To adapt the ICU Mobility Scale (IMS) to the area of intensive care units (ICU) in Spain and to evaluate the metric properties of the Spanish version of the IMS (IMS-Es).
MethodDescriptive metric study developed in two phases. Phase 1, adaptation to Spanish of the IMS by a team of nurses and physiotherapists (translation, pilot, backtranslation and agreement). Phase 2, analysis of metric properties (convergent, divergent and predictive validity, interobserver reliability, sensitivity and minimum important difference) of the IMS-Es. Patient characteristics (Barthel, Charlson, BMI, sex), sedation/agitation level (RASS), ICU and hospital stays, survival, quality of life (SF-12), muscle weakness (MRC-SS) and mobility (IMS-Es) were recorded in the patients of the MOviPre national multicentre study.
ResultsAfter obtaining the IMS-Es, it was implemented in 645 patients from 80 Spanish ICUs between April and June 2017. Convergent validity: moderate correlation between IMS-Es and MRC-SS (r = .389; p < .001) and significant comparison between groups with and without ICU-acquired weakness (p < .001). Divergent validity: no correlation between IMS-Es and BMI [r(95%CI)=-.112((-.232)-(.011))], weight [r(95%CI)=-.098((-.219)-(.026))], Charlson [r(95%CI)=-.122((-.242)-(.001))] and Barthel [r[95%CI]=-.037((-.160)-(.087))] and no differences between sexes (p = .587) or BMI categories (p = .412). Predictive validity: moderate and significant correlations with post-ICU hospital stay [r(95%CI)=-.442((-.502)-(-.377))] and physical component of SF-12 (PCS) [r(95%CI) = .318(.063-.534)]; patients without active mobilisation in ICU increased risk of hospital mortality [OR(95%CI) = 3.769(1.428-9.947)]. Interobserver reliability: very good concordance between nurses [CCI (95%CI) = .987(.983-.990)] and nurse-physiotherapist [CCI (95%CI) = .963(.948-.974)]. Sensitivity to change: small effect on discharge from ICU (d = .273) and moderate effect at 3 months after hospital discharge (d = .709). Minimal difference: 2-point difference cut-off point, 91.1% sensitivity and 100.0% specificity.
ConclusionsThe IMS-Es is useful, valid and reliable for implementation by ICU nurses and physiotherapists in assessing the mobility of critical patients.
Adaptar la ICU Mobility Scale (IMS) al ámbito de las unidades de cuidados intensivos (UCI) de España y evaluar las propiedades métricas de la IMS versión española (IMS-Es).
MétodoEstudio descriptivo de carácter métrico desarrollado en dos fases. Fase 1, adaptación al español de la IMS mediante equipo de enfermeras y fisioterapeutas (traducción, piloto, retrotraducción y acuerdo). Fase 2, análisis de propiedades métricas (validez convergente, divergente y predictiva, fiabilidad interobservador, sensibilidad y diferencia mínima importante) de la IMS-Es. Se registraron características de los pacientes (Barthel, Charlson, IMC, sexo), nivel de sedación/agitación (RASS), estancias en UCI y hospital, supervivencia, calidad de vida (SF-12), debilidad muscular (MRC-SS) y movilidad (IMS-Es) en los pacientes del estudio multicéntrico nacional MOviPre.
ResultadosTras obtener la IMS-Es, se implementó en 645 pacientes de 80 UCI españolas entre abril y junio de 2017. Validez convergente: moderada correlación entre IMS-Es y MRC-SS (r = 0,389; p < 0,001) y comparación significativa entre grupos con y sin debilidad adquirida en la UCI (p < 0,001). Validez divergente: no correlación entre IMS-Es e IMC [r(IC95%)=-0,112((-0,232)–(0,011))], peso [r(IC95%)=-0,098((-0,219)–(0,026))], Charlson [r(IC95%)=-0,122((-0,242)–(0,001))] y Barthel [r[IC95%]=-0,037((-0,160)–(0,087))] y sin diferencias entre sexos (p = 0,587) ni categorías de IMC (p = 0,412). Validez predictiva: moderadas y significativas correlaciones con estancia en hospital post-UCI [r(IC95%)=-0,442((-0,502)–(-0,377))] y componente físico del SF-12 (PCS) [r(IC95%) = 0,318(0,063–0,534)]; pacientes sin movilización activa en UCI mayor riesgo de mortalidad hospitalaria [OR(IC95%) = 3,769(1,428–9,947)]. Fiabilidad interobservador: muy buena concordancia entre enfermeras [CCI(IC95%) = 0,987(0,983–0,990)] y entre enfermera-fisioterapeuta [CCI(IC95%) = 0,963(0,948–0,974)]. Sensibilidad al cambio: efecto pequeño al alta de UCI (d = 0,273) y moderado a los 3 meses del alta hospitalaria (d = 0,709). Diferencia mínima importante: punto de corte de la diferencia de 2 puntos, sensibilidad 91,1% y especificidad 100,0%.
ConclusionesLa IMS-Es es útil, válida y fiable para ser implementada, por enfermeras de UCI y fisioterapeutas, al valorar la movilidad de los pacientes críticos.
To assess the physical function of the intensive care patient and monitor their evolution during their ICU stay validated tools must be used for this population group and with the appropriate metric properties. The ICU Mobility Scale (IMS) is a scale which assesses mobility in critically ill patients with good metric properties. This study aims to adapt the IMS to the Spanish context and validation.
Practice implicationsA valid and reliable scale has been obtained which may be used in the assessment of mobility for critically ill patients in Spain, both by nurses and by physiotherapists. Thus the language between both professions is standardised in the assessment of the degree of activity of the patients, promoting the development and implementation of individualised activity to prevent ICU-acquired weakness syndrome.
Advances in critically ill patient interventions over the last 20 years has made it possible to increase survival after discharge from the intensive care unit (ICU),1 and the aim of care therefore currently centres upon improving the quality of life of patients after admission to the ICU and to hospital.2
Notwithstanding, post-ICU syndrome is a relatively frequent sequela among patients who survive the ICU. It is defined as the physical, functional and cognitive loss of patients who survive the ICU, and is the cause of 47% readmissions or deaths one year after discharge. Even in the best of cases, it affects the capacities and skills of the patients in their return to the workplace.3,4
One of the most recently studied aspects of the post-ICU syndrome is ICU-acquired muscle weakness (ICUAW), which may be diagnosed between 26% and 65% of patients treated with mechanical ventilation (MV) between 5 and 7 days respectively.5,6 ICUAW includes clinical symptoms of myopathy, polyneuopathy and neuro-myopathy, as well as weakness and loss of muscle mass relating to the critical pathology without other explanatory aetiology.
Consequently, validated tools of measurement are required to assess the physical and functional capacity of the critically ill patient in the ICU and to monitor their evolution during their stay and when they return to the community, to be used in our cultural context, with appropriate psychometric properties for the critically ill patient population.7–9
These tools are classified into those which measure muscle mass (anthropometry, bioimpedance, ultrasonography), muscle weakness (manually tested muscle with the Medical Research Council [MRC-SS] or dynamometer and physical function, with this dimension being the one with the most developed tools but fewest appropriately validated. The Chelsea critical care physiotherapy (CPAx), the Physical function in intensive care test (PFIT) and the ICU Mobility Scale (IMS)10 are outstanding as the best due to their psychometric qualities
The latter, the IMS,11,12 was created to replace the 6 minute walking test, not applicable for the critically ill patient8,9 and the only one which aims to standardise the language of nurses and physiotherapists when they describe patient mobilisation during ICU stay. A limitation of many studies is that they assess the effectiveness of early mobilisation in the ICU, but they do not use a validated mobility scale to define the different degrees of activity which are achieved by the patients,13–16 thereby hindering comparison between the different studies that assess efficacy of mobility to prevent ICUAW, shorten ICU and hospital stay or prevent death.
Since the IMS has been validated in the critically ill patient, but in the United Kingdom, the aim of this study was to perform a cross-cultural adaptation of the ICU Mobility Scale to the Spanish intensive care area and to assess the metric properties of this Spanish version.
MethodA descriptive metric study was conducted in two phases: phase 1, adaptation into Spanish of the ICU Mobility Scale; phase 2, analysis of the metric properties of the Spanish version of the scale.
Phase 1. Cross-cultural adaptationThe aim of adaptation into Spanish was to achieve equivalence in the tool at conceptual semantic level, technical content and criterion in a language which differed from that of the original scale.17
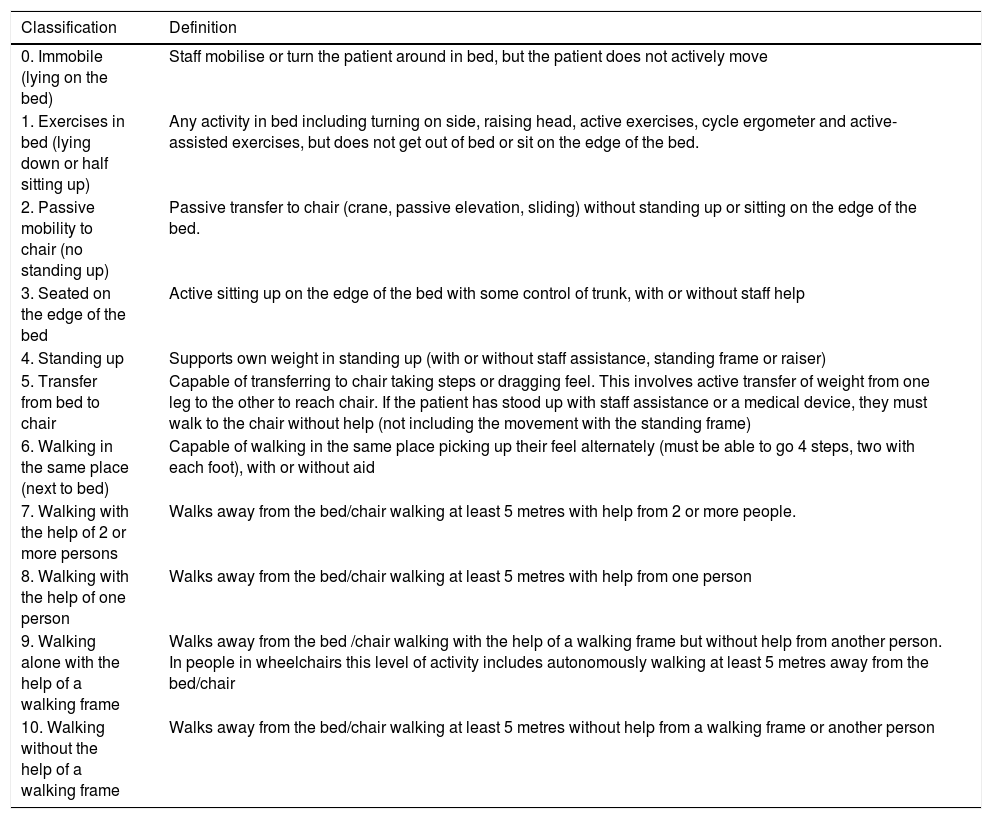
The ICU Mobility Scale (IMS) [Appendix C supplementary material] is a scale that contains 10 items which range from 0 (no mobility) to 10 (walks unaided), and which some authors18 have classed in a binary manner (< 4 passive/active mobilisation in bed and ≥ 4 active mobilisation outside bed).
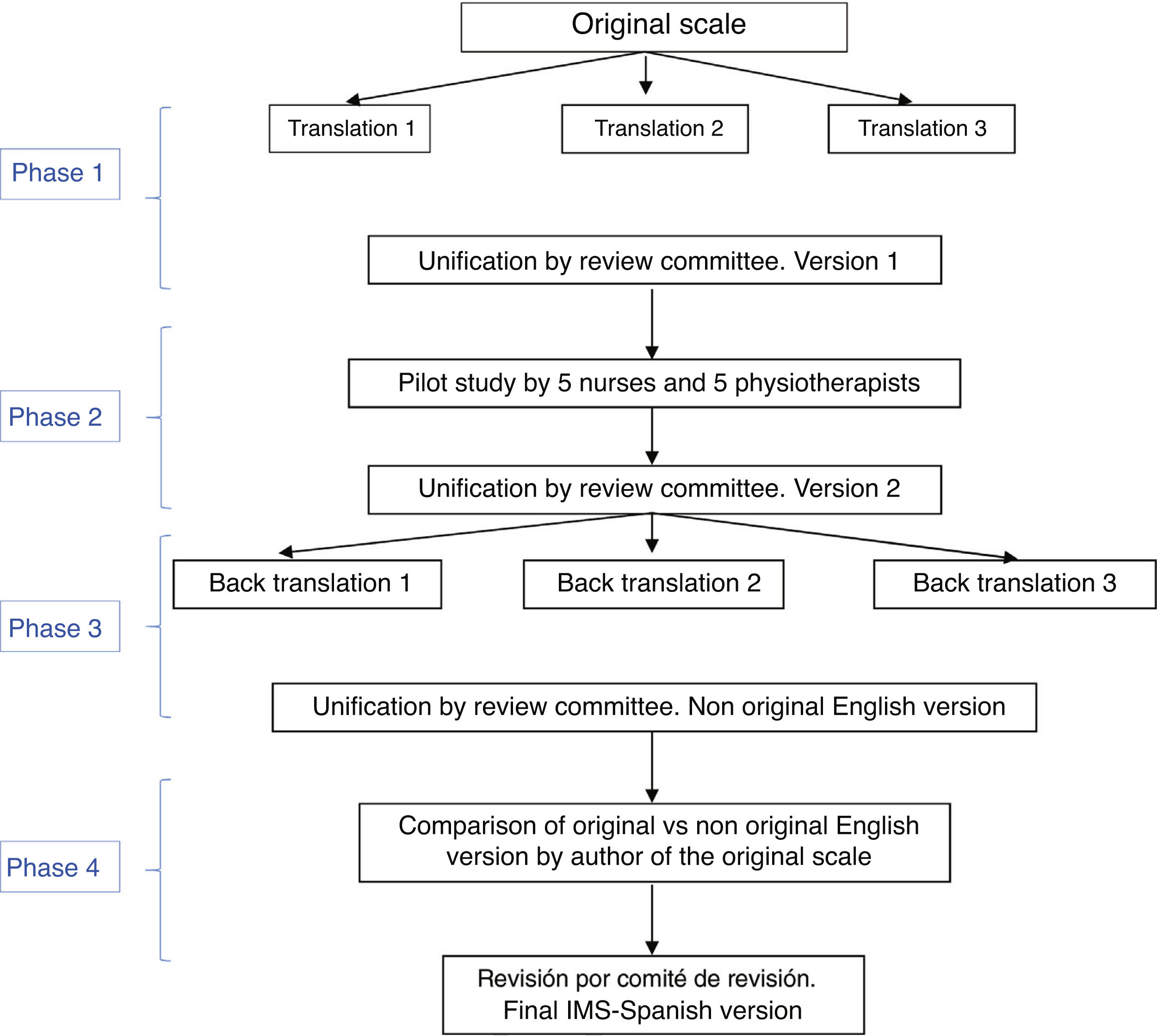
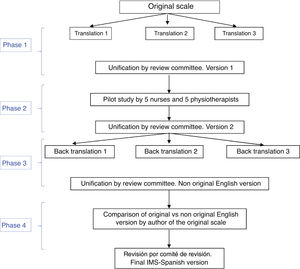
The Spanish adaptation of the IMS was performed in four stages, following different techniques in each (Fig. 1):
Stage one: translation of the IMS and unification for obtaining the first version of the scale into SpanishThe translation from English into Spanish was made by three bilingual health specialist translators who worked independently and whose mother tongue was Spanish. They were given the original version of the tool, together with a brief explanation of its characteristics and uses. Three translations of the tool were obtained into Spanish. After this a review committee was created, formed by two expert nurses and one physiotherapist in the ICU (members of the study research team, with a high level of English) to measure the semantic equivalence of these three versions of the scale translated into Spanish. In the review committee translations were combined, and an initial version of the Spanish IMS was created (IMS-Es-v1).
Stage two: pilot studyThe relevance and comprehensibility of each item of this initial version of the IMS-Es were assessed by 5 nurses and 5 physiotherapists with over 5 years of experience in intensive care. Relevance was determined by a Likert scale in which each item was given a score between 1 (not relevant at all) and 4 (highly relevant. Qualitative appraisals were also collected from the nurses and physiotherapists on each of the items. Text comprehensibility was assessed as bad, acceptable or good.
With this pilot test assessment time was also calculated. Each professional measured the time used in scale application.
With consideration of all contributions, the review committee produced the second version of the IMS-Es (IMS-Es-v2).
Stage three: back-translation of the IMS-EsA back-translation or inverse translation was made from Spanish into English by three independent, health specialist bilingual translators, whose mother tongue was English and who were aware of the IMS scale proposal but not the original version. Justification for veiling the original version was to ensure a translation which had not been contaminated by previous reading of the items. Each one of them did an independent translation from the second version of the IMS-Es. Following this, the review committee, in keeping with the previous procedure, combined the three inverse translations, creating a new version of the IMS into English.
Stage four: correlation of the scales in EnglishThe author of the original scale assessed the correspondence item by time between the original version and that obtained from the inverse translations. They assessed the equivalence of content, syntax, technique, criterion and concept of the items as good, appropriate or bad. After this stage the final version was obtained from the ICU Mobility Scale in Spanish (IMS-Es) (Table 1).
ICU Mobility Scale Spanish version (IMS-Es).
| Classification | Definition |
|---|---|
| 0. Immobile (lying on the bed) | Staff mobilise or turn the patient around in bed, but the patient does not actively move |
| 1. Exercises in bed (lying down or half sitting up) | Any activity in bed including turning on side, raising head, active exercises, cycle ergometer and active-assisted exercises, but does not get out of bed or sit on the edge of the bed. |
| 2. Passive mobility to chair (no standing up) | Passive transfer to chair (crane, passive elevation, sliding) without standing up or sitting on the edge of the bed. |
| 3. Seated on the edge of the bed | Active sitting up on the edge of the bed with some control of trunk, with or without staff help |
| 4. Standing up | Supports own weight in standing up (with or without staff assistance, standing frame or raiser) |
| 5. Transfer from bed to chair | Capable of transferring to chair taking steps or dragging feel. This involves active transfer of weight from one leg to the other to reach chair. If the patient has stood up with staff assistance or a medical device, they must walk to the chair without help (not including the movement with the standing frame) |
| 6. Walking in the same place (next to bed) | Capable of walking in the same place picking up their feel alternately (must be able to go 4 steps, two with each foot), with or without aid |
| 7. Walking with the help of 2 or more persons | Walks away from the bed/chair walking at least 5 metres with help from 2 or more people. |
| 8. Walking with the help of one person | Walks away from the bed/chair walking at least 5 metres with help from one person |
| 9. Walking alone with the help of a walking frame | Walks away from the bed /chair walking with the help of a walking frame but without help from another person. In people in wheelchairs this level of activity includes autonomously walking at least 5 metres away from the bed/chair |
| 10. Walking without the help of a walking frame | Walks away from the bed/chair walking at least 5 metres without help from a walking frame or another person |
This phase took place during the months of April and June 2017 in the participant units of the national Spanish multicentre study on early mobility (MOviPre).19
Study sampleThis included patients in the ICU 48 hours prior to the initiation of invasive mechanical ventilation (IMV).
The following patients were not included: those with a main or secondary diagnosis on entry of neuromuscular pathology (myasthenia gravis, Guillain-Barré syndrome, sarcopenia), inability to walk prior to admission (if they walked with a stick or walking frame they were included), admission for stroke or cerebral vascular accident), pregnant women, major burn patients, underage, re-admission to the ICU or transfers to the ICU from other hospitals. Patients who had previously suffered from particular difficult in mobility were also excluded (absence of limbs de novo, users of orthopaedic devices) and those with a body mass index (BMI) over 35.
Exclusion criteria of the study from the clinical histories were Life Support Limitation (LSL) and patients who were alert but incapable of following simple verbal instructions. Patient withdrawal from the study was understood to be when they did not give their consent or they withdrew their consent during the study period and those who were transferred to another hospital intubated, because they were unable to continue with the follow-up. Approval was obtained from all Ethics Committees and from the Clinical Research departments of the participant centres. Data collected by the researcher of the centre were processed using a user-protected and password database, coding the hospital and patient, ensuring patient confidentiality of all patients in the study.
Type of sampleConvenience sampling during recruitment phase of the MOvipre19 study to include a minimum of 192 patients, according to the original validation of the IMS scale.11 Calculation of the sample (760 patients) was based on the incidence of ICUAW, of 46% according to the systematic review of Stevens et al.,20 with a estimated standard error of 3 and foreseeable losses of 10%.
Study variablesPatient characteristics: Barthel index prior to admission, Charlson comorbidity index, weight and body mass index, sex. Level of agitation-sedation measured daily with the Richmond Agitation Sedation Scale. Hospital stay after discharge from the ICU and survival on hospital discharge. The ICU Mobility Scale (IMS-Es) score was measured by nursing shifts during the whole stay in the ICU and the Medical Research Council Scale sum score (MRC-SS) was measured when the patient woke up for the first time during their stay in the ICU and then consecutively every 7 days until discharge from the ICU and score on the quality of life scale (SF-12) of the physical (PCS) and mental (MCS) components, prior to admission in the unit (direct interview with the patient) and 90 days after hospital discharge (over the phone).
Tools of measurement- •
Barthel index.21 Tool for assessing physical function. The maximum score is 100 points and the patient is considered to be dependent (with different degrees of dependence) if they score 60 or less.
- •
Charlson comorbidity index.22 Ten-year life expectancy evaluation system, according to the comorbidities of the patent on admission and adjusted to age. Scores range between 0 and 33, indicating the highest scores for the greatest severity in chronic illness.
- •
Body mass index (BMI), weight in kilograms relating to the height in centimetres. Measured on admission to the unit. Considered as low weight (BMI < 18.5), normal weight (BMI between 18.5 and 24.99), overweight (BMI between 25 and 29.99) and obese (BMI ≥ 30).
- •
Richmond Agitation Sedation Scale (RASS).23 a scale developed and validated to assess sedation and agitation of critically ill patients. It consists of 10 points with 4 levels of agitation/sedation (+4 combative and +1 anxious) and 5 levels of sedation (―1 sleepy and ―5 very deep sedation). Level 0 indicates that the patient is calm and alert.
- •
Short form 12-item health survey (SF-12).24 Questionnaire on health-related quality of life. Comprises 12 items. It assesses the degree of well-being and functional ability of people over 14, defining a positive and negative status of physical (PCS) and mental health (MCS), through 8 dimensions.
- •
Medical Research Council Scale Sum score (MRC-SS) in keeping with the assessment protocol described by Vane et al.25 it measures muscle strength, in which each muscle group scores between 0 and 5. There are 12 muscle packages and possible scores ranging from 0 to 60. It is considered that the patient presents with clinical symptoms of ICUAW when the score is lower than 48.
Validity indicates whether the information collected by the scales is genuinely what is to be measured.26 For this the following was considered:
Concurrent or convergent validity. The level of agreement between constructs related to one another is valued. The tool to be validated (IMS-Es) has been related to that of reference (MRC-SS), with both being administered by nurses and physiotherapists, respectively, the same day and time each week. Scores obtained in the MRC-SS assessment were categorised, according to the implied diagnosis of ICU-AW (MRC-SS <48) or discarded (MRC-SS ≥48). The Spearman correlation (intensity of lineal correlation) was calculated between the IMS-Es and MRC-SS, with weak correlation being considered to be scores under .30; moderate between .30 and .59, and strong scores above .59.27 Means and interquartile ranges were compared using the Mann-Whitney U-test, [P25-P75] for IMS-ES with relationship with the two categories of the MRC-SS.
Divergent validity. The degree of non correlation between variables which hypothetically bore no relationship with that measured by the new instrument were assessed. The last IMS-Es assessment was correlated simultaneously with the MRC-SS assessment, with body mass index, weight, the Barthel index and the Charlson index. They were also compared with sex and classified BMI. The Spearman correlation was calculated between the IMS-Es and the BMI, weight, Barthel index and Charlson comorbidity index and they were compared, using the Mann-Whitney U-test, medians and interquartile range [P25-P75] for IMS-Es in relation to the patient’s sex and classified BMI.
Predictive validity. Capacity of a tool to predict an event. Analysis of the IMS-Es was performed with the SF-12 quality of life questionnaire, adjusted to age, duration of post-ICU hospital stay and hospital mortality. The IMS-Es was stratified according to the best mobility during ICU admission, and passive/active mobility in bed (IMS-Es <4) or active mobility outside bed (IMS ≥4), and the SF-12 quality of life questionnaire was analysed with scores obtained from the questionnaire at 90 days and with the differences in scores between the two times the questionnaire was applied (prior to admission and on discharge from the ICU). The Spearman correlation was calculated between the best IMS-Es during stay in the ICU with post-ICU hospital stay and the difference in scores between SF-12 prior to hospital admission and after 90 days on discharge home, both in the physical (PCS) and mental (MCS) components. The risk of dying in hospital for ICU survivors was calculated, according to the best IMS-Es during ICU stay (categorised in passive/active mobility in bed [IMS-Es <4] or active mobility outside bed [IMS-Es≥4]), using the chi-square test. The categorized IMS-Es was also compared, through the Mann-Whitney U test with the difference of SF-12 prior to admission and 90 days after discharge.
ReliabilityInterobserver reliability26 Degree of concordance between two or more observers. Scores obtained from the IMS-Es research team nurses and between them and physiotherapists were compared. The maximum level of mobility using direct observation was assessed, at the same time but independently. The scores were not contrasted between observers. Both nurses and physiotherapists had over 5 years experience in the ICU. For comparisons, the intraclass correlation coefficient (ICC) was used, establishing a 95% confidence interval. Accepted ICC scores were between 0 and 1, with scores under .31 indicating nill concordance; between .31 and .5, mediocre concordance; between .51 and .7, moderate; between .71 and .9, good and above .9 very good concordance.28 Total concordance was obtained from the sum of the tables where observer scores coincided, with assessment being measured uisng the corrected kappa index. Poor concordance was considered to be scores under .20; weak concordance between .21 and .40; moderate between .41 and .60; good between .61 and .80 and very good concordance over .81 and 1.29 The Spearman coefficient measured observation correlation.
Sensitivity26Sensitivity to IMS-Es change was assessed in patients who were discharged from hospital and with over 2 days of study inclusion. Scale scores were analysed at different times throughout stay, and size effect and the percentage of patients without change were analysed between the different moments assessed.
Change over time and size effect were assessed using the significance of change in the IMS-Es at three times: on study inclusion, on discharge from the unit and the day in-between. For the patients whose quality of life was assesssed 3 months after hosptial dischage, the three assessment times were: on study inclusion, on discharge from the ICU and 3 months after hosptial discharge. The Wilcoxon signed rank test was used for this, comparing the differences of the medians (interquartile range [IQR]) of the IMS-Es between the first two moments and between the second. Size effect (d) was calculated using the formula d = Z/√n. Scores lower than .49 indicate a small size effect; from .5 to .79, that of moderate magnitude and high as equal to or above .8.30 The comparisons between the proportions of patients who showed no change between both moments were assessed using the chi-square test.
To assess the floor and ceiling effect the porportion of patients with minimal scores was determined (0 points) and the maximum (10 points) in admission and discharge from the ICU. Floor and ceiling effects under 15% were considered acceptable.
Minimal important differenceThis is the smallest difference considered to be clinically relevant for assessing a change in patient management. This was considered the minimal relevant change in level of mobility to assess a change in the rehabilitation programme established by the physiotherapist for each patient. Two methods of analysis was used: a) an external indicator (anchor based method) to analyse the differences of patients by grouping them according to change and show the magnitude of this change over time,31 and b) based on distribution, supported by statistics and metric properties of the measuremens, to determine the slightest change which could be detected by the tool. The minimal important difference was calculated in live patients discharged from the ICU.
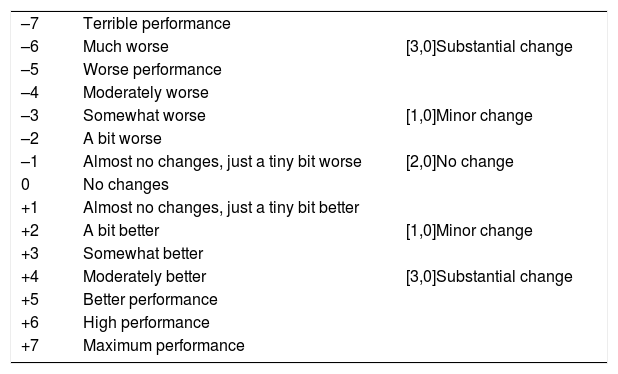
With the anchor based method, when the patient was discharged from the ICU, a research team nurses used a scale from ―7 to 7 (Table 2) to record the overall change in mobility, through the IMS-Es recordings at the inclusion of the study and discharge from the ICU.32 Scores of 0-1 were considered as no change, 2-3 as minor changes and 4-7 as major changes.33 The median (IQR) of the change was compared in the three groups (no change, minor changes and major changes) using the del Kruskal-Wallis test. Sentitivity and specificity of the change in the IMS-Es were calculated to discriminate between the patients with (minor or major) changes and the ones without changes, obtaining a receiver operating characteristic curve (ROC).
Overall change assessment scale.
| –7 | Terrible performance | |
| –6 | Much worse | [3,0]Substantial change |
| –5 | Worse performance | |
| –4 | Moderately worse | |
| –3 | Somewhat worse | [1,0]Minor change |
| –2 | A bit worse | |
| –1 | Almost no changes, just a tiny bit worse | [2,0]No change |
| 0 | No changes | |
| +1 | Almost no changes, just a tiny bit better | |
| +2 | A bit better | [1,0]Minor change |
| +3 | Somewhat better | |
| +4 | Moderately better | [3,0]Substantial change |
| +5 | Better performance | |
| +6 | High performance | |
| +7 | Maximum performance |
To estimate the minimal important difference with the distribution based method, standard mean error (SME) and size effect (SE) of the IMS-Es scores at study inclusion and discharge were used. The SME was calculated from standard deviation (SD) of the means of the measurments and the Spearman rho (r) calculated between the nurses scores (SME = SD √(1-r)). SE was quantified with SD of the difference in scores on study inclusion and discharge (SE = .5*SD). Sensitivity analysis was completed by excluding patients whose condition worsened, according to the overall mobility evaluation.
Usefulness of the scale34This refers to financial cost and time used, simplicity, clarity and if previous training was required for use. The calculation of utility derives from the assessments made by the nurses and physiotherapists during the pilot study in the cross-cultural adaptation phase.
Variables are described as median and interquartile range (IQR) or mean and standard deviation (SD) for the quantitative variables (depending on the parametric behaviour of them). For qualitative variables absolute (n) and relative (%) frequencies were used. The normality of the variables was assessed using the Kolmogorov-Smirnov test. For data analysis the SPSS Statistics for Windows (version 23.0 IBM Corp; U.S.A.) programme and the Stata® (version IC14, StataCorp LLC; U.S.A.) programme were used.
ResultsPhase 1. Cross-cultural adaptationDeveloped between the months of January and February 2017. In the pilot test phase of the IMS-Es-v1 each item scored between 1 (not relevant at all) and 4 (highly relevant). Sixty six per cent of evaluations determined that the items had a relevance of 3 or 4. We found there were differences between the assessments of the nurses and those of the physiotherapists; essentially in the lowest score of relevance (relevance 1: 26% of nurses vs 0% of physiotherapists; p <.001) and in the highest score (relevance 4: 31% of nurses vs 62% of physiotherapists ; p = .001). The items regarded as less relevant by 60% of nurses were those in which the patients carried out active mobility outside bed (IMS-Es≥4), compared with only 20% of physiotherapists.
The time taken to apply the scale was a median of 2'30'' (IQR: 1'45''-4'15''), with no relevant differences found between professionals (nurses vs physiotherapists : 2' [1'30''-4'30'') vs 3' [1'30''-4'20'']).
The item to item agreement of the original scale with that resulting from unification of the back translations or inverse translations was assessed as good in all of its items. After the adaptation process (Fig. 1) we obtained the IMS-Span (MS-Es) (Table 1) scale.
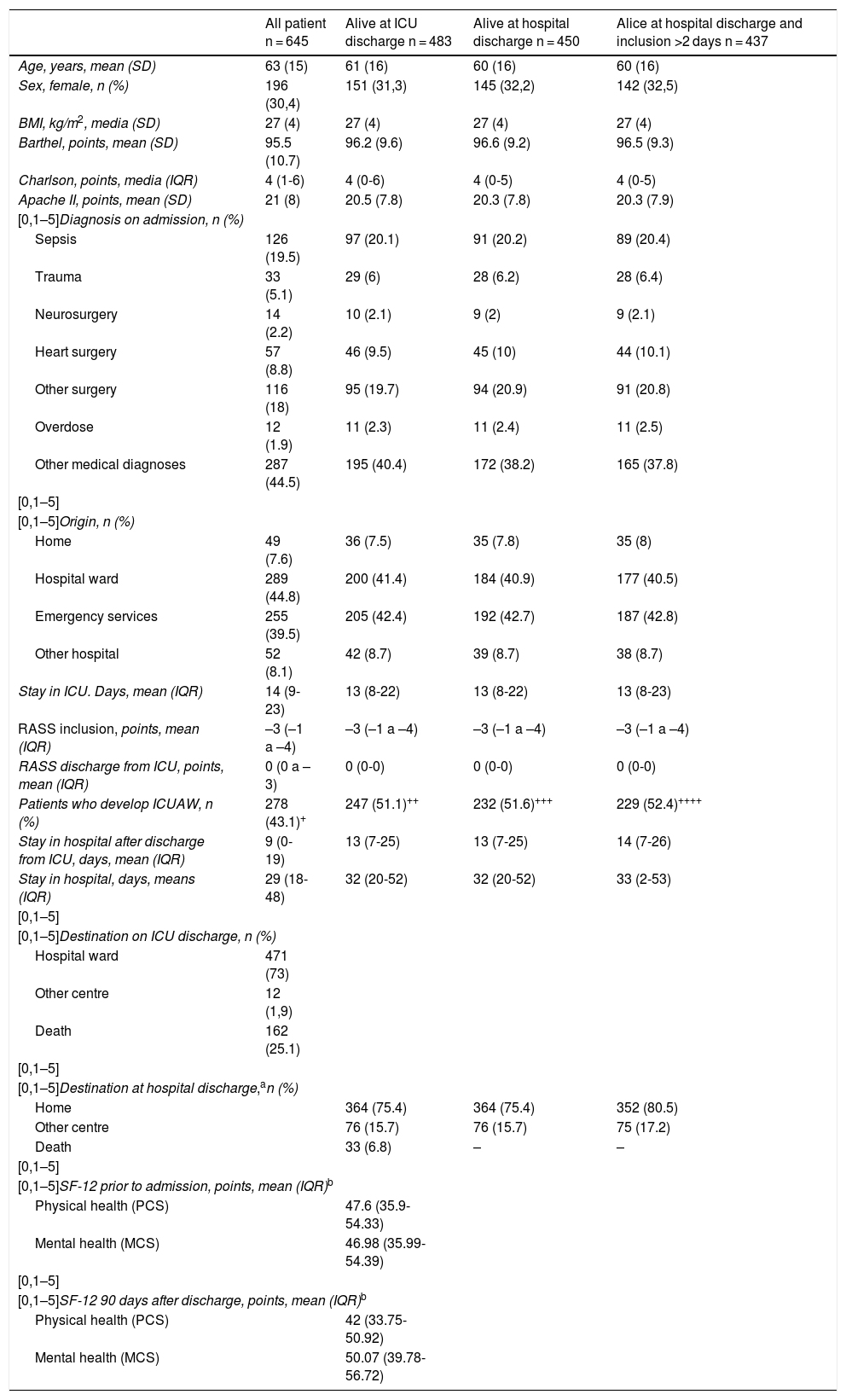
Phase 2. Validity and reliability metric studyThe Spanish version of the IMS (Table 1) was implemented during the months of April to June 2017 in 645 patients from the 80 Spanish ICU participating in the MOvipre study.19 These 645 patients provided 10,133 daily recordings in the ICU. Patient characteristics and measurements of the IMS-Es and MRC-SS are contained in Tables 3 and 4, respectively.
Patient characteristics.
| All patient n = 645 | Alive at ICU discharge n = 483 | Alive at hospital discharge n = 450 | Alice at hospital discharge and inclusion >2 days n = 437 | |
|---|---|---|---|---|
| Age, years, mean (SD) | 63 (15) | 61 (16) | 60 (16) | 60 (16) |
| Sex, female, n (%) | 196 (30,4) | 151 (31,3) | 145 (32,2) | 142 (32,5) |
| BMI, kg/m2, media (SD) | 27 (4) | 27 (4) | 27 (4) | 27 (4) |
| Barthel, points, mean (SD) | 95.5 (10.7) | 96.2 (9.6) | 96.6 (9.2) | 96.5 (9.3) |
| Charlson, points, media (IQR) | 4 (1-6) | 4 (0-6) | 4 (0-5) | 4 (0-5) |
| Apache II, points, mean (SD) | 21 (8) | 20.5 (7.8) | 20.3 (7.8) | 20.3 (7.9) |
| [0,1–5]Diagnosis on admission, n (%) | ||||
| Sepsis | 126 (19.5) | 97 (20.1) | 91 (20.2) | 89 (20.4) |
| Trauma | 33 (5.1) | 29 (6) | 28 (6.2) | 28 (6.4) |
| Neurosurgery | 14 (2.2) | 10 (2.1) | 9 (2) | 9 (2.1) |
| Heart surgery | 57 (8.8) | 46 (9.5) | 45 (10) | 44 (10.1) |
| Other surgery | 116 (18) | 95 (19.7) | 94 (20.9) | 91 (20.8) |
| Overdose | 12 (1.9) | 11 (2.3) | 11 (2.4) | 11 (2.5) |
| Other medical diagnoses | 287 (44.5) | 195 (40.4) | 172 (38.2) | 165 (37.8) |
| [0,1–5] | ||||
| [0,1–5]Origin, n (%) | ||||
| Home | 49 (7.6) | 36 (7.5) | 35 (7.8) | 35 (8) |
| Hospital ward | 289 (44.8) | 200 (41.4) | 184 (40.9) | 177 (40.5) |
| Emergency services | 255 (39.5) | 205 (42.4) | 192 (42.7) | 187 (42.8) |
| Other hospital | 52 (8.1) | 42 (8.7) | 39 (8.7) | 38 (8.7) |
| Stay in ICU. Days, mean (IQR) | 14 (9-23) | 13 (8-22) | 13 (8-22) | 13 (8-23) |
| RASS inclusion, points, mean (IQR) | –3 (–1 a –4) | –3 (–1 a –4) | –3 (–1 a –4) | –3 (–1 a –4) |
| RASS discharge from ICU, points, mean (IQR) | 0 (0 a –3) | 0 (0-0) | 0 (0-0) | 0 (0-0) |
| Patients who develop ICUAW, n (%) | 278 (43.1)+ | 247 (51.1)++ | 232 (51.6)+++ | 229 (52.4)++++ |
| Stay in hospital after discharge from ICU, days, mean (IQR) | 9 (0-19) | 13 (7-25) | 13 (7-25) | 14 (7-26) |
| Stay in hospital, days, means (IQR) | 29 (18-48) | 32 (20-52) | 32 (20-52) | 33 (2-53) |
| [0,1–5] | ||||
| [0,1–5]Destination on ICU discharge, n (%) | ||||
| Hospital ward | 471 (73) | |||
| Other centre | 12 (1,9) | |||
| Death | 162 (25.1) | |||
| [0,1–5] | ||||
| [0,1–5]Destination at hospital discharge,an (%) | ||||
| Home | 364 (75.4) | 364 (75.4) | 352 (80.5) | |
| Other centre | 76 (15.7) | 76 (15.7) | 75 (17.2) | |
| Death | 33 (6.8) | – | – | |
| [0,1–5] | ||||
| [0,1–5]SF-12 prior to admission, points, mean (IQR)b | ||||
| Physical health (PCS) | 47.6 (35.9-54.33) | |||
| Mental health (MCS) | 46.98 (35.99-54.39) | |||
| [0,1–5] | ||||
| [0,1–5]SF-12 90 days after discharge, points, mean (IQR)b | ||||
| Physical health (PCS) | 42 (33.75-50.92) | |||
| Mental health (MCS) | 50.07 (39.78-56.72) | |||
SD: standard deviation; ICUAW: ICU-acquired muscle weakness; BMI: body mass index; RASS: Richmond Agitation Sedation Scale; IQR: interquartile range.
+169.
++45.
+++38.
++++36 lost patients.
a10 lost cases.
bon 57 patients.
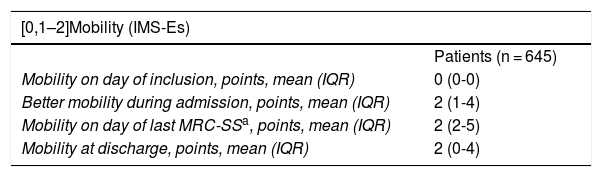
Mobility scores and MRC-SS.
| [0,1–2]Mobility (IMS-Es) | |
|---|---|
| Patients (n = 645) | |
| Mobility on day of inclusion, points, mean (IQR) | 0 (0-0) |
| Better mobility during admission, points, mean (IQR) | 2 (1-4) |
| Mobility on day of last MRC-SSa, points, mean (IQR) | 2 (2-5) |
| Mobility at discharge, points, mean (IQR) | 2 (0-4) |
| Measurements (n = 10.133) | |
|---|---|
| Level of mobility, n (%) | |
| < 4 | 9.232 (91) |
| ≥ 4 | 901 (9) |
| [0,1–2]Weakness (MRC-SS) | |
|---|---|
| Patients (n = 475) | |
| First MRC-SS, points, mean (IQR) | 44 (33-52) |
| Last MRC-SSa, points, mean (IQR) | 49 (41-55) |
| Measurements (n = 929) | |
|---|---|
| MRC-SS, n (%) | |
| < 48 | 518 (55.8) |
| ≥ 48 | 411 (44.2) |
IQR: interquartile range.
aOn 253 patients.
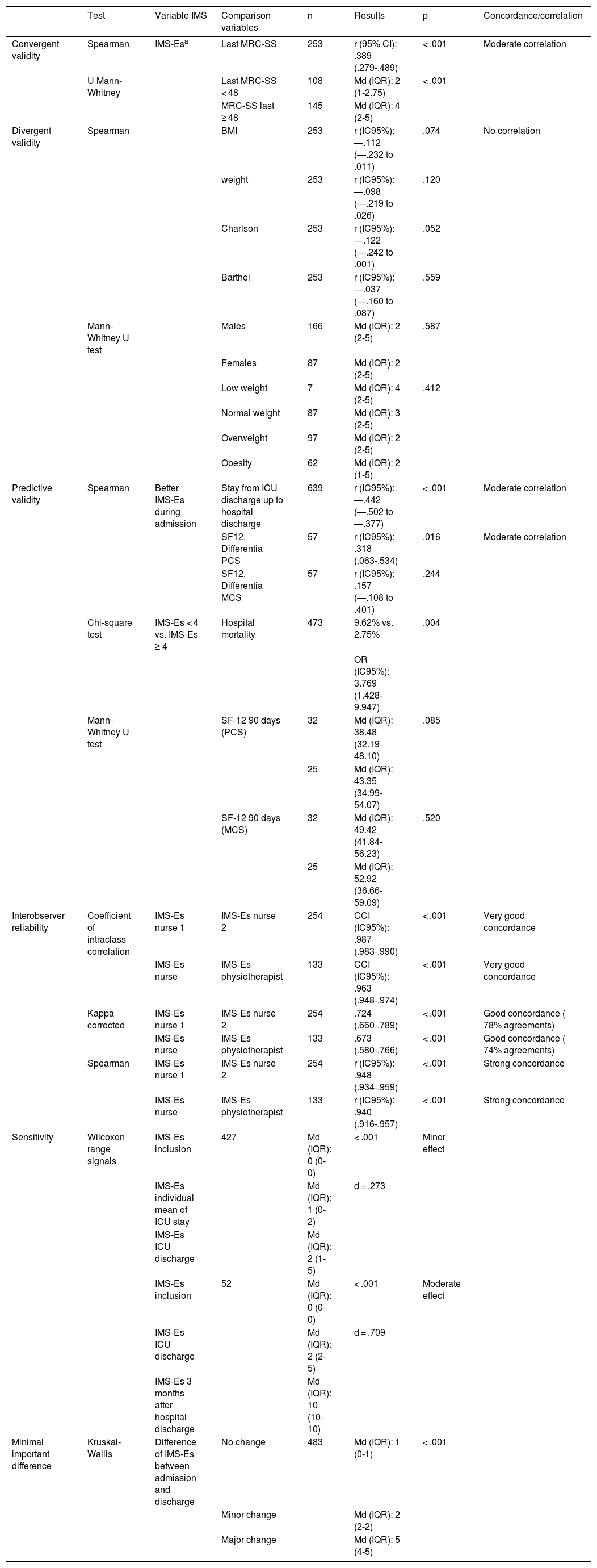
Convergent validity. Of the 645 patients included, MRC-SS was only able to be assessed in 475; the other patients did not become participatory. Out of these, 253 patients were assessed with MRC-SS on more than one occasion. The Spearman correlation coefficient, measured between the last MRC-SS and IMS-Es assessment on the same day, was .389 (p <.001). Among the patients who developed ICUAW during their stay, the Spearman correlation coefficient calculated was .475 (p < .001). The comparison of means (IQR) from the IMS-Es evaluations between groups according to ICUAW (MRC-SS < 48) or non ICUAW (MRC-SS ≥ 48) in the last evaluation of the MRC-SS was statistically significant (p < .001) (Table 5).
Results of the validation analysis.
| Test | Variable IMS | Comparison variables | n | Results | p | Concordance/correlation | |
|---|---|---|---|---|---|---|---|
| Convergent validity | Spearman | IMS-Esa | Last MRC-SS | 253 | r (95% CI): .389 (.279-.489) | < .001 | Moderate correlation |
| U Mann-Whitney | Last MRC-SS < 48 | 108 | Md (IQR): 2 (1-2.75) | < .001 | |||
| MRC-SS last ≥ 48 | 145 | Md (IQR): 4 (2-5) | |||||
| Divergent validity | Spearman | BMI | 253 | r (IC95%): ―.112 (―.232 to .011) | .074 | No correlation | |
| weight | 253 | r (IC95%): ―.098 (―.219 to .026) | .120 | ||||
| Charlson | 253 | r (IC95%): ―.122 (―.242 to .001) | .052 | ||||
| Barthel | 253 | r (IC95%): ―.037 (―.160 to .087) | .559 | ||||
| Mann-Whitney U test | Males | 166 | Md (IQR): 2 (2-5) | .587 | |||
| Females | 87 | Md (IQR): 2 (2-5) | |||||
| Low weight | 7 | Md (IQR): 4 (2-5) | .412 | ||||
| Normal weight | 87 | Md (IQR): 3 (2-5) | |||||
| Overweight | 97 | Md (IQR): 2 (2-5) | |||||
| Obesity | 62 | Md (IQR): 2 (1-5) | |||||
| Predictive validity | Spearman | Better IMS-Es during admission | Stay from ICU discharge up to hospital discharge | 639 | r (IC95%): ―.442 (―.502 to ―.377) | < .001 | Moderate correlation |
| SF12. Differentia PCS | 57 | r (IC95%): .318 (.063-.534) | .016 | Moderate correlation | |||
| SF12. Differentia MCS | 57 | r (IC95%): .157 (―.108 to .401) | .244 | ||||
| Chi-square test | IMS-Es < 4 vs. IMS-Es ≥ 4 | Hospital mortality | 473 | 9.62% vs. 2.75% | .004 | ||
| OR (IC95%): 3.769 (1.428-9.947) | |||||||
| Mann-Whitney U test | SF-12 90 days (PCS) | 32 | Md (IQR): 38.48 (32.19-48.10) | .085 | |||
| 25 | Md (IQR): 43.35 (34.99-54.07) | ||||||
| SF-12 90 days (MCS) | 32 | Md (IQR): 49.42 (41.84-56.23) | .520 | ||||
| 25 | Md (IQR): 52.92 (36.66-59.09) | ||||||
| Interobserver reliability | Coefficient of intraclass correlation | IMS-Es nurse 1 | IMS-Es nurse 2 | 254 | CCI (IC95%): .987 (.983-.990) | < .001 | Very good concordance |
| IMS-Es nurse | IMS-Es physiotherapist | 133 | CCI (IC95%): .963 (.948-.974) | < .001 | Very good concordance | ||
| Kappa corrected | IMS-Es nurse 1 | IMS-Es nurse 2 | 254 | .724 (.660-.789) | < .001 | Good concordance ( 78% agreements) | |
| IMS-Es nurse | IMS-Es physiotherapist | 133 | .673 (.580-.766) | < .001 | Good concordance ( 74% agreements) | ||
| Spearman | IMS-Es nurse 1 | IMS-Es nurse 2 | 254 | r (IC95%): .948 (.934-.959) | < .001 | Strong concordance | |
| IMS-Es nurse | IMS-Es physiotherapist | 133 | r (IC95%): .940 (.916-.957) | < .001 | Strong concordance | ||
| Sensitivity | Wilcoxon range signals | IMS-Es inclusion | 427 | Md (IQR): 0 (0-0) | < .001 | Minor effect | |
| IMS-Es individual mean of ICU stay | Md (IQR): 1 (0-2) | d = .273 | |||||
| IMS-Es ICU discharge | Md (IQR): 2 (1-5) | ||||||
| IMS-Es inclusion | 52 | Md (IQR): 0 (0-0) | < .001 | Moderate effect | |||
| IMS-Es ICU discharge | Md (IQR): 2 (2-5) | d = .709 | |||||
| IMS-Es 3 months after hospital discharge | Md (IQR): 10 (10-10) | ||||||
| Minimal important difference | Kruskal-Wallis | Difference of IMS-Es between admission and discharge | No change | 483 | Md (IQR): 1 (0-1) | < .001 | |
| Minor change | Md (IQR): 2 (2-2) | ||||||
| Major change | Md (IQR): 5 (4-5) |
aLast IMS-Es simultaneously assessed with the last MRC-SS.
MCS: mental component of the SF-12; Md: median ; PCS: physical component of the SF-12; IQR: interquartile range.
Divergent validity. A weak and negative correlation was observed between IMS-Es and weight (r = ―.098), BMI (r = ―.112), the Charlson index (r = ―.122) and the Barthel index (r = ―.043). There were no significant differences between the medians (IQR) of the IMS-Es of men vs women (p = .587), or between the medians (IQR) of classified BMI (p = .412) (Table 5).
Predictive validity. A significant and negative correlation was observed between the best IMS-Es obtained during admission to the ICU and stay in the hospital post-ICU. A significant correlation was also observed in this case positive, between the best IMS-Es and the difference in the physical component of the SF-12 questionnaire, measured prior to admission and 90 days afterwards; with the mental component of the SF-12 we observed no correlation. A significant difference was obtained in the percentage of patients who died in the hospital (on discharge from the ICU), between those who did not have active mobility outside bed during their stay in the ICU (IMS-Es <4) vs those who did actively move (IMS ≥4) (28/291 vs 5/182; OR [95% CI]: 3.769 [1.428-9.947]; p = .004). Although no significant differences were found in the difference between the physical and mental components of the SF-12 between patients with passive/active mobility in bed vs active mobility outside bed, it was observed that patients with active mobility outside bed improved by 5 and 3 points, respectively, in both components (Table 5).
Inter-observer reliabilityTwo hundred and fifty four peer assessments were simultaneously made by two ICU nurses and 133 peer assessments by one ICU nurse and one physiotherapist. Very good concordances and strong correlations were found both in the nurse to nurse peer and in the nurse to physiotherapist peer assessments (Table 5).
SensitivitySize effect in IMS-Es changes between study inclusion and the intermediate point and between this intermediate point and discharge were minor (d = .273) (Table 5). 52.7% of patients presented with an improvement between the day of the inclusion and the intermediate day; 66.3% improved from the intermediate day to discharge from the unit (p < .001).
Size effect observed in the comparison of the medians of the differences between the moments of inclusion and ICU discharge and ICU discharge and 3 months after hospital discharge were moderate (d = .709) (Table 5). 90.4% of patients improved between inclusion and ICU discharge and 100% improved from hospital discharge to 3 months after this (p = .056).
At inclusion the percentage of patients with a 0 score was 76.8%, whilst the percentage of patients with a 10 score was .2%. On discharge from the ICU the IMS-Es presented acceptable scores (<15%) both for the floor and ceiling effect, with percentages of 6.8 and 1.4%, respectively. On inclusion 60.5% of patients had a sedation level (measured with the Richmond Agitation Sedation Scale) of ―3 or under (RASS, median [IQR] =―3 [―1 to ―4]); on discharge there were .9% who had a RASS of ≤ ―3 (RASS, median [IQR] = 0 [0 to 0]).
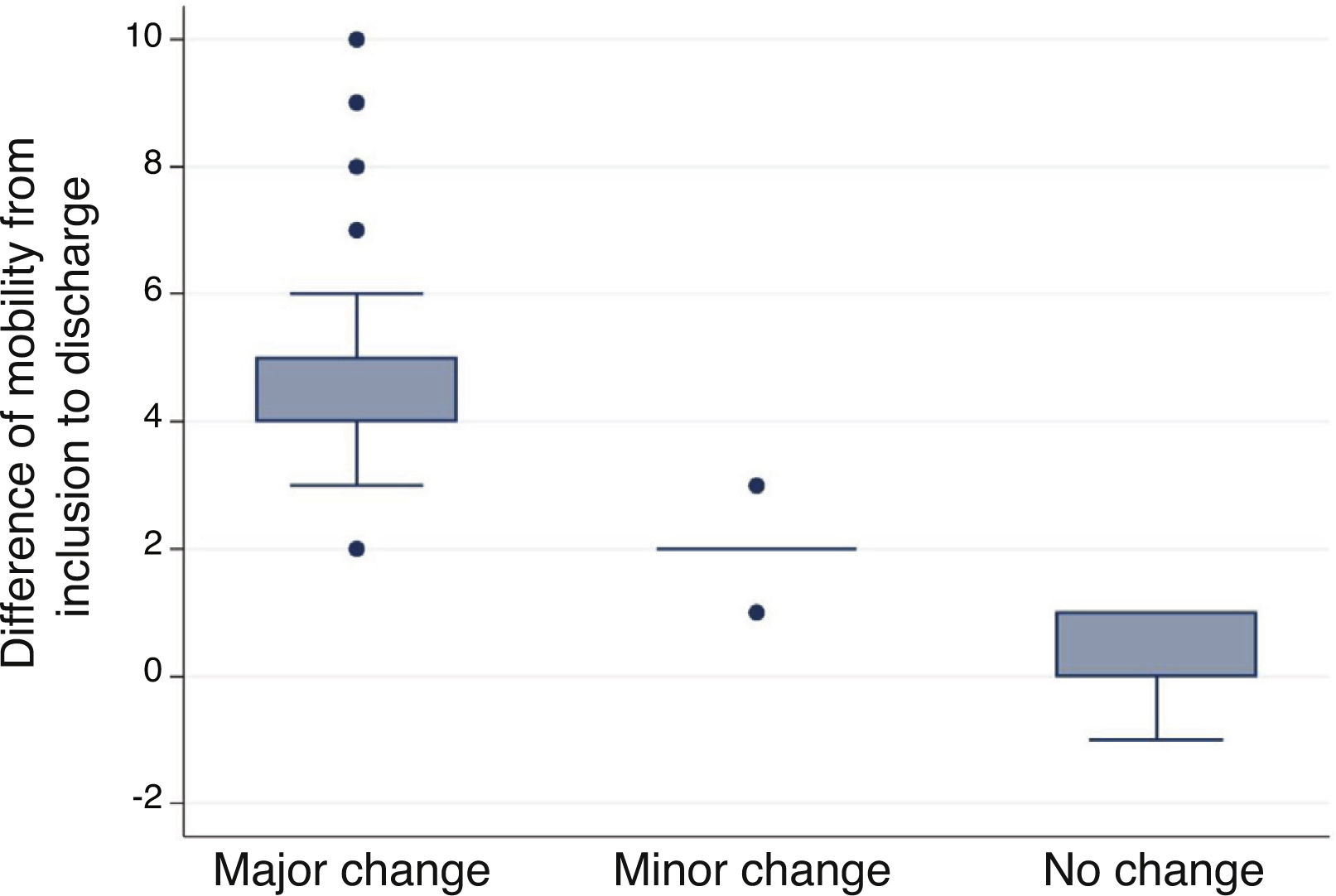
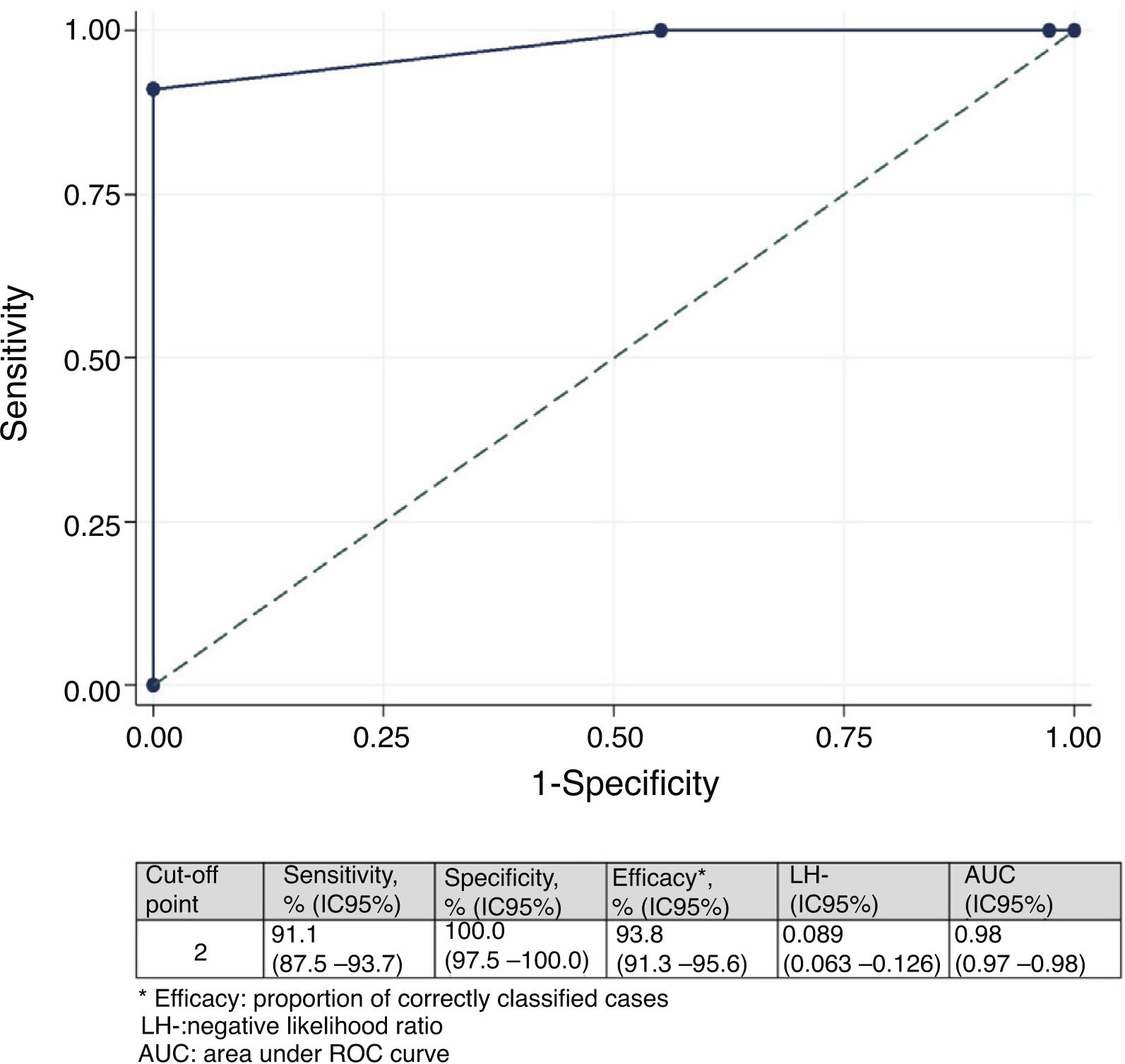
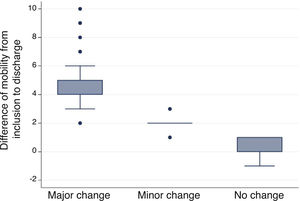
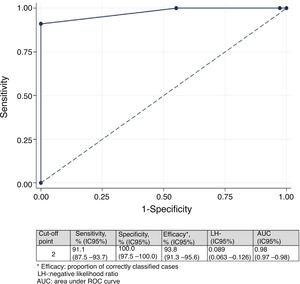
Minimal important differenceMinimal important difference according to the anchor based method. The median (IQR) of IMS-Es on admission of the 483 patients who survived ICU was 0 (0-0) and 2 (1-5) on discharge; the median of the difference of mobility between ICU admission and discharge was 2 (1-4). According to the assessment scale of overall change (Table 2), no change was observed in 147 patients (30.4%); change was minor in 167 (34.6%) and major in 169 (35.0%). Comparison between medians (IQR) of the difference of mobility between admission and discharge between the three groups is significant (Fig. 2). The area below the ROC curve was .98 (95% CI: .97-.98) (Fig. 3). Analysis determined a cut-off point of 2 on the IMS-Es scale as a clinically relevant symptom, with 91.1% sensitivity and 100.0% specificity. In the sensitivity analysis, with the exception of the 4 patients whose mobility worsened between inclusion and discharge, the results obtained were not modified.
Minimal important difference according to distribution. From the standard deviation (SD) of the IMS-Es at time of inclusion (.779) and discharge (2,079), mean standard error (MSE) was .178 and .474, respectively. The size effect calculated from SD of difference (2.039), was 1.020. In sensitivity analysis, excluding the 4 patients whose mobility level deteriorated, the MSE at inclusion was.178 and at discharge .472, with an effect size of 1.011?
Scale utilityThis scale could be considered usefully bearing in mind the time required to implement it, its low cost and that the nurses and physiotherapists who participated in the pilot study stating that it was an easy scale to apply.
DiscussionThis study has led to the adaptation of the IMS scale to the Spanish context and provided a tool which is easy, valid and reliable for nurses and physiotherapists to apply in clinical practice.
The differences observed in the cross-cultural adaptation of the original scale, regarding the relevance of the items assessed by the nurses and physiotherapists, are based on the different skills of each profession. The nurses did not regard high scores as relevant, where the patient walked, because they considered there were few patients who would develop this ability. However, the physiotherapists regard these scores as highly relevant and irrelevant those where the patients remain immobile in bed, which is in keeping with that found in the literature.35–37
With regard to the convergent validity, we obtained a moderate correlation with the latest MRC-SS (r [95% CI]: .389 [.279-.489]; p < .001), which were lower findings than those obtained in the original scale (r [95% CI]: .64 [.49-.75]; p < .001).12 Similarly, the differences in mobility observed between the group which developed ICUAW and the one which did not are equally significant. It is possible for the patients included in this study were less actively mobilized although they had capacity for it, due to the fact that only 14% of units had mobility protocols,19 compared with 36.5% of the units in Australia, where the original scale was validated.12
Furthermore, another justification for low mobility could be the nurse: patient ratio, which in our environment is 1:2 only in 47.7% of units; 1:3 in 25.6% and up to 1:4 in 3.5% of them.19
From analysis of divergent validity we obtained similar results to those obtained in the original scale,12 with an absence of significant correlation with weight and without differences between the sexes. Non comparison of the IMS with the BMI was one of the limitations described in the validation of the original version of the scale; in this version of the IMS-Es we did assess this correlation, and it was not significant considered either as a continuous or categorical variable.
In contrast the IMS-Es scale does have a predictive ability regarding stay and mortality. The patients with the best scores on the IMS-Es during ICU stay have a shorter stay in the hospital following ICU discharge and also non active mobility outside bed carries a higher risk of dying in the hospital. This capacity of the IMS-Es to predict hospital mortality was also observed by Tipping ET al.38 in the original scale.
Regarding quality of life prediction, the IMS38 was unable to demonstrate this validity, but the IMS-Es was. It was proven to have validity regarding the physical component of quality of life 90 days after hospital discharge.
Reliability analysis offers good and very similar concordances to those of the original scale11 (kappa .72 of nurses with senior physiotherapists and .69 with junior physiotherapists) and superior correlations (r = .77-.80).11 Wilches et al.39 obtained similar correlations (CCI between .94 y 1) and better concordances (K between .988 and .992), although in this study only the scores of the physiotherapists were considered, similarly to that of Kawaguchi et al.,40 which obtained higher correlations between physiotherapists (K [95% CI]: .99 [.98-.99]) in the validation of the Portuguese version of the IMS. This correlation between physiotherapists has not been assessed in this study, because we considered that IMS was created to be used in the ICU area and to standardize language between nurses and physiotherapists.
The high floor effect values (76.8%) at time of inclusion may be explained by the level of sedation, since 60.5% of patients had a deep sedation level. Tipping et al.,12 in the original scale, also obtained a high floor effect on admission (96%), with RASS median of ―4. The size effect obtained in the change of IMS-Es between inclusion and discharge was minor (d = .273) and very much lower than that obtained in the original scale12 (d = .8). This small effect observed would possibly be due to the low mobility of the patients included in the study and because in the original scale the moments of inclusion, discharge and 6 months after hospital discharge were analysed, where the mobility level of all patients was 10. In the IMS-Es validation we took into account the moments of inclusion, discharge and the day in-between both. This penalised us because the level of mobility we observed was not as high. We did obtain a moderate size effect (d = .709) in the cohort of patients where the quality of life 3 months after hospital discharge was assessed.
The minimal important difference obtained was between scores of .178 and 2. These scores were below those obtained on the original scale with the population of Australia and New Zeland,12 where scores between .89 and 3 were obtained. The reason for these low data again may stem from the lower mobility of the patients in this study. As a result, clinical relevance in the change was obtained with lower scores.
It should be considered that one limitation of the minimal important difference, measured with the external indicator of the anchor based method is that this method is defined as the smallest change in patient evolution that they consider relevant. In the case of critically ill patients it is not possible for the patients to offer their evaluation of the change, which is why we believe it was most appropriate for the nurse to determine it. Also bearing in mind that the MOviPre19 study included the participation of 80 Spanish ICUs, it was complicated to establish similar criteria to determine the relevance of change, and the evaluation was performed by a single expert ICU nurse, based on the recordings of mobility and bearing in mind age, the Barthel index score on admission, diagnosis on admission, severity and length of ICU stay in days. Although it is true that it may not reflect what patients think, professional evaluation based on clinical data may also be considered a valid method.41
ConclusionsThe scale obtained is useful, valid, and reliable to be used by both ICU nurses and physiotherapists, to evaluate the mobility of critically ill patients and plan personalised activity programmes, to prevent ICU-acquired muscle weakness.
FinancingThis study did not receive any financing.
Conflict of interestThe authors have no conflict of interest to declare.
The Spanish Society of Intensive Nursing and Coronary Units (SEEIUC) was the study promoter.
Natalia Cámara-Conde, Oscar Peñuelas-Rodríguez, Raquel Herrero-Hernández, Mª Mar Sánchez-Sánchez, Mª Pilar Fraile-Gamo (Hospital Universitario de Getafe, Madrid); Candelas López-López, Mª Jesús Frade-Mera, Noelia Regueiro-Díaz, Luis Fernando Carrasco Rodríguez-Rey, Ignacio Zaragoza-García, Francisco Javier Zarza-Bejarano, Virginia Toribio-Rubio, María Catalina Pérez-Muñoz (Hospital Universitario 12 de Octubre, Madrid); María Acevedo Nuevo, Alejandro Barrios (Hospital Universitario Puerta de Hierro Majadahonda, Madrid); Tamara R. Velasco-Sanz (Hospital Universitario Clínico San Carlos, Madrid); Saúl García-González (Hospital Universitario de Móstoles, Madrid); Emilia Romero de San Pío (Hospital Central de Asturias, Oviedo); Joan-Daniel Martí-Romeu, Eva Blázquez-Martínez, Alicia San José-Arribas, Sandra Belmonte (Hospital Clinic de Barcelona, Barcelona); Elisabeth Gallart (Hospital Vall d'Hebrón, Barcelona). Ricardo Rodrigues Gomes (Hospital Alvaro Cunqueiro, Vigo, Pontevedra); Antonio Tomás Ríos Cortés (Hospital General Universitario Santa Lucía, Cartagena, Murcia); Roberto Martínez Alejos (Centre Hospitalier Universitaire, Bordeaux), Ana Rodríguez Merino (Royal Brompton and Harefield Hospital Trust, London), Elsa Afonso (Rosie Hospital, Cambridge).
Coordinadora Andalucía: María Esther Rodríguez Delgado. Autores: Antonia María Contreras Rodríguez; Ester Oreña Cimiano; Alvaro Ortega Guerrero; María del Carmen Martínez del Aguila; Virginia Rodríguez Monsalve; Carlos Leonardo Cano Herrera; Juan Manuel Masegosa Pérez, de los hospitales Poniente, Virgen de Valme, Quirón, Santa Ana.
Coordinadora Aragón: Delia María González de la Cuesta. Autores: María Inmaculada Pardo Artero; Marta Palacios Laseca; Ana Isabel Cabello Casao; María Belén Vicente de Vera Bellostas; Carmen Pérez Martínez; SheilaEscuder González; Amelia Lezcano Cisneros; Antonio Miguel Romeo; Isabel López Alegre, de los hospitales Clínico Universitario Lozano Blesa, Royo Villanova, Universitario Miguel Servet, Obispo Polanco.
Coordinadora Asturias: Emilia Romero de San Pío. Autores: Helena Fernández Alonso; Lara María Rodríguez Villanueva; Roberto Riaño Suárez, Begoña Sánchez Cerviñio, Sergio Carrasco Santos, de los hospitales Universitario Central de Asturias y San Agustín.
Coordinadora Canarias: Alicia San José Arribas. Autores: Miriam González García; Antonio Linares Tavio, del Complejo Hospitalario Universitario de Canarias.
Coordinadora Cantabria: Paz Álvarez García. Autores: Nuria Polo Hernández; Lourdes Gómez Cosío; Isabel Pérez Loza; Ángela Suárez Pérez; Sonia Crespo Rebollo, del Hospital Universitario Marqués de Valdecilla.
Coordinador Castilla La Mancha: Juan Carlos Muñoz Camargo. Autores: Julián García García; César Rojo Aguado; José Gómez López; Laura Sonseca Bartolomé, de los hospitales Universitario de Guadalajara y General de Albacete.
Coordinadora Castilla y León: Alicia San José Arribas. Autores: Sonia del Olmo Nuñez; Patricia García Mazo; Eduardo Siguero Torre; Isabel Muñoz Díez, de los hospitales Universitario Río Ortega y Clínico de Valladolid.
Coordinadora Catalunya: Pilar Delgado Hito. Autores: Mercedes Olalla Garrido Marín; Gemma Marín Vivó; Maria del Mar Eseverri Rovira; Montserrat Guillen Dobon; Montserrat Aran Esteve; Maribel Mirabete Rodríguez; Albert Mariné Méndez; Silvia Rodríguez Fernández; Joan Rosselló Sancho; Valeria Zafra Lamas; Inmaculada Carmona Delgado; Àngels Navarro Arilla; Gustau Zariquiey Esteva; Angel Lucas Bueno Luna; Cristina Lerma Brianso; Rubén Gómez García; Bernat Planas Pascual; Marta Sabaté López; Ana Isabel Mayer Frutos; Roser Roca Escrihuela; Gemma Torrents Albà; Vanesa Garcia Flores; Joan Melis Galmés; Sandra Belmonte Moral; Montserrat Grau Pellicer; Aintzane Ruiz Eizmendi; Carme Garriga Moll; Esteve Bosch de Jaureguízar, de los hospitales Universitari Vall d'Hebrón, Santa Creu i Sant Pau; Clínic de Barcelona; Consorci Sanitari de Terrassa; Universitari de Girona Dr. Josep Trueta; Universitari de Bellvitge.
Coordinador Extremadura: Sergio Cordovilla Guardia, Fidel López Espuela. Autores: Lara Mateos Hinojal, María Isabel Redondo Cantos, del hospital Complejo Hospitalario de Cáceres.
Coordinadora Galicia: Mª del Rosario Villar Redondo. Autores: Jesús Vila Rey, Susana Sánchez Méndez; Yolanda García Fernández; María Cristina Benítez Canosa; Mauricio Díaz Álvarez; José Ramón Cordo Isorna; Ángeles Estébez Penín; Gloria Güeto Rial; Esther Bouzas López, de los hospitales Universitario Lucus Augusti, Complejo Hospitalario Universitario de Ourense, Complejo Universitario Santiago de Compostela y Complejo Hospitalario Universitario de Pontevedra.
Coordinadoras Madrid: Susana Arias Rivera, María Jesús Frade Mera y María Jesús Luengo Alarcia. Autores: Noelia Regueiro Díaz; Luis Fernando Carrasco Rodríguez-Rey; María del Rosario Hernández García; Gema Sala Gómez; Javier Vecino Rubio; Saúl García González; María del Mar Sánchez Sánchez; Carmen Cruzado Franco; Beatriz Martín Rivera; Rocío González Blanco; Ana Belén Sánchez de la Ventana; Maria Luisa Bravo Arcas; Josefa Escobar Lavela; María del Pilar Domingo Moreno; Mercedes García Arias; Inmaculada Concepción Collado Saiz; María Acevedo Nuevo; Alejandro Barrios Suárez; Francisco Javier Zarza Bejarano; María Catalina Pérez Muñoz; Virginia Toribio Rubio; Patricia Martínez Chicharro; Alexandra Pascual Martínez; Sergio López Pozo; Laura Sánchez Infante; Verónica Ocaña García; Daniel Menes Medina; Ana Vadillo Cortázar; Gema Lendínez Burgos; Jesús Díaz Juntádez; María Teresa Godino Olivares; de los hospitales Universitario 12 de Octubre, Universitario de Móstoles, Universitario de Getafe, Universitario La Paz; Universitario Ramon y Cajal; Universitario Clínico San Carlos; Universitario del Sureste; Infanta Leonor, del Henares; Universitario Puerta de Hierro Majadahonda y Universitario Gregorio Marañón.
Coordinador Murcia: Juan José Rodríguez Mondéjar. Autores: Francisco José Martínez Rojo; María Vanessa Ruiz Martínez; Daniel Linares Celdrán; Antonio Ros Molina; Javier Sáez Sánchez; José María Martínez Oliva; Ana Bernal Gilar; María Belén Hernández García; Antonio Tomás Ríos Cortés; Raquel Navarro Méndez; Sebastián Gil García; Juan Sánchez Garre, de los hospitales Universitario Morales Messeguer, Universitario Los Arcos del Mar Menor, Universitario Santa Lucía, Universitario Rafael Méndez, Clínico Universitario Virgen de la Arrixaca y Reina Sofía de Murcia.
Coordinadora Navarra: Miriam del Barrio Linares. Autores: Rosana Goñi Viguria y Raquel Aguirre Santano; de la Clínica Universitaria de Navarra.
Coordinadora País Vasco: Mª Rosario García Díez. Autores: Laura Aparicio Cilla; Mónica Delicado Domingo; César Rodríguez Núñez; Ane Arrasate López, de los hospitales Universitario de Basurto y Universitario Araba (sede Txagorritxu).
Coordinadora Valencia: Ángela Romero Morán. Autores: Rosa Paños Melgoso; Mónica Yañez Cerón; Amparo Mercado Martínez; Beatriz Martínez Llopis; María Josefa Vayá Albelda; Javier Inat Carbonell; m. Rosario Alcayne Senent; Fátima Giménez García; Eva Cristina Fernández Gonzaga; Laura Febrer Puchol; Senén Berenguer Ortuño; María Pastor Martínez; Dunia Valera Talavera; María José Segrera Rovira; Yolanda Langa Revert; Maricruz Espí Pozuelo; María Ángeles de Diego; Beatriz Garijo Aspas; María del Rosario Asensio García; José Ramón Sánchez Muñoz; Quirico Martínez Sánchez; Ramón López Mateu, de los hospitales Universitario Dr. Peset Aleixandre, General de Elda, Universitario de la Ribera, Luís Alcanyis de Xàtiva, Clínico Universitario de Valencia, Provincial de Castellón, de Requena, Universitario San Juan, Universitario de la Plana, de Orihuela y Universitario de Elche.
Please cite this article as: Arias-Rivera S, Raurell-Torredà M, Thuissard-Vasallo IJ, Andreu-Vázquez C, Hodgson CL. Adaptación y validación de la UCI Mobility Scale en España. Enferm Intensiva. 2020;31:131–146.