratories, it achieved a response rate of 92% with 36 respondents. Participation in that study was probably as high as it was because it involved an official questionnaire issued by the Irish Health Service Executive, carrying with it a certain pressure to respond.
In Spain, STI clinics are primarily located in hospitals (usually large ones), operating within a range of different departments and staffed by doctors with very diverse training. Human resources also vary widely depending on the size of the centre. A significant finding was that patients were required to show their health card at 38% of clinics, thus limiting access to care by vulnerable population groups susceptible to these infections4–6.
STIs must be diagnosed early and, more importantly, treated early to prevent the infection from spreading to other individuals. That is why rapid and open access to STI clinics is very important7. However, while 68.2% of the clinics studied did offer direct access, 31.8% of the clinics required the patient to see their general practitioner to then be referred to an STI clinic if deemed necessary. In this regard, it is also important to ascertain the availability of rapid point-of-care testing (POCT) at clinics. Despite its variable sensitivity and specificity, it is vital in order to dispense targeted treatments in the first visit without having to prescribe empirical treatments or schedule a second visit later8. Our study found that 52.4% of clinics perform some kind of rapid diagnostic testing, with 33% equipped with microscopes and the capacity to perform Gram staining or fresh exudate examination. This suggests a high level clinician expertise and commitment, as microscopic examinations entail a certain degree of subjectivity and must be interpreted by a microscopist with extensive experience.
With regard to laboratories, none of those that responded dealt exclusively with STI diagnosis. Laboratory size varied greatly but all generally had a high caseload. Although most laboratories performed rapid diagnostic testing, their actual capacity to offer an instant diagnosis, preferably during the patient's initial consultation, was determined by location, distance and ease of shipping samples from the clinic to the laboratory. These considerations were not examined in this survey.
In terms of PCR techniques, all laboratories were equipped to diagnose CT and NG, yet only 47.8% performed PCR to detect MG. This is very concerning, given that PCR is the only technology capable of detecting this microorganism. This means that at the time this survey was conducted, 52.2% of the laboratories were unable to determine the presence of one of the main causative pathogens of persistent non-gonococcal urethritis.
Another significant finding was that 24% of the laboratories did not confirm PCR results positive for extragenital NG, which may give rise to false positives9.
Despite the fact that 100% of the laboratories had the technology to perform genomic detection of NG by PCR, they all retained the capacity to perform gonorrhoea cultures. This is vital in order to monitor the antimicrobial susceptibility of this micro-organism and detect the appearance of multidrug-resistant strains early, which is critically important in the current context of multidrug-resistant gonorrhoea10. Until reliable molecular techniques to detect resistance are developed, cultures remain the only proven method of susceptibility testing, which can also be used to conduct typing studies to better understand the epidemiology of this infection.
MG resistance can only be detected using molecular techniques, but only four laboratories were equipped with such technology at the time of the study. This is concerning, given that, according to some series, rates of MG resistance to azithromycin are between 16% and 36%11,12.
This study also has certain limitations. Resources available to diagnose all existing STIs were not evaluated as that would have significantly increased the length of an already extensive survey (104 questions). As such, there were no questions specifically about human papillomavirus or hepatitis A (among others). Hepatitis B and C were only touched upon as we feel they are worthy of specific studies, such as the study recently published in this journal on one-step diagnostic testing for hepatitis C virus13. Other limitations derived from the descriptive nature of the study, which yielded a very heterogeneous picture in terms of type of site, study population and resources available. This made it difficult to stratify the sites by particular variables and precluded the conduct of comparative studies.
In conclusion, this study shed some light on current capacity for STI diagnosis and management in Spain. It revealed significant variability in the resources available at each site, but also highlighted the excellent diagnostic capacity of a substantial number of them. The GEITS hopes that the knowledge acquired through this study will drive further collaboration and standardisation of criteria to facilitate a coordinated and effective approach to STI diagnosis.
Conflicts of interestNone.
We would like to thank the hospitals and investigators of each site that filled in the survey for taking part in the study.
We would also like to thank Javier Ávila from the SEIMC Secretariat for developing the online questionnaire and distributing it to the members of the GEITS.
Participating GEITS members
José Ángel Alava Menica (Hospital Universitario de Basurto), Alicia Barreales Fonseca (Hospital Universitario de Burgos), Josefina Belda Ibáñez (Centro de Información y Prevención del VIH/SIDA e ITS [HIV/AIDS and STI Information and Prevention Centre], Alicante), Xabier Beristain Rementería (Complejo Universitario de Navarra), Amaia Cuñado Eizaguirre (Hospital Mendaro, Guipúzcoa), Billie Caceda (Hospital Alto Deba, Guipúzcoa), Cori Gázquez Gómez (Hospital San Juan, Alicante), Araceli Hernández Betancor (Hospital Universitario Insular de Gran Canaria, Las Palmas), Melisa Hernández Febles (Hospital Universitario de Gran Canaria Dr. Negrín, Las Palmas), Leonora Hernández Ragpa (Hospital Universitario de Basurto), Marta Herrero Romero (Hospital Virgen del Rocío, Sevilla), Joaquín López-Contreras González (Hospital de la Santa Creu i San Pau, Barcelona), David Navarro Ortega (Hospital Clínico Universitario de Valencia), María Palomo Lastra (Hospital General Universitario Gregorio Marañón, Madrid), Begoña Palop-Borras (Hospital Regional Universitario de Málaga), Luis Piñeiro (Hospital Universitario Donostia), Carmen Potel Alvarellos (Complejo Hospitalario Universitario de Vigo), Carmen Rodríguez (Centro Sanitario Sandoval, Madrid), Manuel Rodríguez-Iglesias (Hospital Universitario Puerta del Mar/Hospital San Carlos, Cádiz), Juan Romanyk Cabrera (Hospital Príncipe de Asturias, Alcalá de Henares), Jesús Santos (Hospital Virgen de la Victoria, Malaga), Laura Villa Bajo (Hospital Universitario Central de Asturias, Oviedo).
Please cite this article as: Otero-Guerra L, Gil-Alonso L, López-de Munain J, del Romero-Guerrero J, Serra-Pladevall J, Vazquez F. Encuesta de recursos diagnósticos de las ITS en España. Enferm Infecc Microbiol Clin. 2021;39:390–394.
Participating members of the Grupo de Estudio de Infecciones de Transmisión Sexual [Sexually Transmitted Infections Study Group] (GEITS) are listed in Appendix A.